The Future of PET Imaging in Multiple Sclerosis: Characterisation of Individual White Matter Lesions
Abstract
1. Introduction
Search Strategy
2. Multiple Sclerosis: Heterogeneity in Clinical Course and Pathology
2.1. Clinical Heterogeneity
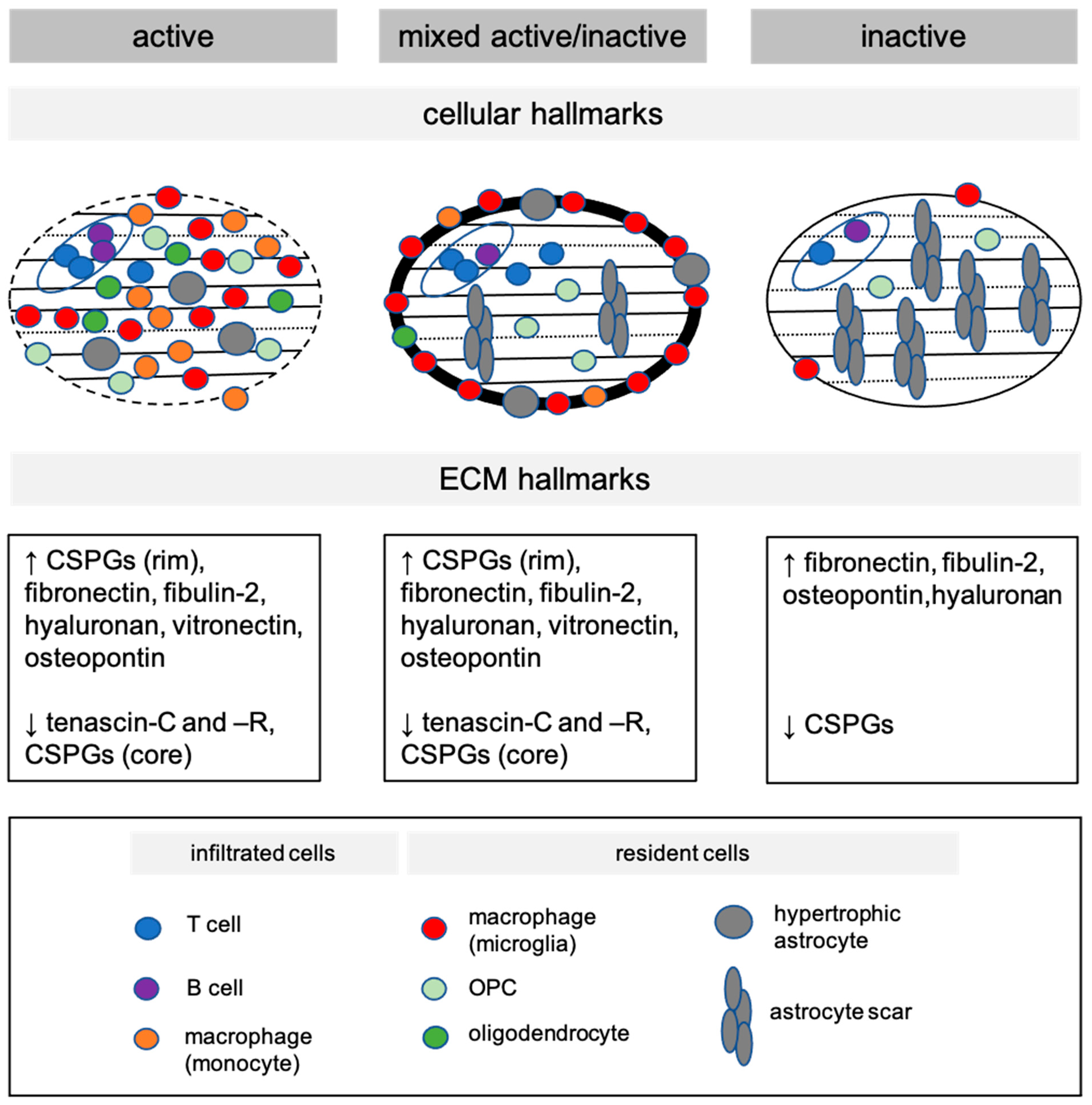
2.2. Distinct Cellular Hallmarks of Demyelinated White Matter MS Lesions
2.3. Distinct Extracellular Matrix Hallmarks of Demyelinated White Matter MS Lesions
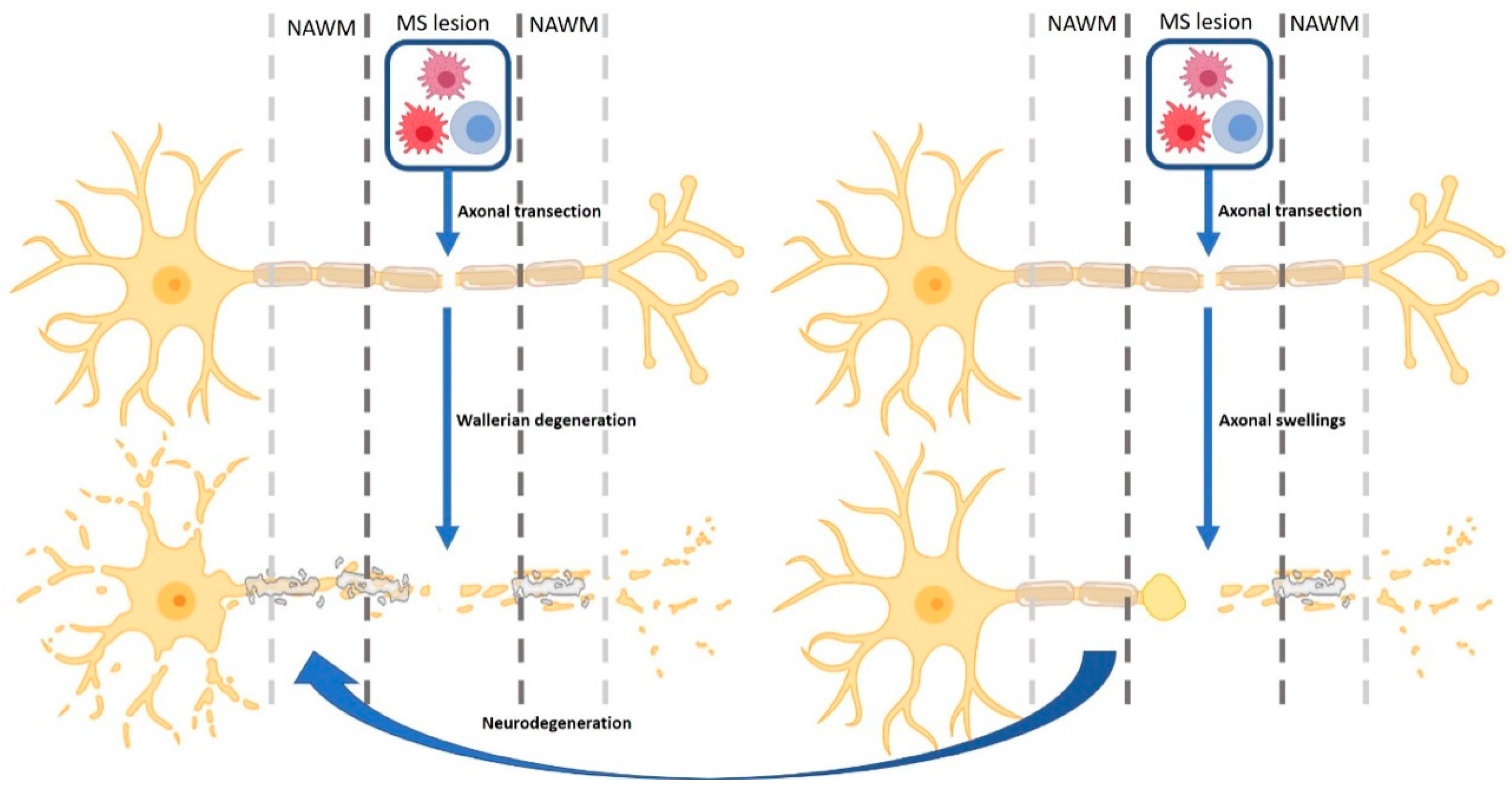
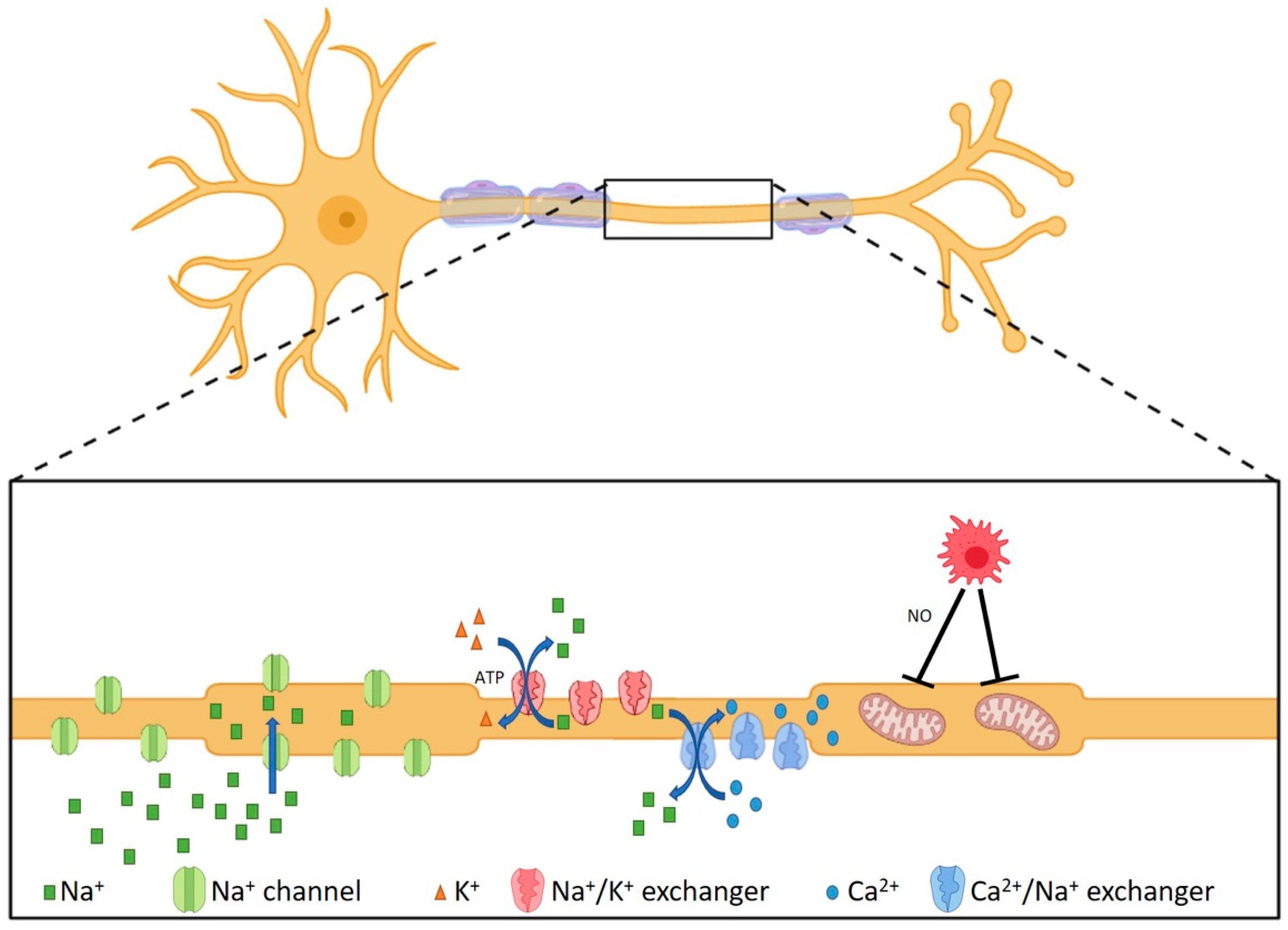
2.4. Neurodegenerative Processes in MS Lesions
3. MS Prognosis and Treatment Options
4. PET Imaging as a Tool to Stratify Distinct Lesions in MS Patients
4.1. PET Tracers for Cellular and Molecular Characteristics of MS Lesions
4.1.1. Inflammatory Activity Status
4.1.2. Myelin Density
4.1.3. Axonal Integrity
4.1.4. Oligodendrocyte Progenitor Cells
4.1.5. Extracellular Matrix
4.2. Potential Avenues for Novel PET Tracers for Brain Imaging: Targeted Blood–Brain Barrier Crossing
5. Considerations for PET Imaging of MS Lesion Characteristics in Clinical Practice
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Ramagopalan, S.V.; Dobson, R.; Meier, U.C.; Giovannoni, G. Multiple Sclerosis: Risk Factors, Prodromes, and Potential Causal Pathways. Lancet Neurol. 2010, 9, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Traugott, U.; Reinherz, E.L.; Raine, C.S. Multiple Sclerosis: Distribution of T Cells, T Cell Subsets and Ia-Positive Macrophages in Lesions of Different Ages. J. Neuroimmunol. 1983, 4, 201–221. [Google Scholar] [CrossRef]
- Huang, W.; Chen, W.; Zhang, X. Multiple Sclerosis: Pathology, Diagnosis and Treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef]
- Miljković, D.; Spasojević, I. Multiple Sclerosis: Molecular Mechanisms and Therapeutic Opportunities. Antioxid. Redox Signal. 2013, 19, 2286–2334. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Hlavica, M.; Schuler, F.A.F.; Good, N.; Good, A.; Baumgartner, L.; Galeno, G.; Schneider, M.P.; Jung, T.; De Vries, R. Remyelination Promoting Therapies in Multiple Sclerosis Animal Models: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- Gingele, S.; Stangel, M. Emerging Myelin Repair Agents in Preclinical and Early Clinical Development for the Treatment of Multiple Sclerosis. Expert Opin. Investig. Drugs 2020, 29, 583–594. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of Multiple Sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef]
- Franklin, R.J.M. Remyelination in the CNS: From Biology to Therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef]
- Lucchinetti, C.; Brück, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. A Quantitative Analysis of Oligodendrocytes in Multiple Sclerosis Lesions: A Study of 113 Cases. Brain 1999, 122, 2279–2295. [Google Scholar] [CrossRef] [PubMed]
- Heß, K.; Starost, L.; Kieran, N.W.; Thomas, C.; Vincenten, M.C.J.; Antel, J.; Martino, G.; Huitinga, I.; Healy, L.; Kuhlmann, T. Lesion Stage-Dependent Causes for Impaired Remyelination in MS. Acta Neuropathol. 2020, 140, 359–375. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Miron, V.; Cuo, Q.; Wegner, C.; Antel, J.; Brück, W. Differentiation Block of Oligodendroglial Progenitor Cells as a Cause for Remyelination Failure in Chronic Multiple Sclerosis. Brain 2008, 131, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Tourtellotte, W.W.; Rudick, R.; Trapp, B.D. Premyelinating Oligodendrocytes in Chronic Lesions of Multiple Sclerosis. N. Engl. J. Med. 2002, 346, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisén, J. Dynamics of Oligodendrocyte Generation in Multiple Sclerosis. Nature 2019, 566, 538–542. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Frisén, J.; Lyons, D.A. Revisiting Remyelination: Towards a Consensus on the Regeneration of CNS Myelin. Semin. Cell Dev. Biol. 2021, 116, 3–9. [Google Scholar] [CrossRef]
- Neely, S.A.; Williamson, J.M.; Klingseisen, A.; Zoupi, L.; Early, J.J.; Williams, A.; Lyons, D.A. New Oligodendrocytes Exhibit More Abundant and Accurate Myelin Regeneration than Those That Survive Demyelination. Nat. Neurosci. 2022, 25, 415–420. [Google Scholar] [CrossRef]
- Jäkel, S.; Agirre, E.; Mendanha Falcão, A.; Van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Williams, A.; Castelo-Branco, G. Altered Human Oligodendrocyte Heterogeneity in Multiple Sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef]
- Schirmer, L.; Velmeshev, D.; Holmqvist, S.; Kaufmann, M.; Werneburg, S.; Jung, D.; Vistnes, S.; Stockley, J.H.; Young, A.; Steindel, M. Neuronal Vulnerability and Multilineage Diversity in Multiple Sclerosis. Nature 2019, 573, 75–82. [Google Scholar] [CrossRef]
- de Jong, J.M.; Wang, P.; Oomkens, M.; Baron, W. Remodeling of the Interstitial Extracellular Matrix in White Matter Multiple Sclerosis Lesions: Implications for Remyelination (Failure). J. Neurosci. Res. 2020, 98, 1370–1397. [Google Scholar] [CrossRef]
- Ghorbani, S.; Yong, V.W. The Extracellular Matrix as Modifier of Neuroinflammation and Remyelination in Multiple Sclerosis. Brain 2021, 144, 1958–1973. [Google Scholar] [CrossRef]
- Gorter, R.P.; Baron, W. Matrix Metalloproteinases Shape the Oligodendrocyte (Niche) during Development and upon Demyelination. Neurosci. Lett. 2020, 729, 134980. [Google Scholar] [CrossRef]
- McCluskey, S.P.; Plisson, C.; Rabiner, E.A.; Howes, O. Advances in CNS PET: The State-of-the-Art for New Imaging Targets for Pathophysiology and Drug Development. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 451–489. [Google Scholar] [CrossRef]
- W Pike, V. Considerations in the Development of Reversibly Binding PET Radioligands for Brain Imaging. Curr. Med. Chem. 2016, 23, 1818–1869. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Coffman, P.; Pulaski, S.; Maloni, H.; Mahan, C.M.; Haselkorn, J.K.; Kurtzke, J.F.; Veterans Affairs Multiple Sclerosis Centres of Excellence Epidemiology Group. The Gulf War Era Multiple Sclerosis Cohort: Age and Incidence Rates by Race, Sex and Service. Brain 2012, 135, 1778–1785. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L. Diagnostic Criteria for Multiple Sclerosis: 2010 Revisions to the McDonald Criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Machado-Santos, J.; Saji, E.; Tröscher, A.R.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The Compartmentalized Inflammatory Response in the Multiple Sclerosis Brain Is Composed of Tissue-Resident CD8+ T Lymphocytes and B Cells. Brain 2018, 141, 2066–2082. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- Cotton, F.; Weiner, H.L.; Jolesz, F.A.; Guttmann, C.R.G. MRI Contrast Uptake in New Lesions in Relapsing-Remitting MS Followed at Weekly Intervals. Neurology 2003, 60, 640–646. [Google Scholar] [CrossRef]
- Scalfari, A.; Neuhaus, A.; Daumer, M.; Muraro, P.A.; Ebers, G.C. Onset of Secondary Progressive Phase and Long-Term Evolution of Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 67–75. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C. Defining the Clinical Course of Multiple Sclerosis: Results of an International Survey. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Hauser, S.L. Reply to “Silent Progression or Bout Onset Progressive Multiple Sclerosis?”. Ann. Neurol. 2019, 86, 472–473. [Google Scholar] [CrossRef]
- Kappos, L.; Butzkueven, H.; Wiendl, H.; Spelman, T.; Pellegrini, F.; Chen, Y.; Dong, Q.; Koendgen, H.; Belachew, S.; Trojano, M. Greater Sensitivity to Multiple Sclerosis Disability Worsening and Progression Events Using a Roving versus a Fixed Reference Value in a Prospective Cohort Study. Mult. Scler. J. 2018, 24, 963–973. [Google Scholar] [CrossRef]
- Luchetti, S.; Fransen, N.L.; van Eden, C.G.; Ramaglia, V.; Mason, M.; Huitinga, I. Progressive Multiple Sclerosis Patients Show Substantial Lesion Activity That Correlates with Clinical Disease Severity and Sex: A Retrospective Autopsy Cohort Analysis. Acta Neuropathol. 2018, 135, 511–528. [Google Scholar] [CrossRef]
- Frischer, J.M.; Weigand, S.D.; Guo, Y.; Kale, N.; Parisi, J.E.; Pirko, I.; Mandrekar, J.; Bramow, S.; Metz, I.; Brück, W. Clinical and Pathological Insights into the Dynamic Nature of the White Matter Multiple Sclerosis Plaque. Ann. Neurol. 2015, 78, 710–721. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Ludwin, S.; Prat, A.; Antel, J.; Brück, W.; Lassmann, H. An Updated Histological Classification System for Multiple Sclerosis Lesions. Acta Neuropathol. 2017, 133, 13–24. [Google Scholar] [CrossRef]
- Fransen, N.L.; de Jong, B.A.; Heß, K.; Kuhlmann, T.; Vincenten, M.C.J.; Hamann, J.; Huitinga, I.; Smolders, J. Absence of B Cells in Brainstem and White Matter Lesions Associates with Less Severe Disease and Absence of Oligoclonal Bands in MS. Neurol. Neuroinflamm. 2021, 8, e955. [Google Scholar] [CrossRef]
- Zrzavy, T.; Hametner, S.; Wimmer, I.; Butovsky, O.; Weiner, H.L.; Lassmann, H. Loss of ‘Homeostatic’Microglia and Patterns of Their Activation in Active Multiple Sclerosis. Brain 2017, 140, 1900–1913. [Google Scholar] [CrossRef]
- Fransen, N.L.; Hsiao, C.-C.; van der Poel, M.; Engelenburg, H.J.; Verdaasdonk, K.; Vincenten, M.C.J.; Remmerswaal, E.B.M.; Kuhlmann, T.; Mason, M.R.J.; Hamann, J. Tissue-Resident Memory T Cells Invade the Brain Parenchyma in Multiple Sclerosis White Matter Lesions. Brain 2020, 143, 1714–1730. [Google Scholar] [CrossRef]
- Lassmann, H. Axonal Injury in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2003, 74, 695–697. [Google Scholar] [CrossRef]
- Häusser-Kinzel, S.; Weber, M.S. The Role of B Cells and Antibodies in Multiple Sclerosis, Neuromyelitis Optica, and Related Disorders. Front. Immunol. 2019, 10, 201. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Wucherpfennig, K.W. B Cells and Autoantibodies in the Pathogenesis of Multiple Sclerosis and Related Inflammatory Demyelinating Diseases. Adv. Immunol. 2008, 98, 121–149. [Google Scholar]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Chang, A.; Nishiyama, A.; Peterson, J.; Prineas, J.; Trapp, B.D. NG2-Positive Oligodendrocyte Progenitor Cells in Adult Human Brain and Multiple Sclerosis Lesions. J. Neurosci. 2000, 20, 6404–6412. [Google Scholar] [CrossRef]
- Singh, S.; Dallenga, T.; Winkler, A.; Roemer, S.; Maruschak, B.; Siebert, H.; Brück, W.; Stadelmann, C. Relationship of Acute Axonal Damage, Wallerian Degeneration, and Clinical Disability in Multiple Sclerosis. J. Neuroinflamm. 2017, 14, 57. [Google Scholar] [CrossRef]
- Chang, A.; Staugaitis, S.M.; Dutta, R.; Batt, C.E.; Easley, K.E.; Chomyk, A.M.; Yong, V.W.; Fox, R.J.; Kidd, G.J.; Trapp, B.D. Cortical Remyelination: A New Target for Repair Therapies in Multiple Sclerosis. Ann. Neurol. 2012, 72, 918–926. [Google Scholar] [CrossRef]
- Jolanda Münzel, E.; Williams, A. Promoting Remyelination in Multiple Sclerosis—Recent Advances. Drugs 2013, 73, 2017–2029. [Google Scholar] [CrossRef]
- Jäckle, K.; Zeis, T.; Schaeren-Wiemers, N.; Junker, A.; van der Meer, F.; Kramann, N.; Stadelmann, C.; Brück, W. Molecular Signature of Slowly Expanding Lesions in Progressive Multiple Sclerosis. Brain 2020, 143, 2073–2088. [Google Scholar] [CrossRef]
- Macnair, W.; Calini, D.; Agirre, E.; Bryois, J.; Jäkel, S.; Smith, R.S.; Kukanja, P.; Stokar-Regenscheit, N.; Ott, V.; Foo, L.C. SnRNA-Seq Stratifies Multiple Sclerosis Patients into Distinct White Matter Glial Responses. Neuron 2025, 113, 396–410. [Google Scholar] [CrossRef]
- Lau, L.W.; Keough, M.B.; Haylock-Jacobs, S.; Cua, R.; Döring, A.; Sloka, S.; Stirling, D.P.; Rivest, S.; Yong, V.W. Chondroitin Sulfate Proteoglycans in Demyelinated Lesions Impair Remyelination. Ann. Neurol. 2012, 72, 419–432. [Google Scholar] [CrossRef]
- Hibbits, N.; Yoshino, J.; Le, T.Q.; Armstrong, R.C. Astrogliosis during Acute and Chronic Cuprizone Demyelination and Implications for Remyelination. ASN Neuro 2012, 4, AN20120062. [Google Scholar] [CrossRef]
- Keough, M.B.; Rogers, J.A.; Zhang, P.; Jensen, S.K.; Stephenson, E.L.; Chen, T.; Hurlbert, M.G.; Lau, L.W.; Rawji, K.S.; Plemel, J.R. An Inhibitor of Chondroitin Sulfate Proteoglycan Synthesis Promotes Central Nervous System Remyelination. Nat. Commun. 2016, 7, 11312. [Google Scholar] [CrossRef]
- Zhao, C.; Fancy, S.P.J.; Franklin, R.J.M.; ffrench-Constant, C. Up-regulation of Oligodendrocyte Precursor Cell AV Integrin and Its Extracellular Ligands during Central Nervous System Remyelination. J. Neurosci. Res. 2009, 87, 3447–3455. [Google Scholar] [CrossRef]
- Espitia Pinzón, N.; Brevé, J.J.P.; Bol, J.G.J.M.; Drukarch, B.; Baron, W.; van Dam, A.-M. Tissue Transglutaminase in Astrocytes Is Enhanced by Inflammatory Mediators and Is Involved in the Formation of Fibronectin Fibril-like Structures. J. Neuroinflamm. 2017, 14, 260. [Google Scholar] [CrossRef]
- Stoffels, J.M.J.; De Jonge, J.C.; Stancic, M.; Nomden, A.; Van Strien, M.E.; Ma, D.; Šiškova, Z.; Maier, O.; Ffrench-Constant, C.; Franklin, R.J.M. Fibronectin Aggregation in Multiple Sclerosis Lesions Impairs Remyelination. Brain 2013, 136, 116–131. [Google Scholar] [CrossRef]
- Selvaraju, R.; Bernasconi, L.; Losberger, C.; Graber, P.; Kadi, L.; Avellana-Adalid, V.; Picard-Riera, N.; Van Evercooren, A.B.; Cirillo, R.; Kosco-Vilbois, M. Osteopontin Is Upregulated during in Vivo Demyelination and Remyelination and Enhances Myelin Formation in Vitro. Mol. Cell. Neurosci. 2004, 25, 707–721. [Google Scholar] [CrossRef]
- Gutowski, N.J.; Newcombe, J.; Cuzner, M.L. Tenascin-R and C in Multiple Sclerosis Lesions: Relevance to Extracellular Matrix Remodelling. Neuropathol. Appl. Neurobiol. 1999, 25, 207–214. [Google Scholar] [CrossRef]
- Satoh, J.I.; Tabunoki, H.; Yamamura, T. Molecular Network of the Comprehensive Multiple Sclerosis Brain-Lesion Proteome. Mult. Scler. J. 2009, 15, 531–541. [Google Scholar] [CrossRef]
- Sobel, R.A.; Mitchell, M.E. Fibronectin in Multiple Sclerosis Lesions. Am. J. Pathol. 1989, 135, 161. [Google Scholar]
- van Horssen, J.; Bö, L.; Vos, C.M.P.; Virtanen, I.; de Vries, H.E. Basement Membrane Proteins in Multiple Sclerosis-Associated Inflammatory Cuffs: Potential Role in Influx and Transport of Leukocytes. J. Neuropathol. Exp. Neurol. 2005, 64, 722–729. [Google Scholar] [CrossRef]
- Ghorbani, S.; Li, C.; Lozinski, B.M.; Moezzi, D.; D’Mello, C.; Dong, Y.; Visser, F.; Li, H.; Silva, C.; Khakpour, M. Fibulin-2 Is an Extracellular Matrix Inhibitor of Oligodendrocytes Relevant to Multiple Sclerosis. J. Clin. Investig. 2024, 134, e176910. [Google Scholar] [CrossRef]
- Sobel, R.A.; Ahmed, A.S. White Matter Extracellular Matrix Chondroitin Sulfate/Dermatan Sulfate Proteoglycans in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2001, 60, 1198–1207. [Google Scholar] [CrossRef]
- Dahl, D.; Perides, G.; Bignami, A. Axonal Regeneration in Old Multiple Sclerosis Plaques. Acta Neuropathol. 1989, 79, 154–159. [Google Scholar] [CrossRef]
- Ghorbani, S.; Jelinek, E.; Jain, R.; Buehner, B.; Li, C.; Lozinski, B.M.; Sarkar, S.; Kaushik, D.K.; Dong, Y.; Wight, T.N. Versican Promotes T Helper 17 Cytotoxic Inflammation and Impedes Oligodendrocyte Precursor Cell Remyelination. Nat. Commun. 2022, 13, 2445. [Google Scholar] [CrossRef]
- Back, S.A.; Tuohy, T.M.F.; Chen, H.; Wallingford, N.; Craig, A.; Struve, J.; Luo, N.L.; Banine, F.; Liu, Y.; Chang, A. Hyaluronan Accumulates in Demyelinated Lesions and Inhibits Oligodendrocyte Progenitor Maturation. Nat. Med. 2005, 11, 966–972. [Google Scholar] [CrossRef]
- Wang, P.; Gorter, R.P.; de Jonge, J.C.; Nazmuddin, M.; Zhao, C.; Amor, S.; Hoekstra, D.; Baron, W. MMP 7 Cleaves Remyelination-impairing Fibronectin Aggregates and Its Expression Is Reduced in Chronic Multiple Sclerosis Lesions. Glia 2018, 66, 1625–1643. [Google Scholar] [CrossRef]
- Maeda, A.; Sobel, R.A. Matrix Metalloproteinases in the Normal Human Central Nervous System, Microglial Nodules, and Multiple Sclerosis Lesions. J. Neuropathol. Exp. Neurol. 1996, 55, 300–309. [Google Scholar] [CrossRef]
- Vos, C.M.P.; van Haastert, E.S.; de Groot, C.J.A.; van der Valk, P.; de Vries, H.E. Matrix Metalloproteinase-12 Is Expressed in Phagocytotic Macrophages in Active Multiple Sclerosis Lesions. J. Neuroimmunol. 2003, 138, 106–114. [Google Scholar] [CrossRef]
- Cuzner, M.L.; Gveric, D.; Strand, C.; Loughlin, A.J.; Paemen, L.; Opdenakker, G.; Newcombe, J. The Expression of Tissue-Type Plasminogen Activator, Matrix Metalloproteases and Endogenous Inhibitors in the Central Nervous System in Multiple Sclerosis: Comparison of Stages in Lesion Evolution. J. Neuropathol. Exp. Neurol. 1996, 55, 1194–1204. [Google Scholar] [CrossRef]
- Anthony, D.C.; Ferguson, B.; Matyzak, M.K.; Miller, K.M.; Esiri, M.M.; Perry, V.H. Differential Matrix Metalloproteinase Expression in Cases of Multiple Sclerosis and Stroke. Neuropathol. Appl. Neurobiol. 1997, 23, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Sanchez, M.; Williams, K.; DeLuca, G.C.; Esiri, M.M. Protein Co-Expression with Axonal Injury in Multiple Sclerosis Plaques. Acta Neuropathol. 2006, 111, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Vos, C.M.P.; Admiraal, L.; Van Haastert, E.S.; Montagne, L.; Van der Valk, P.; De Vries, H.E. Matrix Metalloproteinase-19 Is Highly Expressed in Active Multiple Sclerosis Lesions. Neuropathol. Appl. Neurobiol. 2006, 32, 585–593. [Google Scholar] [CrossRef]
- Werkman, I.; Sikkema, A.H.; Versluijs, J.B.; Qin, J.; de Boer, P.; Baron, W. TLR3 Agonists Induce Fibronectin Aggregation by Activated Astrocytes: A Role of pro-Inflammatory Cytokines and Fibronectin Splice Variants. Sci. Rep. 2020, 10, 532. [Google Scholar] [CrossRef]
- Sikkema, A.H.; Stoffels, J.M.J.; Wang, P.; Basedow, F.J.; Bulsink, R.; Bajramovic, J.J.; Baron, W. Fibronectin Aggregates Promote Features of a Classically and Alternatively Activated Phenotype in Macrophages. J. Neuroinflamm. 2018, 15, 218. [Google Scholar] [CrossRef]
- Dyck, S.; Kataria, H.; Alizadeh, A.; Santhosh, K.T.; Lang, B.; Silver, J.; Karimi-Abdolrezaee, S. Perturbing Chondroitin Sulfate Proteoglycan Signaling through LAR and PTPσ Receptors Promotes a Beneficial Inflammatory Response Following Spinal Cord Injury. J. Neuroinflamm. 2018, 15, 90. [Google Scholar] [CrossRef]
- Rolls, A.; Shechter, R.; London, A.; Segev, Y.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Schwartz, M. Two Faces of Chondroitin Sulfate Proteoglycan in Spinal Cord Repair: A Role in Microglia/Macrophage Activation. PLoS Med. 2008, 5, e171. [Google Scholar] [CrossRef]
- Bjartmar, C.; Wujek, J.R.; Trapp, B.D. Axonal Loss in the Pathology of MS: Consequences for Understanding the Progressive Phase of the Disease. J. Neurol. Sci. 2003, 206, 165–171. [Google Scholar] [CrossRef]
- Haines, J.D.; Inglese, M.; Casaccia, P. Axonal Damage in Multiple Sclerosis. Mt. Sinai J. Med. A J. Transl. Pers. Med. 2011, 78, 231–243. [Google Scholar] [CrossRef]
- Stassart, R.M.; Möbius, W.; Nave, K.-A.; Edgar, J.M. The Axon-Myelin Unit in Development and Degenerative Disease. Front. Neurosci. 2018, 12, 467. [Google Scholar] [CrossRef]
- Micu, I.; Plemel, J.R.; Caprariello, A.V.; Nave, K.-A.; Stys, P.K. Axo-Myelinic Neurotransmission: A Novel Mode of Cell Signalling in the Central Nervous System. Nat. Rev. Neurosci. 2018, 19, 49–58. [Google Scholar] [CrossRef]
- Saab, A.S.; Nave, K.-A. Myelin Dynamics: Protecting and Shaping Neuronal Functions. Curr. Opin. Neurobiol. 2017, 47, 104–112. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.-W. Oligodendroglia Metabolically Support Axons and Contribute to Neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W. Glycolytic Oligodendrocytes Maintain Myelin and Long-Term Axonal Integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef]
- Su, K.G.; Banker, G.; Bourdette, D.; Forte, M. Axonal Degeneration in Multiple Sclerosis: The Mitochondrial Hypothesis. Curr. Neurol. Neurosci. Rep. 2009, 9, 411–417. [Google Scholar] [CrossRef]
- Gironi, M.; Arnò, C.; Comi, G.; Penton-Rol, G.; Furlan, R. Multiple Sclerosis and Neurodegenerative Diseases. In Immune Rebalancing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 63–84. [Google Scholar]
- Criste, G.; Trapp, B.; Dutta, R. Axonal Loss in Multiple Sclerosis: Causes and Mechanisms. Handb. Clin. Neurol. 2014, 122, 101–113. [Google Scholar]
- Lisak, R.P. Neurodegeneration in Multiple Sclerosis: Defining the Problem. Neurology 2007, 68, S5–S12. [Google Scholar] [CrossRef]
- Perry, V.H.; Anthony, D.C. Axon Damage and Repair in Multiple Sclerosis. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999, 354, 1641–1647. [Google Scholar] [CrossRef]
- Ontaneda, D. Progressive Multiple Sclerosis. Contin. Lifelong Learn. Neurol. 2019, 25, 736–752. [Google Scholar] [CrossRef]
- Roemer, S.F.; Lucchinetti, C.F. Immune Effector Heterogeneity in Multiple Sclerosis and Related CNS Inflammatory Demyelinating Disorders. In Inflammatory Diseases of the Central Nervous System; Ransohoff, R.M., Wesselingh, S., Kilpatrick, T., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 47–74. ISBN 9780521888745. [Google Scholar]
- Lubetzki, C.; Zalc, B.; Kremer, D.; Küry, P. Endogenous Clues Promoting Remyelination in Multiple Sclerosis. Curr. Opin. Neurol. 2022, 35, 307–312. [Google Scholar] [CrossRef]
- Zhao, X.; Jacob, C. Mechanisms of Demyelination and Remyelination Strategies for Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 6373. [Google Scholar] [CrossRef] [PubMed]
- López-Muguruza, E.; Villar-Gómez, N.; Matias-Guiu, J.A.; Selma-Calvo, B.; Moreno-Jiménez, L.; Sancho-Bielsa, F.; Lopez-Carbonero, J.; Benito-Martín, M.S.; García-Flores, S.; Bonel-García, N. The Integration of Cell Therapy and Biomaterials as Treatment Strategies for Remyelination. Life 2022, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C. 2021 MAGNIMS–CMSC–NAIMS Consensus Recommendations on the Use of MRI in Patients with Multiple Sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef]
- Maggi, P.; Kuhle, J.; Schädelin, S.; van der Meer, F.; Weigel, M.; Galbusera, R.; Mathias, A.; Lu, P.-J.; Rahmanzadeh, R.; Benkert, P.; et al. Chronic White Matter Inflammation and Serum Neurofilament Levels in Multiple Sclerosis. Neurology 2021, 97, e543–e553. [Google Scholar] [CrossRef]
- Absinta, M.; Sati, P.; Schindler, M.; Leibovitch, E.C.; Ohayon, J.; Wu, T.; Meani, A.; Filippi, M.; Jacobson, S.; Cortese, I.C.M. Persistent 7-Tesla Phase Rim Predicts Poor Outcome in New Multiple Sclerosis Patient Lesions. J. Clin. Investig. 2016, 126, 2597–2609. [Google Scholar] [CrossRef]
- Bagnato, F.; Sati, P.; Hemond, C.C.; Elliott, C.; Gauthier, S.A.; Harrison, D.M.; Mainero, C.; Oh, J.; Pitt, D.; Shinohara, R.T. Imaging Chronic Active Lesions in Multiple Sclerosis: A Consensus Statement. Brain 2024, 147, 2913–2933. [Google Scholar] [CrossRef]
- Bruschi, N.; Boffa, G.; Inglese, M. Ultra-High-Field 7-T MRI in Multiple Sclerosis and Other Demyelinating Diseases: From Pathology to Clinical Practice. Eur. Radiol. Exp. 2020, 4, 59. [Google Scholar] [CrossRef]
- Lou, C.; Sati, P.; Absinta, M.; Clark, K.; Dworkin, J.D.; Valcarcel, A.M.; Schindler, M.K.; Reich, D.S.; Sweeney, E.M.; Shinohara, R.T. Fully Automated Detection of Paramagnetic Rims in Multiple Sclerosis Lesions on 3T Susceptibility-Based MR Imaging. NeuroImage. Clin. 2021, 32, 102796. [Google Scholar] [CrossRef] [PubMed]
- Absinta, M.; Sati, P.; Fechner, A.; Schindler, M.K.; Nair, G.; Reich, D.S. Identification of Chronic Active Multiple Sclerosis Lesions on 3T MRI. AJNR. Am. J. Neuroradiol. 2018, 39, 1233–1238. [Google Scholar] [CrossRef]
- Montalban, X.; Lebrun-Frenay, C.; Oh, J.; Arrambide, G.; Moccia, M.; Pia Amato, M.; Amezcua, L.; Banwell, B.; Bar-Or, A.; Barkhof, F.; et al. 2024 Revisions of the McDonald Diagnostic Criteria for MS. Available online: https://ectrims.eu/mcdonald-diagnostic-criteria (accessed on 15 May 2025).
- van der Weijden, C.W.J.; García, D.V.; Borra, R.J.H.; Thurner, P.; Meilof, J.F.; van Laar, P.-J.; Dierckx, R.A.J.O.; Gutmann, I.W.; de Vries, E.F.J. Myelin Quantification with MRI: A Systematic Review of Accuracy and Reproducibility. Neuroimage 2021, 226, 117561. [Google Scholar] [CrossRef]
- van der Weijden, C.W.J.; Biondetti, E.; Gutmann, I.W.; Dijkstra, H.; McKerchar, R.; de Paula Faria, D.; de Vries, E.F.J.; Meilof, J.F.; Dierckx, R.A.J.O.; Prevost, V.H. Quantitative Myelin Imaging with MRI and PET: An Overview of Techniques and Their Validation Status. Brain 2022, 146, 1243–1266. [Google Scholar] [CrossRef]
- van der Weijden, C.W.J.; van der Hoorn, A.; Potze, J.H.; Renken, R.J.; Borra, R.J.H.; Dierckx, R.A.J.O.; Gutmann, I.W.; Ouaalam, H.; Karimi, D.; Gholipour, A. Diffusion-Derived Parameters in Lesions, Peri-Lesion and Normal-Appearing White Matter in Multiple Sclerosis Using Tensor, Kurtosis and Fixel-Based Analysis. J. Cereb. Blood Flow Metab. 2022, 42, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- van der Hoorn, A.; Manusiwa, L.E.; van der Weide, H.L.; Sinnige, P.F.; Huitema, R.B.; Brouwer, C.L.; Klos, J.; Borra, R.J.H.; Dierckx, R.A.J.O.; Rakers, S.E.; et al. Assessing the Validity of Diffusion Weighted Imaging Models: A Study in Patients with Post-Surgical Lower-Grade Glioma. J. Clin. Med. 2025, 14, 551. [Google Scholar] [CrossRef] [PubMed]
- Villoslada, P.; Steinman, L. New Targets and Therapeutics for Neuroprotection, Remyelination and Repair in Multiple Sclerosis. Expert Opin. Investig. Drugs 2020, 29, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Pu, A.; Stephenson, E.L.; Yong, V.W. The Extracellular Matrix: Focus on Oligodendrocyte Biology and Targeting CSPG s for Remyelination Therapies. Glia 2018, 66, 1809–1825. [Google Scholar] [CrossRef]
- Frost, E.E.; Buttery, P.C.; Milner, R. Integrins Mediate a Neuronal Survival Signal for Oligodendrocytes. Curr. Biol. 1999, 9, 1251-S1. [Google Scholar] [CrossRef]
- Colognato, H.; Baron, W.; Avellana-Adalid, V.; Relvas, J.B.; Evercooren, A.B.-V.; Georges-Labouesse, E. CNS Integrins Switch Growth Factor Signalling to Promote Target-Dependent Survival. Nat. Cell Biol. 2002, 4, 833–841. [Google Scholar] [CrossRef]
- Colognato, H.; Tzvetanova, I.D. Glia Unglued: How Signals from the Extracellular Matrix Regulate the Development of Myelinating Glia. Dev. Neurobiol. 2011, 71, 924–955. [Google Scholar] [CrossRef]
- Relucio, J.; Tzvetanova, I.D.; Ao, W.; Lindquist, S.; Colognato, H. Laminin Alters Fyn Regulatory Mechanisms and Promotes Oligodendrocyte Development. J. Neurosci. 2009, 29, 11794–11806. [Google Scholar] [CrossRef]
- Relucio, J.; Menezes, M.J.; Miyagoe-Suzuki, Y.; Takeda, S.; Colognato, H. Laminin Regulates Postnatal Oligodendrocyte Production by Promoting Oligodendrocyte Progenitor Survival in the Subventricular Zone. Glia 2012, 60, 1451–1467. [Google Scholar] [CrossRef]
- Pendleton, J.C.; Shamblott, M.J.; Gary, D.S.; Belegu, V.; Hurtado, A.; Malone, M.L.; McDonald, J.W. Chondroitin Sulfate Proteoglycans Inhibit Oligodendrocyte Myelination through PTPσ. Exp. Neurol. 2013, 247, 113–121. [Google Scholar] [CrossRef]
- Siebert, J.R.; Osterhout, D.J. The Inhibitory Effects of Chondroitin Sulfate Proteoglycans on Oligodendrocytes. J. Neurochem. 2011, 119, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Preston, M.; Gong, X.; Su, W.; Matsumoto, S.G.; Banine, F.; Winkler, C.; Foster, S.; Xing, R.; Struve, J.; Dean, J. Digestion Products of the PH20 Hyaluronidase Inhibit Remyelination. Ann. Neurol. 2013, 73, 266–280. [Google Scholar] [CrossRef]
- Srivastava, T.; Diba, P.; Dean, J.M.; Banine, F.; Shaver, D.; Hagen, M.; Gong, X.; Su, W.; Emery, B.; Marks, D.L. A TLR/AKT/FoxO3 Immune Tolerance–like Pathway Disrupts the Repair Capacity of Oligodendrocyte Progenitors. J. Clin. Investig. 2018, 128, 2025–2041. [Google Scholar] [CrossRef] [PubMed]
- Šišková, Z.; Baron, W.; de Vries, H.; Hoekstra, D. Fibronectin Impedes “Myelin” Sheet-Directed Flow in Oligodendrocytes: A Role for a Beta 1 Integrin-Mediated PKC Signaling Pathway in Vesicular Trafficking. Mol. Cell. Neurosci. 2006, 33, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Šišková, Z.; Yong, V.W.; Nomden, A.; van Strien, M.; Hoekstra, D.; Baron, W. Fibronectin Attenuates Process Outgrowth in Oligodendrocytes by Mislocalizing MMP-9 Activity. Mol. Cell. Neurosci. 2009, 42, 234–242. [Google Scholar] [CrossRef]
- Baron, W.; Bijlard, M.; Nomden, A.; de Jonge, J.C.; Teunissen, C.E.; Hoekstra, D. Sulfatide-mediated Control of Extracellular Matrix-dependent Oligodendrocyte Maturation. Glia 2014, 62, 927–942. [Google Scholar] [CrossRef]
- Stoffels, J.M.J.; Hoekstra, D.; Franklin, R.J.M.; Baron, W.; Zhao, C. The EIIIA Domain from Astrocyte-derived Fibronectin Mediates Proliferation of Oligodendrocyte Progenitor Cells Following CNS Demyelination. Glia 2015, 63, 242–256. [Google Scholar] [CrossRef]
- Pu, A.; Mishra, M.K.; Dong, Y.; Ghorbanigazar, S.; Stephenson, E.L.; Rawji, K.S.; Silva, C.; Kitagawa, H.; Sawcer, S.; Yong, V.W. The Glycosyltransferase EXTL2 Promotes Proteoglycan Deposition and Injurious Neuroinflammation Following Demyelination. J. Neuroinflamm. 2020, 17, 220. [Google Scholar] [CrossRef]
- Feliu, A.; Mestre, L.; Carrillo-Salinas, F.J.; Yong, V.W.; Mecha, M.; Guaza, C. 2-arachidonoylglycerol Reduces Chondroitin Sulphate Proteoglycan Production by Astrocytes and Enhances Oligodendrocyte Differentiation under Inhibitory Conditions. Glia 2020, 68, 1255–1273. [Google Scholar] [CrossRef]
- Stephenson, E.L.; Zhang, P.; Ghorbani, S.; Wang, A.; Gu, J.; Keough, M.B.; Rawji, K.S.; Silva, C.; Yong, V.W.; Ling, C.-C. Targeting the Chondroitin Sulfate Proteoglycans: Evaluating Fluorinated Glucosamines and Xylosides in Screens Pertinent to Multiple Sclerosis. ACS Cent. Sci. 2019, 5, 1223–1234. [Google Scholar] [CrossRef]
- Su, W.; Matsumoto, S.; Banine, F.; Srivastava, T.; Dean, J.; Foster, S.; Pham, P.; Hammond, B.; Peters, A.; Girish, K.S. A Modified Flavonoid Accelerates Oligodendrocyte Maturation and Functional Remyelination. Glia 2020, 68, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Tran, A.P.; Xin, L.; Sanapala, C.; Lang, B.T.; Silver, J.; Yang, Y. Modulation of Proteoglycan Receptor PTPσ Enhances MMP-2 Activity to Promote Recovery from Multiple Sclerosis. Nat. Commun. 2018, 9, 4126. [Google Scholar] [CrossRef]
- Qin, J.; Sikkema, A.H.; van der Bij, K.; de Jonge, J.C.; Klappe, K.; Nies, V.; Jonker, J.W.; Kok, J.W.; Hoekstra, D.; Baron, W. GD1a Overcomes Inhibition of Myelination by Fibronectin via Activation of Protein Kinase A: Implications for Multiple Sclerosis. J. Neurosci. 2017, 37, 9925–9938. [Google Scholar] [CrossRef] [PubMed]
- Brier, M.R.; Taha, F. Measuring Pathology in Patients with Multiple Sclerosis Using Positron Emission Tomography. Curr. Neurol. Neurosci. Rep. 2023, 23, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Goulart, M.T.; Queiroz, D.T.U.; Ribeiro, F.M. The Dual Role of Microglia in Multiple Sclerosis and Its Implications for Diagnostics and Repair. Curr. Neuropharmacol. 2025. [Google Scholar] [CrossRef]
- Stankoff, B.; Poirion, E.; Tonietto, M.; Bodini, B. Exploring the Heterogeneity of MS Lesions Using Positron Emission Tomography: A Reappraisal of Their Contribution to Disability. Brain Pathol. 2018, 28, 723–734. [Google Scholar] [CrossRef]
- Bodini, B.; Tonietto, M.; Airas, L.; Stankoff, B. Positron Emission Tomography in Multiple Sclerosis—Straight to the Target. Nat. Rev. Neurol. 2021, 17, 663–675. [Google Scholar] [CrossRef]
- Poutiainen, P.; Jaronen, M.; Quintana, F.J.; Brownell, A.-L. Precision Medicine in Multiple Sclerosis: Future of PET Imaging of Inflammation and Reactive Astrocytes. Front. Mol. Neurosci. 2016, 9, 85. [Google Scholar] [CrossRef]
- Airas, L.; Rissanen, E.; Rinne, J. Imaging of Microglial Activation in MS Using PET: Research Use and Potential Future Clinical Application. Mult. Scler. J. 2017, 23, 496–504. [Google Scholar] [CrossRef]
- Högel, H.; Rissanen, E.; Vuorimaa, A.; Airas, L. Positron Emission Tomography Imaging in Evaluation of MS Pathology in Vivo. Mult. Scler. J. 2018, 24, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M.; Capitanio, S.; Raffa, S.; Roccatagliata, L.; Pardini, M.; Lapucci, C.; Marini, C.; Sambuceti, G.; Inglese, M.; Gallo, P. Molecular Imaging of Multiple Sclerosis: From the Clinical Demand to Novel Radiotracers. EJNMMI Radiopharm. Chem. 2019, 4, 6. [Google Scholar] [CrossRef]
- Sucksdorff, M.; Matilainen, M.; Tuisku, J.; Polvinen, E.; Vuorimaa, A.; Rokka, J.; Nylund, M.; Rissanen, E.; Airas, L. Brain TSPO-PET Predicts Later Disease Progression Independent of Relapses in Multiple Sclerosis. Brain 2020, 143, 3318–3330. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, E.; Tuisku, J.; Vahlberg, T.; Sucksdorff, M.; Paavilainen, T.; Parkkola, R.; Rokka, J.; Gerhard, A.; Hinz, R.; Talbot, P.S.; et al. Microglial Activation, White Matter Tract Damage, and Disability in MS. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e443. [Google Scholar] [CrossRef]
- Debruyne, J.C.; Versijpt, J.; Van Laere, K.J.; De Vos, F.; Keppens, J.; Strijckmans, K.; Achten, E.; Slegers, G.; Dierckx, R.A.; Korf, J.; et al. PET Visualization of Microglia in Multiple Sclerosis Patients Using [11C]PK11195. Eur. J. Neurol. 2003, 10, 257–264. [Google Scholar] [CrossRef]
- Polvinen, E.; Matilainen, M.; Nylund, M.; Sucksdorff, M.; Airas, L.M. TSPO-Detectable Chronic Active Lesions Predict Disease Progression in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200133. [Google Scholar] [CrossRef]
- Sucksdorff, M.; Tuisku, J.; Matilainen, M.; Vuorimaa, A.; Smith, S.; Keitilä, J.; Rokka, J.; Parkkola, R.; Nylund, M.; Rinne, J.; et al. Natalizumab Treatment Reduces Microglial Activation in the White Matter of the MS Brain. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e574. [Google Scholar] [CrossRef] [PubMed]
- Nutma, E.; Stephenson, J.A.; Gorter, R.P.; de Bruin, J.; Boucherie, D.M.; Donat, C.K.; Breur, M.; van der Valk, P.; Matthews, P.M.; Owen, D.R. A Quantitative Neuropathological Assessment of Translocator Protein Expression in Multiple Sclerosis. Brain 2019, 142, 3440–3455. [Google Scholar] [CrossRef]
- Chauveau, F.; Winkeler, A.; Chalon, S.; Boutin, H.; Becker, G. PET Imaging of Neuroinflammation: Any Credible Alternatives to TSPO Yet? Mol. Psychiatry 2025, 30, 213–228. [Google Scholar] [CrossRef]
- Hagens, M.H.J.; Golla, S.S.V.; Janssen, B.; Vugts, D.J.; Beaino, W.; Windhorst, A.D.; O’Brien-Brown, J.; Kassiou, M.; Schuit, R.C.; Schwarte, L.A. The P2X7 Receptor Tracer [11C]SMW139 as an in Vivo Marker of Neuroinflammation in Multiple Sclerosis: A First-in Man Study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 379–389. [Google Scholar] [CrossRef]
- Burnstock, G. P2X Ion Channel Receptors and Inflammation. Purinergic Signal. 2016, 12, 59–67. [Google Scholar] [CrossRef]
- de Torre-Minguela, C.; Barberà-Cremades, M.; Gómez, A.I.; Martín-Sánchez, F.; Pelegrín, P. Macrophage Activation and Polarization Modify P2X7 Receptor Secretome Influencing the Inflammatory Process. Sci. Rep. 2016, 6, 22586. [Google Scholar] [CrossRef]
- Rissanen, E.; Virta, J.R.; Paavilainen, T.; Tuisku, J.; Helin, S.; Luoto, P.; Parkkola, R.; Rinne, J.O.; Airas, L. Adenosine A2A Receptors in Secondary Progressive Multiple Sclerosis: A [11C] TMSX Brain PET Study. J. Cereb. Blood Flow Metab. 2013, 33, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Waggan, I.; Rissanen, E.; Tuisku, J.; Matilainen, M.; Parkkola, R.; Rinne, J.O.; Airas, L. Adenosine A2A Receptor Availability in Cerebral Gray and White Matter of Patients with Parkinson’s Disease. Park. Relat. Disord. 2023, 113, 105766. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.R.; Cunha, R.A.; Agostinho, P. Astrocytes and Adenosine A2 A Receptors: Active Players in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 666710. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.-H.; Song, J. Adenosine and Adenosine Receptors in Metabolic Imbalance-Related Neurological Issues. Biomed. Pharmacother. 2024, 177, 116996. [Google Scholar] [CrossRef]
- Bours, M.J.L.; Swennen, E.L.R.; Di Virgilio, F.; Cronstein, B.N.; Dagnelie, P.C. Adenosine 5′-Triphosphate and Adenosine as Endogenous Signaling Molecules in Immunity and Inflammation. Pharmacol. Ther. 2006, 112, 358–404. [Google Scholar] [CrossRef]
- Madeira, M.H.; Rashid, K.; Ambrósio, A.F.; Santiago, A.R.; Langmann, T. Blockade of Microglial Adenosine A2A Receptor Impacts Inflammatory Mechanisms, Reduces ARPE-19 Cell Dysfunction and Prevents Photoreceptor Loss in Vitro. Sci. Rep. 2018, 8, 2272. [Google Scholar] [CrossRef]
- Rajasundaram, S. Adenosine A2A Receptor Signaling in the Immunopathogenesis of Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Olsen, M.; Aguilar, X.; Sehlin, D.; Fang, X.T.; Antoni, G.; Erlandsson, A.; Syvänen, S. Astroglial Responses to Amyloid-Beta Progression in a Mouse Model of Alzheimer’s Disease. Mol. Imaging Biol. 2018, 20, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Jaisa-Aad, M.; Muñoz-Castro, C.; Healey, M.A.; Hyman, B.T.; Serrano-Pozo, A. Characterization of Monoamine Oxidase-B (MAO-B) as a Biomarker of Reactive Astrogliosis in Alzheimer’s Disease and Related Dementias. Acta Neuropathol. 2024, 147, 66. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.M.; Roy, A.; Varshney, M.; Kumar, A. Mapping Reactive Astrogliosis in Parkinson’s Brain with Astroglial Tracers BU99008 and Deprenyl: New Insights from a Multi-marker Postmortem Study. Alzheimer’s Dement. 2025, 21, e14488. [Google Scholar] [CrossRef]
- Horti, A.G.; Naik, R.; Foss, C.A.; Minn, I.; Misheneva, V.; Du, Y.; Wang, Y.; Mathews, W.B.; Wu, Y.; Hall, A. PET Imaging of Microglia by Targeting Macrophage Colony-Stimulating Factor 1 Receptor (CSF1R). Proc. Natl. Acad. Sci. USA 2019, 116, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ji, B.; Seki, C.; Nagai, Y.; Minamimoto, T.; Fujinaga, M.; Zhang, M.-R.; Saito, T.; Saido, T.C.; Suhara, T.; et al. PET Imaging of Colony-Stimulating Factor 1 Receptor: A Head-to-Head Comparison of a Novel Radioligand, (11)C-GW2580, and (11)C-CPPC, in Mouse Models of Acute and Chronic Neuroinflammation and a Rhesus Monkey. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2021, 41, 2410–2422. [Google Scholar] [CrossRef]
- Altomonte, S.; Yan, X.; Morse, C.L.; Liow, J.-S.; Jenkins, M.D.; Montero Santamaria, J.A.; Zoghbi, S.S.; Innis, R.B.; Pike, V.W. Discovery of a High-Affinity Fluoromethyl Analog of [11C] 5-Cyano-N-(4-(4-Methylpiperazin-1-Yl)-2-(Piperidin-1-Yl) Phenyl) Furan-2-Carboxamide ([11C] CPPC) and Their Comparison in Mouse and Monkey as Colony-Stimulating Factor 1 Receptor Positron Emission. ACS Pharmacol. Transl. Sci. 2023, 6, 614–632. [Google Scholar] [CrossRef]
- Narayanaswami, V.; Dahl, K.; Bernard-Gauthier, V.; Josephson, L.; Cumming, P.; Vasdev, N. Emerging PET Radiotracers and Targets for Imaging of Neuroinflammation in Neurodegenerative Diseases: Outlook Beyond TSPO. Mol. Imaging 2018, 17, 1536012118792317. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Chaney, A.M.; Carlson, M.L.; Jackson, I.M.; Rao, A.; James, M.L. Neuroinflammation PET Imaging: Current Opinion and Future Directions. J. Nucl. Med. 2020, 61, 1107–1112. [Google Scholar] [CrossRef]
- Giannetti, P.; Politis, M.; Su, P.; Turkheimer, F.E.; Malik, O.; Keihaninejad, S.; Wu, K.; Waldman, A.; Reynolds, R.; Nicholas, R. Increased PK11195-PET Binding in Normal-Appearing White Matter in Clinically Isolated Syndrome. Brain 2015, 138, 110–119. [Google Scholar] [CrossRef]
- Vowinckel, E.; Reutens, D.; Becher, B.; Verge, G.; Evans, A.; Owens, T.; Antel, J.P. PK11195 Binding to the Peripheral Benzodiazepine Receptor as a Marker of Microglia Activation in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. J. Neurosci. Res. 1997, 50, 345–353. [Google Scholar] [CrossRef]
- Hagens, M.H.J.; Golla, S.V.; Wijburg, M.T.; Yaqub, M.; Heijtel, D.; Steenwijk, M.D.; Schober, P.; Brevé, J.J.P.; Schuit, R.C.; Reekie, T.A. In Vivo Assessment of Neuroinflammation in Progressive Multiple Sclerosis: A Proof of Concept Study with [18F]DPA714 PET. J. Neuroinflamm. 2018, 15, 314. [Google Scholar] [CrossRef]
- Rissanen, E.; Tuisku, J.; Rokka, J.; Paavilainen, T.; Parkkola, R.; Rinne, J.O.; Airas, L. In Vivo Detection of Diffuse Inflammation in Secondary Progressive Multiple Sclerosis Using PET Imaging and the Radioligand 11C-PK11195. J. Nucl. Med. 2014, 55, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Oh, U.; Fujita, M.; Ikonomidou, V.N.; Evangelou, I.E.; Matsuura, E.; Harberts, E.; Ohayon, J.; Pike, V.W.; Zhang, Y.; Zoghbi, S.S. Translocator Protein PET Imaging for Glial Activation in Multiple Sclerosis. J. Neuroimmune Pharmacol. 2011, 6, 354–361. [Google Scholar] [CrossRef]
- Herranz, E.; Giannì, C.; Louapre, C.; Treaba, C.A.; Govindarajan, S.T.; Ouellette, R.; Loggia, M.L.; Sloane, J.A.; Madigan, N.; Izquierdo-Garcia, D. Neuroinflammatory Component of Gray Matter Pathology in Multiple Sclerosis. Ann. Neurol. 2016, 80, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T.; O’Connor, K.; Dubey, S.; Belanger, A.P.; Hurwitz, S.; Chu, R.; Tauhid, S.; Kijewski, M.F.; DiCarli, M.F.; Weiner, H.L. 18F-PBR06 versus 11C-PBR28 PET for Assessing White Matter Translocator Protein Binding in Multiple Sclerosis. Clin. Nucl. Med. 2018, 43, e289–e295. [Google Scholar] [CrossRef] [PubMed]
- Datta, G.; Colasanti, A.; Kalk, N.; Owen, D.; Scott, G.; Rabiner, E.A.; Gunn, R.N.; Lingford-Hughes, A.; Malik, O.; Ciccarelli, O. 11C-PBR28 and 18F-PBR111 Detect White Matter Inflammatory Heterogeneity in Multiple Sclerosis. J. Nucl. Med. 2017, 58, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Colasanti, A.; Guo, Q.; Muhlert, N.; Giannetti, P.; Onega, M.; Newbould, R.D.; Ciccarelli, O.; Rison, S.; Thomas, C.; Nicholas, R. In Vivo Assessment of Brain White Matter Inflammation in Multiple Sclerosis with 18F-PBR111 PET. J. Nucl. Med. 2014, 55, 1112–1118. [Google Scholar] [CrossRef]
- Unterrainer, M.; Mahler, C.; Vomacka, L.; Lindner, S.; Havla, J.; Brendel, M.; Böning, G.; Ertl-Wagner, B.; Kümpfel, T.; Milenkovic, V.M. TSPO PET with [18F] GE-180 Sensitively Detects Focal Neuroinflammation in Patients with Relapsing–Remitting Multiple Sclerosis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1423–1431. [Google Scholar] [CrossRef]
- Bunai, T.; Terada, T.; Kono, S.; Yokokura, M.; Yoshikawa, E.; Futatsubashi, M.; Miyajima, H.; Ouchi, Y. Neuroinflammation Following Disease Modifying Therapy in Multiple Sclerosis: A Pilot Positron Emission Tomography Study. J. Neurol. Sci. 2018, 385, 30–33. [Google Scholar] [CrossRef]
- Takata, K.; Kato, H.; Shimosegawa, E.; Okuno, T.; Koda, T.; Sugimoto, T.; Mochizuki, H.; Hatazawa, J.; Nakatsuji, Y. 11C-Acetate PET Imaging in Patients with Multiple Sclerosis. PLoS ONE 2014, 9, e111598. [Google Scholar] [CrossRef]
- Rissanen, E.; Tuisku, J.; Luoto, P.; Arponen, E.; Johansson, J.; Oikonen, V.; Parkkola, R.; Airas, L.; Rinne, J.O. Automated Reference Region Extraction and Population-Based Input Function for Brain [11C]TMSX PET Image Analyses. J. Cereb. Blood Flow Metab. 2015, 35, 157–165. [Google Scholar] [CrossRef]
- Matías-Guiu, J.A.; Cabrera-Martín, M.N.; Matías-Guiu, J.; Oreja-Guevara, C.; Riola-Parada, C.; Moreno-Ramos, T.; Arrazola, J.; Carreras, J.L. Amyloid PET Imaging in Multiple Sclerosis: An 18F-Florbetaben Study. BMC Neurol. 2015, 15, 243. [Google Scholar] [CrossRef]
- Bodini, B.; Veronese, M.; García-Lorenzo, D.; Battaglini, M.; Poirion, E.; Chardain, A.; Freeman, L.; Louapre, C.; Tchikviladze, M.; Papeix, C. Dynamic Imaging of Individual Remyelination Profiles in Multiple Sclerosis. Ann. Neurol. 2016, 79, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Pietroboni, A.M.; Carandini, T.; Colombi, A.; Mercurio, M.; Ghezzi, L.; Giulietti, G.; Scarioni, M.; Arighi, A.; Fenoglio, C.; De Riz, M.A. Amyloid PET as a Marker of Normal-Appearing White Matter Early Damage in Multiple Sclerosis: Correlation with CSF β-Amyloid Levels and Brain Volumes. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Matías-Guiu, J.A.; Cabrera-Martín, M.N.; Cortés-Martínez, A.; Pytel, V.; Moreno-Ramos, T.; Oreja-Guevara, C.; Carreras, J.L.; Matías-Guiu, J. Amyloid PET in Pseudotumoral Multiple Sclerosis. Mult. Scler. Relat. Disord. 2017, 15, 15–17. [Google Scholar] [CrossRef]
- Zeydan, B.; Lowe, V.J.; Schwarz, C.G.; Przybelski, S.A.; Tosakulwong, N.; Zuk, S.M.; Senjem, M.L.; Gunter, J.L.; Roberts, R.O.; Mielke, M.M. Pittsburgh Compound-B PET White Matter Imaging and Cognitive Function in Late Multiple Sclerosis. Mult. Scler. J. 2018, 24, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ni, Y.; Zhou, Q.; He, L.; Meng, H.; Gao, Y.; Huang, X.; Meng, H.; Li, P.; Chen, M. 18F-Florbetapir PET/MRI for Quantitatively Monitoring Myelin Loss and Recovery in Patients with Multiple Sclerosis: A Longitudinal Study. EClinicalMedicine 2021, 37, 100982. [Google Scholar] [CrossRef]
- van der Weijden, C.W.J.; Meilof, J.F.; van der Hoorn, A.; Zhu, J.; Wu, C.; Wang, Y.; Willemsen, A.; Dierckx, R.A.J.O.; Lammertsma, A.A.; de Vries, E.F.J. Quantitative Assessment of Myelin Density Using [11C]MeDAS PET in Patients with Multiple Sclerosis: A First-in-Human Study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3492–3507. [Google Scholar] [CrossRef]
- De Paula Faria, D.; Copray, S.; Sijbesma, J.W.A.; Willemsen, A.T.M.; Buchpiguel, C.A.; Dierckx, R.A.J.O.; De Vries, E.F.J. PET Imaging of Focal Demyelination and Remyelination in a Rat Model of Multiple Sclerosis: Comparison of [11C]MeDAS, [11C]CIC and [11C]PIB. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 995–1003. [Google Scholar] [CrossRef]
- de Paula Faria, D.; De Vries, E.F.J.; Sijbesma, J.W.A.; Dierckx, R.A.J.O.; Buchpiguel, C.A.; Copray, S. PET Imaging of Demyelination and Remyelination in the Cuprizone Mouse Model for Multiple Sclerosis: A Comparison between [11C]CIC and [11C]MeDAS. Neuroimage 2014, 87, 395–402. [Google Scholar] [CrossRef]
- de Paula Faria, D.; de Vries, E.F.; Sijbesma, J.W.; Buchpiguel, C.A.; Dierckx, R.A.; Copray, S.C. PET Imaging of Glucose Metabolism, Neuroinflammation and Demyelination in the Lysolecithin Rat Model for Multiple Sclerosis. Mult. Scler. J. 2014, 20, 1443–1452. [Google Scholar] [CrossRef]
- de Paula Faria, D.; Vlaming, M.L.H.; Copray, S.C.V.M.; Tielen, F.; Anthonijsz, H.J.A.; Sijbesma, J.W.A.; Buchpiguel, C.A.; Dierckx, R.A.J.O.; van der Hoorn, J.W.A.; de Vries, E.F.J. PET Imaging of Disease Progression and Treatment Effects in the Experimental Autoimmune Encephalomyelitis Rat Model. J. Nucl. Med. 2014, 55, 1330–1336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van der Weijden, C.W.J.; van der Hoorn, A.; Wang, Y.; Willemsen, A.T.M.; Dierckx, R.A.J.O.; Lammertsma, A.A.; de Vries, E.F.J. Investigation of Image-Derived Input Functions for Non-Invasive Quantification of Myelin Density Using [11C] MeDAS PET. Neuroimage 2022, 264, 119772. [Google Scholar] [CrossRef] [PubMed]
- van der Weijden, C.W.J.; Ahmed, A.K.M.A.; van der Hoorn, A.; Zhu, J.; Wu, C.; Wang, Y.; Stormezand, G.N.; Dierckx, R.A.J.O.; Meilof, J.F.; de Vries, E.F.J. Myelin Imaging of the Spinal Cord in Animal Models and Patients with Multiple Sclerosis Using [11C] MeDAS PET: A Translational Study. J. Nucl. Med. 2024, 66, 136–141. [Google Scholar] [CrossRef]
- Guehl, N.J.; Ramos-Torres, K.M.; Linnman, C.; Moon, S.-H.; Dhaynaut, M.; Wilks, M.Q.; Han, P.K.; Ma, C.; Neelamegam, R.; Zhou, Y.-P. Evaluation of the Potassium Channel Tracer [18F] 3F4AP in Rhesus Macaques. J. Cereb. Blood Flow Metab. 2021, 41, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Bahri, M.A.; Plenevaux, A.; Aerts, J.; Bastin, C.; Becker, G.; Mercier, J.; Valade, A.; Buchanan, T.; Mestdagh, N.; Ledoux, D.; et al. Measuring Brain Synaptic Vesicle Protein 2A with Positron Emission Tomography and [18F]UCB-H. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 481–486. [Google Scholar] [CrossRef]
- Bakshi, R.; Miletich, R.S.; Kinkel, P.R.; Emmet, M.L.; Kinkel, W.R. High-resolution Fluorodeoxyglucose Positron Emission Tomography Shows Both Global and Regional Cerebral Hypometabolism in Multiple Sclerosis. J. Neuroimaging 1998, 8, 228–234. [Google Scholar] [CrossRef]
- Derache, N.; Grassiot, B.; Mézenge, F.; Dugué, A.E.; Desgranges, B.; Constans, J.-M.; Defer, G.-L. Fatigue Is Associated with Metabolic and Density Alterations of Cortical and Deep Gray Matter in Relapsing-Remitting-Multiple Sclerosis Patients at the Earlier Stage of the Disease: A PET/MR Study. Mult. Scler. Relat. Disord. 2013, 2, 362–369. [Google Scholar] [CrossRef]
- Roelcke, U.; Kappos, L.; Lechner-Scott, J.; Brunnschweiler, H.; Huber, S.; Ammann, W.; Plohmann, A.; Dellas, S.; Maguire, R.P.; Missimer, J. Reduced Glucose Metabolism in the Frontal Cortex and Basal Ganglia of Multiple Sclerosis Patients with Fatigue: A 18F-fluorodeoxyglucose Positron Emission Tomography Study. Neurology 1997, 48, 1566–1571. [Google Scholar] [CrossRef]
- Kindred, J.H.; Ketelhut, N.B.; Rudroff, T. Glucose Uptake Heterogeneity of the Leg Muscles Is Similar between Patients with Multiple Sclerosis and Healthy Controls during Walking. Clin. Biomech. 2015, 30, 159–165. [Google Scholar] [CrossRef][Green Version]
- Blinkenberg, M.; Rune, K.; Jensen, C.V.; Ravnborg, M.H.; Kyllingsbaek, S.; Holm, S.; Paulson, O.B.; Sørensen, P.S. Reduced Metabolism in Cerebral Cortex Correlates with MRI Changes and Cognitive Dysfunction in Patients with Disseminated Sclerosis. Ugeskr. Laeger 2001, 163, 3788–3792. [Google Scholar]
- Golla, S.S.V.; Wolters, E.E.; Timmers, T.; Ossenkoppele, R.; van der Weijden, C.W.J.; Scheltens, P.; Schwarte, L.; Mintun, M.A.; Devous Sr, M.D.; Schuit, R.C. Parametric Methods for [18F]Flortaucipir PET. J. Cereb. Blood Flow Metab. 2020, 40, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET Imaging in Neurodegenerative Tauopathies—Still a Challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef]
- Hagens, M.H.J.; Killestein, J.; Yaqub, M.M.; van Dongen, G.A.M.S.; Lammertsma, A.A.; Barkhof, F.; van Berckel, B.N.M. Cerebral Rituximab Uptake in Multiple Sclerosis: A 89Zr-ImmunoPET Pilot Study. Mult. Scler. J. 2018, 24, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Moek, K.L.; Waaijer, S.J.H.; Kok, I.C.; Suurs, F.V.; Brouwers, A.H.; Menke-van der Houven van Oordt, C.W.; Wind, T.T.; Gietema, J.A.; Schröder, C.P.; Mahesh, S.V.K. 89Zr-Labeled Bispecific T-Cell Engager AMG 211 PET Shows AMG 211 Accumulation in CD3-Rich Tissues and Clear, Heterogeneous Tumor Uptake. Clin. Cancer Res. 2019, 25, 3517–3527. [Google Scholar] [CrossRef]
- Kist de Ruijter, L.; van de Donk, P.P.; Hooiveld-Noeken, J.S.; Giesen, D.; Elias, S.G.; Lub-de Hooge, M.N.; Oosting, S.F.; Jalving, M.; Timens, W.; Brouwers, A.H. Whole-Body CD8+ T Cell Visualization before and during Cancer Immunotherapy: A Phase 1/2 Trial. Nat. Med. 2022, 28, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Pishesha, N.; Harmand, T.J.; Heston, H.; Woodham, A.W.; Cheloha, R.W.; Bousbaine, D.; Rashidian, M.; Ploegh, H.L. Converting an Anti-Mouse CD4 Monoclonal Antibody into an ScFv Positron Emission Tomography Imaging Agent for Longitudinal Monitoring of CD4+ T Cells. J. Immunol. 2021, 207, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, P.P.; Wind, T.T.; Hooiveld-Noeken, J.S.; van der Veen, E.L.; Glaudemans, A.W.J.M.; Diepstra, A.; Jalving, M.; de Vries, E.G.E.; de Vries, E.F.J.; Hospers, G.A.P. Interleukin-2 PET Imaging in Patients with Metastatic Melanoma before and during Immune Checkpoint Inhibitor Therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4369–4376. [Google Scholar] [CrossRef]
- Tonietto, M.; Poirion, E.; Lazzarotto, A.; Ricigliano, V.; Papeix, C.; Bottlaender, M.; Bodini, B.; Stankoff, B. Periventricular Remyelination Failure in Multiple Sclerosis: A Substrate for Neurodegeneration. Brain 2023, 146, 182–194. [Google Scholar] [CrossRef]
- Lazzarotto, A.; Tonietto, M.; Poirion, E.; Battaglini, M.; Palladino, R.; Benoit, C.; Ricigliano, V.A.G.; Maillart, E.; De Stefano, N.; Stankoff, B. Clinically Relevant Profiles of Myelin Content Changes in Patients with Multiple Sclerosis: A Multimodal and Multicompartment Imaging Study. Mult. Scler. J. 2022, 28, 1881–1890. [Google Scholar] [CrossRef]
- Pytel, V.; Matias-Guiu, J.A.; Matías-Guiu, J.; Cortés-Martínez, A.; Montero, P.; Moreno-Ramos, T.; Arrazola, J.; Carreras, J.L.; Cabrera-Martín, M.N. Amyloid PET Findings in Multiple Sclerosis Are Associated with Cognitive Decline at 18 Months. Mult. Scler. Relat. Disord. 2020, 39, 101926. [Google Scholar] [CrossRef]
- Mouihate, A.; Kalakh, S. Ganaxolone Enhances Microglial Clearance Activity and Promotes Remyelination in Focal Demyelination in the Corpus Callosum of Ovariectomized Rats. CNS Neurosci. Ther. 2020, 26, 240–250. [Google Scholar] [CrossRef]
- Cantoni, C.; Bollman, B.; Licastro, D.; Xie, M.; Mikesell, R.; Schmidt, R.; Yuede, C.M.; Galimberti, D.; Olivecrona, G.; Klein, R.S. TREM2 Regulates Microglial Cell Activation in Response to Demyelination in Vivo. Acta Neuropathol. 2015, 129, 429–447. [Google Scholar] [CrossRef]
- Matsuo, A.; Akiguchi, I.; Lee, G.C.; McGeer, E.G.; McGeer, P.L.; Kimura, J. Myelin Degeneration in Multiple System Atrophy Detected by Unique Antibodies. Am. J. Pathol. 1998, 153, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Brugarolas, P.; Sánchez-Rodríguez, J.E.; Tsai, H.-M.; Basuli, F.; Cheng, S.-H.; Zhang, X.; Caprariello, A.V.; Lacroix, J.J.; Freifelder, R.; Murali, D.; et al. Development of a PET Radioligand for Potassium Channels to Image CNS Demyelination. Sci. Rep. 2018, 8, 607. [Google Scholar] [CrossRef]
- Nestor, P.J.; Altomare, D.; Festari, C.; Drzezga, A.; Rivolta, J.; Walker, Z.; Bouwman, F.; Orini, S.; Law, I.; Agosta, F. Clinical Utility of FDG-PET for the Differential Diagnosis among the Main Forms of Dementia. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1509–1525. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; La Fougere, C.; Pope, W.; Law, I.; Arbizu, J. Response Assessment in Neuro-Oncology Working Group and European Association for Neuro-Oncology Recommendations for the Clinical Use of PET Imaging in Gliomas. Neuro. Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Glaudemans, A.W.J.M.; de Vries, E.F.J.; Galli, F.; Dierckx, R.A.J.O.; Slart, R.H.J.A.; Signore, A. The Use of 18F-FDG-PET/CT for Diagnosis and Treatment Monitoring of Inflammatory and Infectious Diseases. Clin. Dev. Immunol. 2013, 2013, 623036. [Google Scholar] [CrossRef]
- Kapaki, E.; Paraskevas, G.P.; Michalopoulou, M.; Kilidireas, K. Increased Cerebrospinal Fluid Tau Protein in Multiple Sclerosis. Eur. Neurol. 2000, 43, 228–232. [Google Scholar] [CrossRef]
- Nabulsi, N.B.; Mercier, J.; Holden, D.; Carré, S.; Najafzadeh, S.; Vandergeten, M.-C.; Lin, S.-F.; Deo, A.; Price, N.; Wood, M.; et al. Synthesis and Preclinical Evaluation of 11C-UCB-J as a PET Tracer for Imaging the Synaptic Vesicle Glycoprotein 2A in the Brain. J. Nucl. Med. 2016, 57, 777–784. [Google Scholar] [CrossRef]
- Cai, Z.; Li, S.; Matuskey, D.; Nabulsi, N.; Huang, Y. PET Imaging of Synaptic Density: A New Tool for Investigation of Neuropsychiatric Diseases. Neurosci. Lett. 2019, 691, 44–50. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, X.; Zheng, K.; Li, H.; Huang, H.; Zhang, Z.; Mastracci, T.; Wegner, M.; Chen, Y.; Sussel, L. Genetic Evidence That Nkx2. 2 and Pdgfra Are Major Determinants of the Timing of Oligodendrocyte Differentiation in the Developing CNS. Development 2014, 141, 548–555. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Liu, Q.; Wang, A. Tyrphostin AG1296, a Platelet-Derived Growth Factor Receptor Inhibitor, Induces Apoptosis, and Reduces Viability and Migration of PLX4032-Resistant Melanoma Cells. Onco. Targets. Ther. 2015, 8, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.; Kino, Y.; Yanaizu, M.; Tosaki, Y.; Sakai, K.; Ishida, T.; Saito, Y. Expression of GPR17, a Regulator of Oligodendrocyte Differentiation and Maturation, in Nasu-Hakola Disease Brains. Intractable Rare Dis. Res. 2017, 6, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Ayaki, T.; Maki, T.; Yasuda, K.; Yoshii, D.; Kaji, S.; Takahashi, R. Evaluation of BCAS1-Positive Immature Oligodendrocytes after Cerebral Ischemic Stroke and SVD. Neurosci. Lett. 2023, 812, 137405. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Castaneda, A.; Gaultier, A. Adult Oligodendrocyte Progenitor Cells–Multifaceted Regulators of the CNS in Health and Disease. Brain. Behav. Immun. 2016, 57, 1–7. [Google Scholar] [CrossRef]
- Jacobson, O.; Yan, X.; Niu, G.; Weiss, I.D.; Ma, Y.; Szajek, L.P.; Shen, B.; Kiesewetter, D.O.; Chen, X. PET Imaging of Tenascin-C with a Radiolabeled Single-Stranded DNA Aptamer. J. Nucl. Med. 2015, 56, 616–621. [Google Scholar] [CrossRef]
- Han, Z.; Lu, Z.-R. Targeting Fibronectin for Cancer Imaging and Therapy. J. Mater. Chem. B 2017, 5, 639–654. [Google Scholar] [CrossRef]
- Rangasamy, L.; Di Geronimo, B.; Ortín, I.; Coderch, C.; Zapico, J.M.; Ramos, A.; de Pascual-Teresa, B. Molecular Imaging Probes Based on Matrix Metalloproteinase Inhibitors (MMPIs). Molecules 2019, 24, 2982. [Google Scholar] [CrossRef]
- Désogère, P.; Tapias, L.F.; Rietz, T.A.; Rotile, N.; Blasi, F.; Day, H.; Elliott, J.; Fuchs, B.C.; Lanuti, M.; Caravan, P. Optimization of a Collagen-Targeted PET Probe for Molecular Imaging of Pulmonary Fibrosis. J. Nucl. Med. 2017, 58, 1991–1996. [Google Scholar] [CrossRef]
- Fridén, M.; Wennerberg, M.; Antonsson, M.; Sandberg-Ställ, M.; Farde, L.; Schou, M. Identification of Positron Emission Tomography (PET) Tracer Candidates by Prediction of the Target-Bound Fraction in the Brain. EJNMMI Res. 2014, 4, 50. [Google Scholar] [CrossRef]
- Hultqvist, G.; Syvänen, S.; Fang, X.T.; Lannfelt, L.; Sehlin, D. Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor. Theranostics 2017, 7, 308. [Google Scholar] [CrossRef]
- Syvänen, S.; Fang, X.T.; Hultqvist, G.; Meier, S.R.; Lannfelt, L.; Sehlin, D. A Bispecific Tribody PET Radioligand for Visualization of Amyloid-Beta Protofibrils–a New Concept for Neuroimaging. Neuroimage 2017, 148, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sehlin, D.; Fang, X.T.; Meier, S.R.; Jansson, M.; Syvänen, S. Pharmacokinetics, Biodistribution and Brain Retention of a Bispecific Antibody-Based PET Radioligand for Imaging of Amyloid-β. Sci. Rep. 2017, 7, 17254. [Google Scholar] [CrossRef]
- Pakula, R.J.; Scott, P.J.H. Applications of Radiolabeled Antibodies in Neuroscience and Neuro-oncology. J. Label. Compd. Radiopharm. 2023, 66, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Sehlin, D.; Syvänen, S. Engineered Antibodies: New Possibilities for Brain PET? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2848–2858. [Google Scholar] [CrossRef] [PubMed]
- Razpotnik, R.; Novak, N.; Čurin Šerbec, V.; Rajcevic, U. Targeting Malignant Brain Tumors with Antibodies. Front. Immunol. 2017, 8, 1181. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Syrmos, N.C.; D’Arco, F.; Ganau, L.; Chibbaro, S.; Prisco, L.; Ligarotti, G.K.I.; Ambu, R.; Soddu, A. Enhancing Contrast Agents and Radiotracers Performance through Hyaluronic Acid-Coating in Neuroradiology and Nuclear Medicine. Hell. J. Nucl. Med. 2017, 20, 166–168. [Google Scholar]
- Huntemann, N.; Rolfes, L.; Pawlitzki, M.; Ruck, T.; Pfeuffer, S.; Wiendl, H.; Meuth, S.G. Failed, Interrupted, or Inconclusive Trials on Neuroprotective and Neuroregenerative Treatment Strategies in Multiple Sclerosis: Update 2015–2020. Drugs 2021, 81, 1031–1063. [Google Scholar] [CrossRef]
- Ricigliano, V.A.G.; Tonietto, M.; Hamzaoui, M.; Poirion, É.; Lazzarotto, A.; Bottlaender, M.; Gervais, P.; Maillart, E.; Stankoff, B.; Bodini, B. Spontaneous Remyelination in Lesions Protects the Integrity of Surrounding Tissues over Time in Multiple Sclerosis. Eur. J. Neurol. 2022, 29, 1719–1729. [Google Scholar] [CrossRef]
- Lubetzki, C.; Zalc, B.; Williams, A.; Stadelmann, C.; Stankoff, B. Remyelination in Multiple Sclerosis: From Basic Science to Clinical Translation. Lancet. Neurol. 2020, 19, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Sutiwisesak, R.; Burns, T.C.; Rodriguez, M.; Warrington, A.E. Remyelination Therapies for Multiple Sclerosis: Optimizing Translation from Animal Models into Clinical Trials. Expert Opin. Investig. Drugs 2021, 30, 857–876. [Google Scholar] [CrossRef] [PubMed]


| Imaging Target | Relevant Process/Cells | Tracer | Status | References |
|---|---|---|---|---|
| Inflammation | ||||
| TSPO | activated microglia/macrophages, astrocytes, endothelial cells | [11C]PK11195 [11C]PBR28 [18F]PBR06 [18F]PBR111 [18F]GE-180 [11C]DPA713 [18F]DPA714 | clinical research | [161,162,163,164,165,166,167,168,169,170,171] |
| P2X7 receptor | pro-inflammatory microglia, astrocytes, OPCs | [11C]SMW139 | clinical research | [143] |
| CSF1 receptor | activated microglia | [11C]GW2580 | Experimental models | [156,157] |
| MCT1 | activated astrocytes | [11C]acetate | clinical research | [172] |
| Adenosine 2A receptor | T cells, macrophages, microglia, monocytes, NK cells, endothelial cells, neurons | [11C]TMSX | Clinical research | [146,173] |
| monoamine oxidase-B | activated astrocytes | [11C]DED | experimental models | [153] |
| Myelin Density | ||||
| amyloid beta & MBP | in MS pathology, assessment of myelin integrity | [11C]PiB [18F]Florbetaben [18F]Florbetapir | clinical research | [174,175,176,177,178,179] |
| MBP | assessment of myelin integrity | [11C]MeDAS | clinical research | [180,181,182,183,184,185,186] |
| axonal potassium channels | assessment of myelin integrity | [18F]3F4AP | Experimental models | [187] |
| Axonal Integrity | ||||
| synaptic vesicle protein 2a | synaptic density | [11C]UCB-J | clinical research | [188] |
| glucose consumption | neurodegeneration | [18F]FDG | clinical research | [189,190,191,192,193] |
| tau | neurodegeneration | [18F]Flortaucipir [18F]MK-6240 | clinical research | [194,195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Weijden, C.W.J.; Meilof, J.F.; van der Hoorn, A.; de Vries, E.F.J.; Baron, W. The Future of PET Imaging in Multiple Sclerosis: Characterisation of Individual White Matter Lesions. J. Clin. Med. 2025, 14, 4439. https://doi.org/10.3390/jcm14134439
van der Weijden CWJ, Meilof JF, van der Hoorn A, de Vries EFJ, Baron W. The Future of PET Imaging in Multiple Sclerosis: Characterisation of Individual White Matter Lesions. Journal of Clinical Medicine. 2025; 14(13):4439. https://doi.org/10.3390/jcm14134439
Chicago/Turabian Stylevan der Weijden, Chris W. J., Jan F. Meilof, Anouk van der Hoorn, Erik F. J. de Vries, and Wia Baron. 2025. "The Future of PET Imaging in Multiple Sclerosis: Characterisation of Individual White Matter Lesions" Journal of Clinical Medicine 14, no. 13: 4439. https://doi.org/10.3390/jcm14134439
APA Stylevan der Weijden, C. W. J., Meilof, J. F., van der Hoorn, A., de Vries, E. F. J., & Baron, W. (2025). The Future of PET Imaging in Multiple Sclerosis: Characterisation of Individual White Matter Lesions. Journal of Clinical Medicine, 14(13), 4439. https://doi.org/10.3390/jcm14134439







