Time Course of Functional Recovery Following Single-Level Anterior Lumbar Interbody Fusion with and Without Posterior Instrumentation: A Retrospective Single-Institution Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Inclusion
2.2. Data Collection
- Demographic data: age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) class, age-adjusted Charlson Comorbidity Index (CCI)
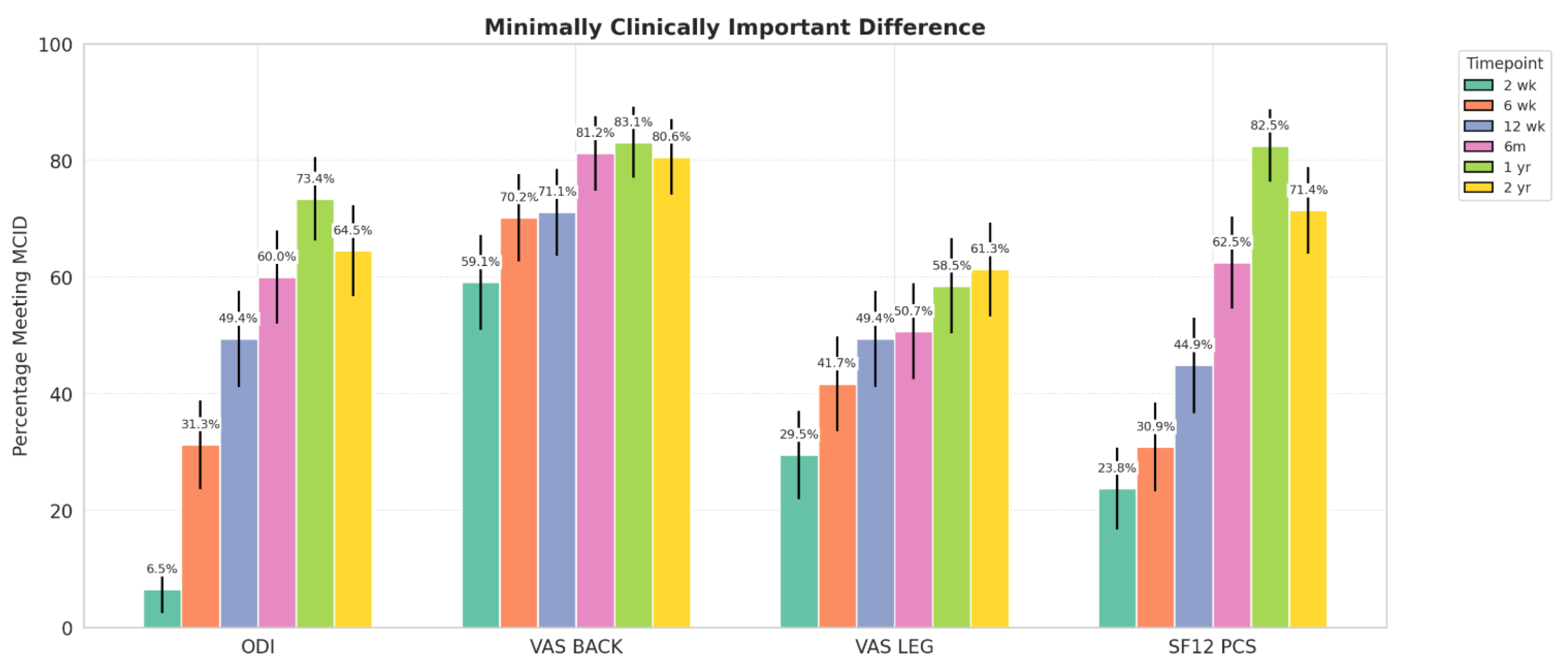
- Patient-reported outcome measures (PROMs): Oswestry Disability Index (ODI), Visual Analog Scale (VAS) back and leg, and 12-Item Short Form Survey Physical Component Score (SF-12 PCS) were collected at each follow-up time point (2 weeks, 6 weeks, 12 weeks, 6 months, and 1 year). Minimal clinically important difference (MCID) achievement was calculated for each PROM using thresholds of 12.8 ODI, 1.2 for VAS back, 1.6 for VAS leg, and 4.9 SF-12 PCS, as described by Copay et al. [10]
- Global Rating Change (GRC): Patients were assessed at each follow-up time point using a single-question survey: “Compared to before your surgery, how do you feel?” with the following response options: (1) much better; (2) somewhat better; (3) the same; (4) somewhat worse; and (5) much worse. For analysis, responses of “much better” and “somewhat better” were categorized as “better,” while “somewhat worse” and “much worse” were classified as “worse”.
- Operative and perioperative data: operative time, operated levels, estimated blood loss (EBL), length of stay (LOS)
- Return to activities (RTA): Postoperative recovery was monitored by tracking patients’ return to daily activities. Those driving or working before surgery were asked at each follow-up visit whether they had resumed these activities, with tracking continuing until their return. Additionally, patients who required postoperative opioid analgesia were asked to report the date they discontinued use, if applicable.
2.3. Statistical Analysis
2.4. Cohort Demographics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar] [CrossRef]
- Tung, K.K.; Tseng, W.C.; Wu, Y.C.; Chen, K.-H.; Pan, C.-C.; Lu, W.-X.; Shih, C.-M.; Lee, C.-H. Comparison of radiographic and clinical outcomes between ALIF, OLIF, and TLIF over 2-year follow-up: A comparative study. J. Orthop. Surg. Res. 2023, 18, 158. [Google Scholar] [CrossRef]
- Alhaug, O.K.; Dolatowski, F.C.; Thyrhaug, A.M.; Mjønes, S.; Dos Reis, J.A.B.P.R.; Austevoll, I. Long-term comparison of anterior (ALIF) versus transforaminal (TLIF) lumbar interbody fusion: A propensity score-matched register-based study. Eur. Spine J. 2024, 33, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Ehrlich, A.; Omurzakov, A.; Asada, T.; Singleton, Q.; Colon, L.; Owusu-Sarpong, S.; Musharbash, F.; Halayqeh, S.; Lui, A.M.; et al. Temporal Changes in PROMs Improvement and Recovery Kinetics Following Transforaminal Endoscopic Lumbar Discectomy. Spine 2024. [Google Scholar] [CrossRef] [PubMed]
- Shahi, P.; Subramanian, T.; Tuma, O.; Singh, S.; Araghi, K.B.; Asada, T.; Korsun, M.B.; Singh, N.; Simon, C.B.; Vaishnav, A.; et al. Temporal Trends of Improvement After Minimally Invasive Transforaminal Lumbar Interbody Fusion. Spine 2024, 50, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, R.J.; Lennox, A.; Ho, Y.T.; Phan, K.; Choy, W.J. L5/S1 anterior lumbar interbody fusion technique. J. Spine Surg. 2017, 3, 429–432. [Google Scholar] [CrossRef]
- Phan, K.; Xu, J.; Scherman, D.B.; Rao, P.J.; Mobbs, R.J. Anterior Lumbar Interbody Fusion With and Without an “Access Surgeon”: A Systematic Review and Meta-analysis. Spine 2017, 42, E592–E601. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Copay, A.G.; Glassman, S.D.; Subach, B.R.; Berven, S.; Schuler, T.C.; Carreon, L.Y. Minimum clinically important difference in lumbar spine surgery patients: A choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008, 8, 968–974. [Google Scholar] [CrossRef]
- Pereira, P.; Buzek, D.; Franke, J.; Senker, W.; Kosmala, A.; Hubbe, U.; Manson, N.; Rosenberg, W.; Assietti, R.; Martens, F.; et al. Surgical data and early postoperative outcomes after minimally invasive lumbar interbody fusion: Results of a prospective, multicenter, observational data-monitored study. PLoS ONE 2015, 10, e0122312. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.C.; Patel, M.R.; Ribot, M.A.; Parsons, A.W.; Vanjani, N.N.; Pawlowski, H.; Prabhu, M.C.; Singh, K. Single-Level Minimally Invasive Transforaminal Lumbar Interbody Fusion versus Anterior Lumbar Interbody Fusion with Posterior Instrumentation at L5/S1. World Neurosurg. 2022, 157, e111–e122. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.J.F.; Cady-McCrea, C.I.; Menga, E.N.; Haddas, R.; Molinari, R.N.; Mesfin, A.; Rubery, P.T.; Puvanesarajah, V. Clinical Improvement After Lumbar Fusion: Using PROMIS to Assess Recovery Kinetics. Spine 2024, 49, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.W.; Hartman, T.J.; Oyetayo, O.O.; MacGregor, K.R.; Zheng, E.; Massel, D.H.; Singh, K. Recovery ratios and minimum clinically important difference for clinical outcomes in workers’ compensation recipients undergoing MIS-TLIF versus ALIF. Acta Neurochir. 2023, 165, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.D.K.; Lynch, C.P.; Patel, M.R.; Jacob, K.C.; Geoghegan, C.E.; Jadczak, C.N.; Mohan, S.; Singh, K. Preoperative Duration of Symptoms Does Not Affect Outcomes of Anterior Lumbar Interbody Fusion. Neurosurgery 2022, 90, 215–220. [Google Scholar] [CrossRef]
- Subramanian, T.; Shahi, P.; Hirase, T.; Korsun, M.; Zhang, J.; Kim, E.; Kwas, C.; Kaidi, A.C.; Boddapati, V.; Song, J.; et al. High Modified 5 Factor Frailty Index is Associated With Worse PROMs and Decreased Return to Activities After 1 or 2 Level MI-TLIF. Glob. Spine J. 2025, 21925682251314380. [Google Scholar] [CrossRef]
- Wang, X.; Borgman, B.; Vertuani, S.; Nilsson, J. A systematic literature review of time to return to work and narcotic use after lumbar spinal fusion using minimal invasive and open surgery techniques. BMC Health Serv. Res. 2017, 17, 446. [Google Scholar] [CrossRef]
- Behmanesh, B.; Wempe, H.; Kilinc, F.; Dubinski, D.; Won, S.-Y.; Czabanka, M.; Setzer, M.; Schuss, P.; Schneider, M.; Freiman, T.; et al. Return to Work Following Anterior Lumbar Interbody Fusion with Percutaneous Posterior Pedicle Fixation: A Retrospective Analysis from Two Academic Centers in Germany. J. Clin. Med. 2024, 13, 5636. [Google Scholar] [CrossRef]
- Gornet, M.F.; Burkus, J.K.; Dryer, R.F.; Peloza, J.H. Lumbar disc arthroplasty with Maverick disc versus stand-alone interbody fusion: A prospective, randomized, controlled, multicenter investigational device exemption trial. Spine 2011, 36, E1600–E1611. [Google Scholar] [CrossRef]
- Kim, B.D.; Hsu, W.K.; De Oliveira, G.S.; Saha, S.; Kim, J.Y.S. Operative duration as an independent risk factor for postoperative complications in single-level lumbar fusion: An analysis of 4588 surgical cases. Spine 2014, 39, 510–520. [Google Scholar] [CrossRef]
- Daniels, A.H.; Daher, M.; Singh, M.; Balmaceno-Criss, M.; Lafage, R.; Diebo, B.G.; Hamilton, D.K.; Smith, J.S.; Eastlack, R.K.; Fessler, R.G.; et al. The Case for Operative Efficiency in Adult Spinal Deformity Surgery: Impact of Operative Time on Complications, Length of Stay, Alignment, Fusion Rates, and Patient-Reported Outcomes. Spine 2024, 49, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, R.D.; East, J.W.; Winters, K.; Tackett, J.; Hajovsky-Pietla, A. Anterior and posterior lumbar interbody fusion with percutaneous pedicle screws: Comparison to muscle damage and minimally invasive techniques. Spine 2009, 34, E923–E925. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Ebata, S.; Haro, H. Comparison of serum markers for muscle damage, surgical blood loss, postoperative recovery, and surgical site pain after extreme lateral interbody fusion with percutaneous pedicle screws or traditional open posterior lumbar interbody fusion. BMC Musculoskelet. Disord. 2017, 18, 415. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, T.; Shahi, P.; Araghi, K.; Mayaan, O.; Amen, T.B.; Iyer, S.; Qureshi, S. Using Artificial Intelligence to Answer Common Patient-Focused Questions in Minimally Invasive Spine Surgery. J. Bone Jt. Surg. Am. 2023, 105, 1649–1653. [Google Scholar] [CrossRef]
- Mancuso, C.A.; Cammisa, F.P.; Sama, A.A.; Hughes, A.P.; Ghomrawi, H.M.; Girardi, F.P. Development and testing of an expectations survey for patients undergoing lumbar spine surgery. J. Bone Jt. Surg. Am. 2013, 95, 1793–1800. [Google Scholar] [CrossRef]
- Stokes, O.M.; Cole, A.A.; Breakwell, L.M.; Lloyd, A.J.; Leonard, C.M.; Grevitt, M. Do we have the right PROMs for measuring outcomes in lumbar spinal surgery? Eur. Spine J. 2017, 26, 816–824. [Google Scholar] [CrossRef]
- Beighley, A.; Zhang, A.; Huang, B.; Carr, C.; Mathkour, M.; Werner, C.; Scullen, T.; Kilgore, M.D.; Maulucci, C.M.; Dallapiazza, R.F.; et al. Patient-reported outcome measures in spine surgery: A systematic review. J. Craniovertebr. Junction Spine 2022, 13, 378–389. [Google Scholar] [CrossRef]
| Variable | Overall | Stand Alone | A-P | p-Value |
|---|---|---|---|---|
| n | 143 | 90 | 53 | |
| Age | 52.78 ± 13.07 | 50.00 ± 12.17 | 57.51 ± 13.29 | <0.001 |
| Male | 67 (46.9%) | 40 (44.4%) | 27 (50.9%) | 0.812 |
| BMI | 27.70 ± 5.04 | 27.35 ± 4.90 | 28.31 ± 5.25 | 0.272 |
| ASA Class | ||||
| 1 | 15 (10.5%) | 12 (75%) | 3 (5.7%) | 0.009 |
| 2 | 116 (81.1%) | 75 (83.3%) | 41 (77.4%) | |
| 3 | 11 (7.7%) | 2 (2.2%) | 9 (17%) | |
| 4 | 1 (0.7%) | 1 (1.1%) | 0 (0%) | |
| CCI | 1.51 ± 1.59 | 1.22 ± 1.55 | 2 ± 1.56 | 0.004 |
| EBL | 118.87 ± 228.11 | 110.84 ± 275.18 | 132.36 ± 112.49 | 0.588 |
| Operative Time | 156.51 ± 80.73 | 103.27 ± 28.83 | 247.91 ± 54.88 | <0.001 |
| LOS (hours) | 57.75 ± 37.93 | 47.26 ± 24.68 | 75.56 ± 48.71 | <0.001 |
| Surgical Level | 0.273 | |||
| L4-L5 | 13 (9.1%) | 10 (11.1%) | 3 (5.7%) | |
| L5-S1 | 130 (90.9%) | 80 (88.9%) | 50 (94.3%) |
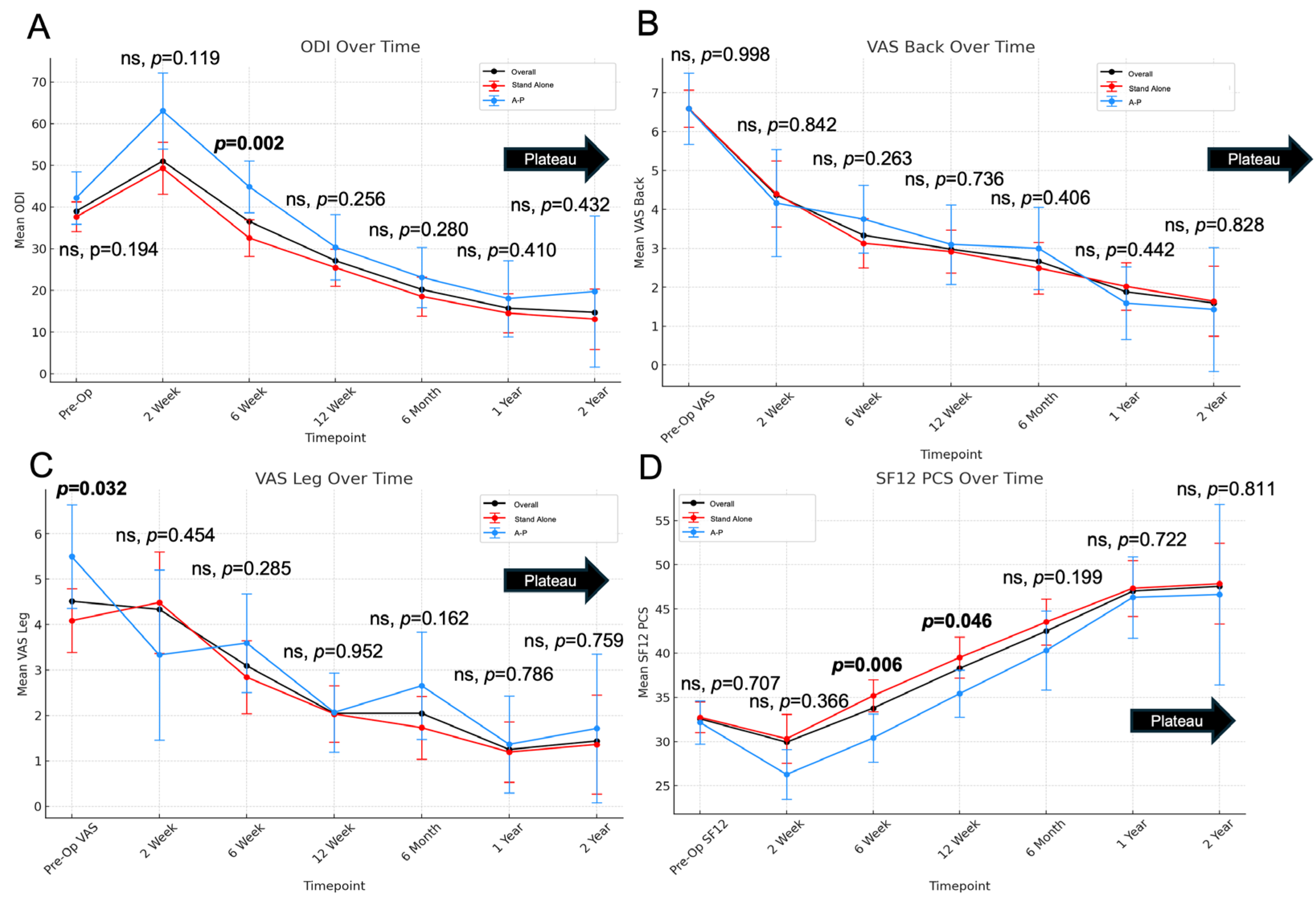
| ODI | VAS-Back | VAS-Leg | SF12-PCS | |
|---|---|---|---|---|
| Preoperative | 39.02 ± 16.85 | 6.59 ± 2.34 | 4.49 ± 3.3 | 32.57 ± 7.36 |
| 2 Weeks | 51.05 ± 20.11 | 4.37 ± 2.62 | 4.34 ± 3.47 | 29.93 ± 8.38 |
| <0.001 | <0.001 | 0.345 | 0.050 | |
| 6 Weeks | 36.55 ± 18.53 | 3.34 ± 2.55 | 3.09 ± 3.22 | 33.78 ± 7.61 |
| <0.001 | <0.001 | <0.001 | 0.001 | |
| 3 Months | 27.12 ± 19.46 | 2.98 ± 2.41 | 2.04 ± 2.44 | 38.27 ± 8.77 |
| <0.001 | 0.843 | 0.023 | <0.001 | |
| 6 Months | 20.23 ± 18.33 | 2.67 ± 2.51 | 2.05 ± 2.73 | 42.49 ± 9.88 |
| <0.001 | 0.058 | 0.692 | 0.002 | |
| 1 Year | 15.47 ± 18.75 | 1.88 ± 2.14 | 1.25 ± 2.37 | 47.02 ± 10.77 |
| 0.021 | 0.023 | 0.004 | 0.004 | |
| 2 Years | 14.72 ± 20.37 | 1.59 ± 2.23 | 1.44 ± 2.64 | 47.57 ± 11.43 |
| 0.135 | 0.013 | 0.608 | 0.817 |
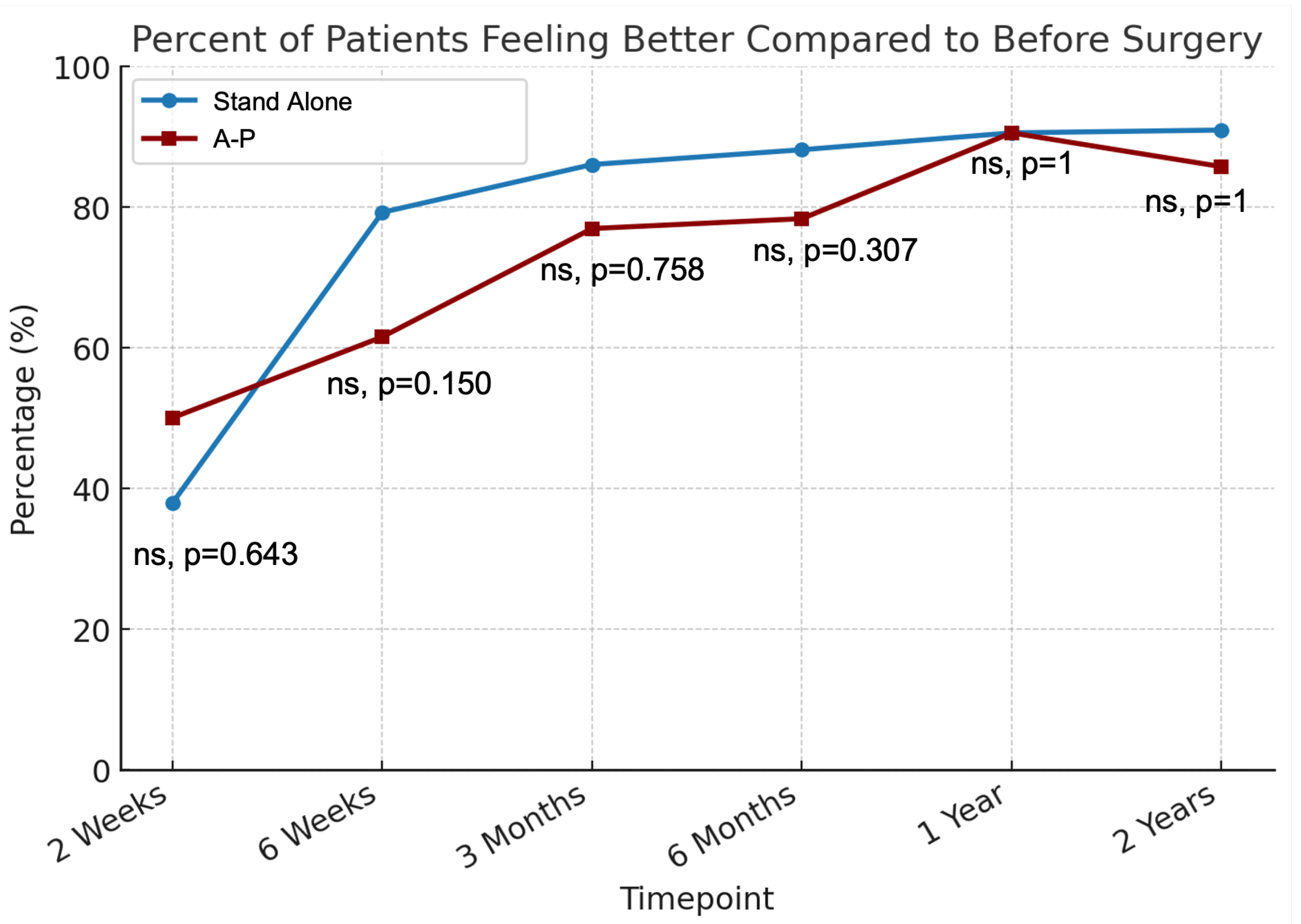
| Overall | Stand Alone | A-P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Timepoint | Better | Same | Worse | Better | Same | Worse | Better | Same | Worse |
| 2 Weeks | 39.4% | 15.2% | 45.5% | 37.9% | 13.8% | 48.3% | 50.0% | 25.0% | 25.0% |
| 6 Weeks | 73.4% | 12.7% | 13.9% | 79.2% | 13.2% | 7.5% | 61.5% | 11.5% | 26.9% |
| 3 Months | 82.9% | 6.6% | 10.5% | 86.0% | 6.0% | 8.0% | 76.9% | 7.7% | 15.4% |
| 6 Months | 84.6% | 3.1% | 12.3% | 88.1% | 2.4% | 9.5% | 78.3% | 4.3% | 17.4% |
| 1 Year | 90.5% | 1.6% | 7.9% | 90.5% | 0.0% | 9.5% | 90.5% | 4.8% | 4.8% |
| 2 Years | 89.7% | 3.4% | 6.9% | 90.9% | 0.0% | 9.1% | 85.7% | 14.3% | 0.0% |
| Stand Alone | A-P | |||||||
|---|---|---|---|---|---|---|---|---|
| ODI | VAS-Back | VAS-Leg | SF-12 PCS | ODI | VAS-Back | VAS-Leg | SF-12 PCS | |
| 2 Weeks | 7.1% | 57.5% | 25.0% | 26.3% | 0.0% | 75.0% | 75.0% | 0.0% |
| 6 Weeks | 31.0% | 70.7% | 41.4% | 33.3% | 32.0% | 69.2% | 42.3% | 25.0% |
| 12 Weeks | 46.6% | 75.4% | 43.9% | 48.1% | 56.0% | 61.5% | 61.5% | 37.5% |
| 6 Months | 60.4% | 85.1% | 48.9% | 65.1% | 59.1% | 72.7% | 54.5% | 57.1% |
| 1 Year | 69.8% | 81.8% | 54.5% | 78.6% | 81.0% | 85.7% | 66.7% | 90.5% |
| 2 Years | 62.5% | 79.2% | 50% * | 71.4% | 71.4% | 85.7% | 100% * | 71.4% |
| Variable | Overall | Stand Alone | A-P | p-Value |
| Driving | n = 81 | n = 58 | n = 23 | |
| Return to Driving | 91.36% | 93.10% | 86.96% | 0.375 |
| Days Required | 29 [14–48.5] | 28 [12–46] | 39 [24–62] | 0.080 |
| Working | n = 70 | n = 51 | n = 19 | |
| Return to Working | 85.71% | 88.24% | 78.95% | 0.323 |
| Days Required | 29 [12–61] | 27 [12–57.5] | 35.5 [13–91] | 0.267 |
| Opioids | n = 88 | n = 62 | n = 26 | |
| Discontinuation of Narcotics | 93.18% | 93.55% | 92.31% | 0.903 |
| Days Required | 12 [4.5–40] | 9 [3–21] | 22 [7–60] | 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanian, T.; Owusu-Sarpong, S.; Kush, S.; Ehrlich, A.M.; Asada, T.; Zhao, E.R.; Araghi, K.; Hirase, T.; Kaidi, A.C.; Kazarian, G.S.; et al. Time Course of Functional Recovery Following Single-Level Anterior Lumbar Interbody Fusion with and Without Posterior Instrumentation: A Retrospective Single-Institution Study. J. Clin. Med. 2025, 14, 4397. https://doi.org/10.3390/jcm14134397
Subramanian T, Owusu-Sarpong S, Kush S, Ehrlich AM, Asada T, Zhao ER, Araghi K, Hirase T, Kaidi AC, Kazarian GS, et al. Time Course of Functional Recovery Following Single-Level Anterior Lumbar Interbody Fusion with and Without Posterior Instrumentation: A Retrospective Single-Institution Study. Journal of Clinical Medicine. 2025; 14(13):4397. https://doi.org/10.3390/jcm14134397
Chicago/Turabian StyleSubramanian, Tejas, Stephane Owusu-Sarpong, Sophie Kush, Adin M. Ehrlich, Tomoyuki Asada, Eric R. Zhao, Kasra Araghi, Takashi Hirase, Austin C. Kaidi, Gregory S. Kazarian, and et al. 2025. "Time Course of Functional Recovery Following Single-Level Anterior Lumbar Interbody Fusion with and Without Posterior Instrumentation: A Retrospective Single-Institution Study" Journal of Clinical Medicine 14, no. 13: 4397. https://doi.org/10.3390/jcm14134397
APA StyleSubramanian, T., Owusu-Sarpong, S., Kush, S., Ehrlich, A. M., Asada, T., Zhao, E. R., Araghi, K., Hirase, T., Kaidi, A. C., Kazarian, G. S., Musharbash, F., Colón, L. F., Lui, A. T. H., Durbas, A., Tuma, O. C., Shahi, P., Morse, K. W., Lovecchio, F. C., Sheha, E. D., ... Iyer, S. (2025). Time Course of Functional Recovery Following Single-Level Anterior Lumbar Interbody Fusion with and Without Posterior Instrumentation: A Retrospective Single-Institution Study. Journal of Clinical Medicine, 14(13), 4397. https://doi.org/10.3390/jcm14134397







