Evaluation of the Diagnostic and Predictive Significance of Postoperative C-Reactive Protein to Transferrin or Albumin Ratio in Identifying Septic Events Following Major Abdominal Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Establishment of Postoperative Septic Event Diagnosis
2.3. Study Protocol
2.4. Inclusion Criteria
2.5. Exclusion Criteria
- (I)
- Lack of microbiological evidence of SSI,
- (II)
- Refusal of the patient to attend the study,
- (III)
- Reoperation prior to postoperative day 3,
- (IV)
- ALB infusion either preoperatively or within 2 postoperative days,
- (V)
- Known autoimmune disease under treatment or not,
- (VI)
- Incomplete laboratory data,
- (VII)
- Anemia of chronic disease.
2.6. Blood Sampling
2.7. Data Collection
2.8. Control Group
2.9. Statistical Analysis
2.10. Theory/Calculation
3. Results
3.1. Patient Characteristics
3.2. Establishment of Postoperative Septic Event Diagnosis
3.3. Evaluation of the Correlation Between Patient Characteristics, Laboratory Tests, and Postoperative Septic Events
- Laboratory tests
- Correlation of preoperative ALB levels with SSI incidence
- Multiparametric analysis
- Multiple logistic regression analysis
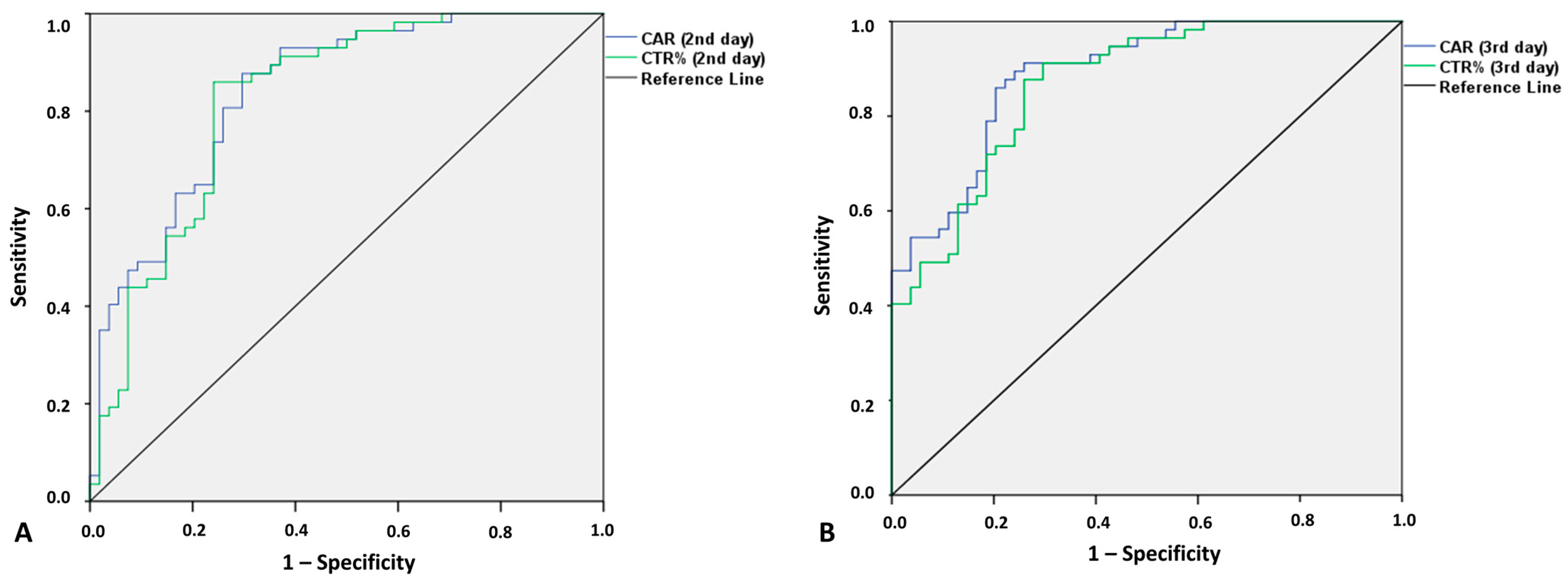
3.4. Predictive Value of CRP, CAR, and CTR for Postoperative Septic Complications
3.5. Correlation Between Examiner’s and CAR/CTR Diagnostic/Predictive Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caroff, D.A.; Chan, C.; Kleinman, K.; Calderwood, M.S.; Wolf, R.; Wick, E.C.; Platt, R.; Huang, S. Association of Open Approach vs Laparoscopic Approach With Risk of Surgical Site Infection After Colon Surgery. JAMA Netw. Open 2019, 2, E1913570. [Google Scholar] [CrossRef] [PubMed]
- Jatoliya, H.; Pipal, R.K.; Pipal, D.K.; Biswas, P.; Pipal, V.R.; Yadav, S.; Verma, B.; Vardhan, V. Surgical Site Infections in Elective and Emergency Abdominal Surgeries: A Prospective Observational Study About Incidence, Risk Factors, Pathogens, and Antibiotic Sensitivity at a Government Tertiary Care Teaching Hospital in India. Cureus 2023, 15, e48071. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, H.; Lv, P.; Peng, X.; Wu, C.; Ren, J.; Wang, P. Prospective multicenter study on the incidence of surgical site infection after emergency abdominal surgery in China. Sci. Rep. 2021, 11, 7794. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, A.; Ademuyiwa, A.O.; Aguilera, M.L.; Alexander, P.; Al-Saqqa, S.W.; Borda-Luque, G.; Costas-Chavarri, A.; Drake, T.M.; Ntirenganya, F.; Fitzgerald, J.E.; et al. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: A prospective, international, multicentre cohort study. Lancet Infect Dis 2018, 18, 516–525. [Google Scholar]
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Abbas, M.; Atema, J.J.; Gans, S.; van Rijen, M.; et al. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e276–e287. [Google Scholar] [CrossRef]
- de Vries, F.E.E.; Gans, S.L.; Solomkin, J.S.; Allegranzi, B.; Egger, M.; Dellinger, E.P.; Boermeester, M.A. Meta-analysis of lower perioperative blood glucose target levels for reduction of surgical-site infection. Br. J. Surg. 2017, 104, e95–e105. [Google Scholar] [CrossRef]
- Jenks, P.J.; Laurent, M.; McQuarry, S.; Watkins, R. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. J. Hosp. Infect. 2014, 86, 24–33. [Google Scholar] [CrossRef]
- Strobel, R.M.; Leonhardt, M.; Förster, F.; Neumann, K.; Lobbes, L.A.; Seifarth, C.; Lee, L.D.; Schineis, C.H.W.; Kamphues, C.; Weixler, B.; et al. The impact of surgical site infection—A cost analysis. Langenbeck’s Arch. Surg. 2022, 407, 819–828. [Google Scholar] [CrossRef]
- Hummel, R.; Bausch, D. Anastomotic Leakage after Upper Gastrointestinal Surgery: Surgical Treatment. Visc. Med. 2017, 33, 207–211. [Google Scholar] [CrossRef]
- Telem, D.A.; Chin, E.H.; Nguyen, S.Q.; Divino, C.M. Risk factors for anastomotic leak following colorectal surgery: A case-control study. Arch. Surg. 2010, 145, 371–376. [Google Scholar] [CrossRef]
- Litchinko, A.; Buchs, N.; Balaphas, A.; Toso, C.; Liot, E.; Meurette, G.; Ris, F.; Meyer, J. Score prediction of anastomotic leak in colorectal surgery: A systematic review. Surg. Endosc. 2024, 38, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Karliczek, A.; Harlaar, N.J.; Zeebregts, C.J.; Wiggers, T.; Baas, P.C.; van Dam, G.M. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int. J. Color. Dis. 2009, 24, 569–576. [Google Scholar] [CrossRef]
- Klose, J.; Tarantino, I.; von Fournier, A.; Stowitzki, M.J.; Kulu, Y.; Bruckner, T.; Volz, C.; Schmidt, T.; Schneider, M.; Büchler, M.W.; et al. A Nomogram to Predict Anastomotic Leakage in Open Rectal Surgery—Hope or Hype? J. Gastrointest. Surg. 2018, 22, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- McKenna, N.P.; Bews, K.A.; Cima, R.R.; Crowson, C.S.; Habermann, E.B. Development of a Risk Score to Predict Anastomotic Leak After Left-Sided Colectomy: Which Patients Warrant Diversion? J. Gastrointest. Surg. 2020, 24, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, V.; van Ramshorst, B.; van Dieren, S.; van Geloven, N.; Boermeester, M.; Boerma, D. Early complication detection after colorectal surgery (CONDOR): Study protocol for a prospective clinical diagnostic study. Int. J. Color. Dis. 2016, 31, 459–464. [Google Scholar] [CrossRef]
- El Zaher, H.A.; Ghareeb, W.M.; Fouad, A.M.; Madbouly, K.; Fathy, H.; Vedin, T.; Edelhamre, M.; Emile, S.H.; Faisal, M. Role of the triad of procalcitonin, C-reactive protein, and white blood cell count in the prediction of anastomotic leak following colorectal resections. World J. Surg. Oncol. 2022, 20, 33. [Google Scholar] [CrossRef]
- Choi, J.D.W.; Kwik, C.; Shanmugalingam, A.; Allan, L.; Khoury, T.E.; Pathmanathan, N.; Toh, J.W.T. C-Reactive Protein as a Predictive Marker for Anastomotic Leak Following Restorative Colorectal Surgery in an Enhanced Recovery After Surgery Program. J. Gastrointest. Surg. 2023, 27, 2604–2607. [Google Scholar] [CrossRef]
- Yeung, D.E.; Peterknecht, E.; Hajibandeh, S.; Hajibandeh, S.; Torrance, A.W. C-reactive protein can predict anastomotic leak in colorectal surgery: A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 1147–1162. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Deidda, S.; Maslyankov, S.; Paycheva, T.; Farag, A.; Mashhour, A.; Misiakos, E.; Papakonstantinou, D.; Mik, M.; Losinska, J.; et al. C reactive protein to albumin ratio (CAR) as predictor of anastomotic leakage in colorectal surgery. Surg. Oncol. 2021, 38, 101621. [Google Scholar] [CrossRef]
- Ge, X.; Cao, Y.; Wang, H.; Ding, C.; Tian, H.; Zhang, X.; Gong, J.; Zhu, W.; Li, N. Diagnostic accuracy of the postoperative ratio of C-reactive protein to albumin for complications after colorectal surgery. World J. Surg. Oncol. 2017, 15, 15. [Google Scholar] [CrossRef]
- Sparreboom, C.L.; Wu, Z.; Dereci, A.; Boersema, G.S.A.; Menon, A.G.; Ji, J.; Kleinrensink, G.J.; Lange, J.F. Cytokines as early markers of colorectal anastomotic leakage: A systematic review and meta-analysis. Gastroenterol. Res. Pract. 2016, 2016, 3786418. [Google Scholar] [CrossRef] [PubMed]
- Hajong, R.; Newme, K.; Nath, C.; Moirangthem, T.; Dhal, M.; Pala, S. Role of serum C-reactive protein and interleukin-6 as a predictor of intra-abdominal and surgical site infections after elective abdominal surgery. J. Fam. Med. Prim. Care 2021, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Azzini, A.M.; Dorizzi, R.M.; Sette, P.; Vecchi, M.; Coledan, I.; Righi, E.; Tacconelli, E. A 2020 review on the role of procalcitonin in different clinical settings: An update conducted with the tools of the Evidence Based Laboratory Medicine. Ann. Transl. Med. 2020, 8, 610. [Google Scholar] [CrossRef]
- Desborough, J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Ogawa, E.; Wada, H.; Hirata, T. Systemic inflammatory response syndrome and surgical stress in thoracic surgery. J. Crit. Care 2006, 21, 48–53. [Google Scholar] [CrossRef]
- Talmor, M.; Hydo, L.; Barie, P.S. Relationship of Systemic Inflammatory Response Syndrome to Organ Dysfunction, Length of Stay, and Mortality in Critical Surgical Illness: Effect of Intensive Care Unit Resuscitation. Arch. Surg. 1999, 134, 81–87. [Google Scholar] [CrossRef]
- Lin, L.; Pantapalangkoor, P.; Tan, B.; Bruhn, K.W.; Ho, T.; Nielsen, T.; Skaar, E.P.; Zhang, Y.; Bai, R.; Wang, A.; et al. Transferrin Iron Starvation Therapy for Lethal Bacterial and Fungal Infections. J. Infect. Dis. 2014, 210, 254–264. [Google Scholar] [CrossRef]
- Claise, C.; Saleh, J.; Rezek, M.; Vaulont, S.; Peyssonnaux, C.; Edeas, M. Low transferrin levels predict heightened inflammation in patients with COVID-19: New insights. Int. J. Infect. Dis. 2022, 116, 74–79. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Ginoya, S.; Tandon, P.; Gohel, T.D.; Guirguis, J.; Vallabh, H.; Jevenn, A.; Hanouneh, I. Malnutrition: Laboratory markers vs nutritional assessment. Gastroenterol. Rep. 2016, 4, 272–280. [Google Scholar] [CrossRef]
- Sharma, A.; Giraddi, G.; Krishnan, G.; Shahi, A.K. Efficacy of Serum Prealbumin and CRP Levels as Monitoring Tools for Patients with Fascial Space Infections of Odontogenic Origin: A Clinicobiochemical Study. J. Maxillofac. Oral Surg. 2014, 13, 1–9. [Google Scholar] [CrossRef]
- Ogun, A.S.; Adeyinka, A. Biochemistry, Transferrin. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Aisen, P.; Enns, C.; Wessling-Resnick, M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001, 33, 940–959. [Google Scholar] [CrossRef]
- Weinberg, E.D. Iron availability and infection. Biochim. Biophys. Acta 2009, 1790, 600–605. [Google Scholar] [CrossRef]
- Siddiqui, K.; Uqaili, A.A.; Memon, S.; Shah, T.; Shaikh, S.N.; Memon, A.R. Association of Serum Albumin, Globulin, and Transferrin Levels in Children of Poorly Managed Celiac Disease. BioMed Res. Int. 2023, 2023, 5081303. [Google Scholar] [CrossRef] [PubMed]
- Ogle, C.K.; Wesley Alexander, J.; Macmillan, B.G. The relationship of bacteremia to levels of transferrin, albumin and total serum protein in burn patients. Burns 1981, 8, 32–38. [Google Scholar] [CrossRef]
- CDC. Surgical Site Infection Event (SSI) Introduction; National Healthcare Safety Network: Atlanta, GA, USA, 2022; pp. 1–39.
- Macefield, R.; Blazeby, J.; Reeves, B.; Brookes, S.; Avery, K.; Rogers, C.; Woodward, M.; Welton, N.; Rooshenas, L.; Mathers, J.; et al. Validation of the Bluebelle Wound Healing Questionnaire for assessment of surgical-site infection in closed primary wounds after hospital discharge. Br. J. Surg. 2019, 106, 226–235. [Google Scholar] [CrossRef]
- Courtney, A.; Clymo, J.; Dorudi, Y.; Moonesinghe, S.R.; Dorudi, S. Scoping review: The terminology used to describe major abdominal surgical procedures. World J. Surg. 2024, 48, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Courtney, A.; Dorudi, Y.; Clymo, J.; Cosentino, D.; Cross, T.; Moonesinghe, S.R.; Dorudi, S. Novel approach to defining major abdominal surgery. Br. J. Surg. 2024, 111, znad355. [Google Scholar] [CrossRef]
- Guest, J.F.; Fuller, G.W.; Griffiths, B. Cohort study to characterise surgical site infections after open surgery in the UK’s National Health Service. BMJ Open 2023, 13, e076735. [Google Scholar] [CrossRef]
- Hou, Y.; Collinsworth, A.; Hasa, F.; Griffin, L. Incidence and impact of surgical site infections on length of stay and cost of care for patients undergoing open procedures. Surg. Open Sci. 2023, 11, 1–18. [Google Scholar] [CrossRef]
- Eriksen, M.T.; Wibe, A.; Norstein, J.; Haffner, J.; Wiig, J.N. Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Color. Dis. 2005, 7, 51–57. [Google Scholar] [CrossRef]
- Seidelman, J.L.; Mantyh, C.R.; Anderson, D.J. Surgical Site Infection Prevention: A Review. JAMA 2023, 329, 244–252. [Google Scholar] [CrossRef]
- Zwicky, S.N.; Gloor, S.; Tschan, F.; Candinas, D.; Demartines, N.; Weber, M.; Beldi, G. Impact of gender on surgical site infections in abdominal surgery: A multi-center study. Br. J. Surg. 2022, 109 (Suppl. 3), 17–21. [Google Scholar] [CrossRef]
- Aghdassi, S.J.S.; Schröder, C.; Gastmeier, P. Gender-related risk factors for surgical site infections. Results from 10 years of surveillance in Germany. Antimicrob. Resist. Infect. Control 2019, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Offner, P.J.; Moore, E.E.; Biffl, W.L. Male gender is a risk factor for major infections after surgery. Arch. Surg. 1999, 134, 935–940. [Google Scholar] [CrossRef]
- Gagen, B.; Hall, C. Preventing Surgical Site Infections in Emergency General Surgery: Current Strategies and Recommendations. Curr. Surg. Rep. 2024, 12, 227–237. [Google Scholar] [CrossRef]
- Pellino, G.; Sciaudone, G.; Selvaggi, F.; Canonico, S. Prophylactic negative pressure wound therapy in colorectal surgery. Effects on surgical site events: Current status and call to action. Updates Surg. 2015, 67, 235–245. [Google Scholar] [CrossRef]
- Wells, C.I.; Ratnayake, C.B.B.; Perrin, J.; Pandanaboyana, S. Prophylactic Negative Pressure Wound Therapy in Closed Abdominal Incisions: A Meta-analysis of Randomised Controlled Trials. World J. Surg. 2019, 43, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.F.; Dellinger, E.P.; Ko, C.Y.; Duane, T.M. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J. Am. Coll. Surg. 2017, 224, 59–74. [Google Scholar] [CrossRef]
- De Simone, B.; Sartelli, M.; Coccolini, F.; Ball, C.G.; Brambillasca, P.; Chiarugi, M.; Campanile, F.C.; Nita, G.; Corbella, D.; Leppaniemi, A.; et al. Intraoperative surgical site infection control and prevention: A position paper and future addendum to WSES intra-abdominal infections guidelines. World J. Emerg. Surg. 2020, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Winfield, R.D.; Reese, S.; Bochicchio, K.; Mazuski, J.E.; Bochicchio, G.V. Obesity and the risk for surgical site infection in abdominal surgery. Am. Surg. 2016, 82, 331–336. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.A.; Favelyukis, S.; Nguyen, A.K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef]
- Luck, H.; Khan, S.; Kim, J.H.; Copeland, J.K.; Revelo, X.S.; Tsai, S.; Chakraborty, M.; Cheng, K.; Tao Chan, Y.; Nøhr, M.K.; et al. Gut-associated IgA+ immune cells regulate obesity-related insulin resistance. Nat. Commun. 2019, 10, 3650. [Google Scholar] [CrossRef]
- Lauterbach, M.A.R.; Wunderlich, F.T. Macrophage function in obesity-induced inflammation and insulin resistance. Pflugers Arch. Eur. J. Physiol. 2017, 469, 385–396. [Google Scholar] [CrossRef]
- Pugliese, G.; Liccardi, A.; Graziadio, C.; Barrea, L.; Muscogiuri, G.; Colao, A. Obesity and infectious diseases: Pathophysiology and epidemiology of a double pandemic condition. Int. J. Obes. 2022, 46, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Ralph, N.; Brown, L.; McKillop, K.L.; Duff, J.; Osborne, S.; Terry, V.R.; Edward, K.L.; King, R.; Barui, E. Oral nutritional supplements for preventing surgical site infections: Protocol for a systematic review and meta-analysis. Syst. Rev. 2020, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Bucataru, A.; Balasoiu, M.; Ghenea, A.E.; Zlatian, O.M.; Vulcanescu, D.D.; Horhat, F.G.; Bagiu, I.C.; Sorop, V.B.; Sorop, M.I.; Oprisoni, A.; et al. Factors Contributing to Surgical Site Infections: A Comprehensive Systematic Review of Etiology and Risk Factors. Clin. Pract. 2023, 14, 52–68. [Google Scholar] [CrossRef]
- Khalil, R.H.; Al-Humadi, N. Types of acute phase reactants and their importance in vaccination. Biomed. Rep. 2020, 12, 143–152. [Google Scholar] [CrossRef]
- Gruys, E.; Toussaint, M.J.M.; Niewold, T.A.; Koopmans, S.J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. 2005, 6, 1045–1056. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef] [PubMed]
- Calabro, P.; Chang, D.W.; Willerson, J.T.; Yeh, E.T.H. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: Linking obesity to vascular inflammation. J. Am. Coll. Cardiol. 2005, 46, 1112–1113. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron Sequestration and Anemia of Inflammation. Semin. Hematol. 2009, 46, 387–393. [Google Scholar] [CrossRef]
- Van Der Poll, T.; Van De Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Rollier, A.; DiPersio, C.M.; Cereghini, S.; Stevens, K.; Tronche, F.; Zaret, K.; Weiss, M.C. Regulation of albumin gene expression in hepatoma cells of fetal phenotype: Dominant inhibition of HNF1 function and role of ubiquitous transcription factors. Mol. Biol. Cell 1993, 4, 59–69. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Meier-Ewert, H.K.; Ridker, P.M.; Rifai, N.; Price, N.; Dinges, D.F.; Mullington, J.M. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin. Chem. 2001, 47, 426–430. [Google Scholar] [CrossRef]
- Andersen, I.B.; Brasen, C.L.; Christensen, H.; Noehr-Jensen, L.; Nielsen, D.E.; Brandslund, I.; Madsen, J.S. Standardised Resting Time Prior to Blood Sampling and Diurnal Variation Associated with Risk of Patient Misclassification: Results from Selected Biochemical Components. PLoS ONE 2015, 10, e0140475. [Google Scholar] [CrossRef]
- Bennett, C.; Pettikiriarachchi, A.; McLean, A.R.D.; Harding, R.; Blewitt, M.E.; Seillet, C.; Pasricha, S.R. Serum iron and transferrin saturation variation are circadian regulated and linked to the harmonic circadian oscillations of erythropoiesis and hepatic Tfrc expression in mice. Am. J. Hematol. 2024, 99, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.; Carlsson, A.; Gerdle, B.; Ernberg, M.; Ghafouri, B. Diurnal variation of inflammatory plasma proteins involved in pain. Pain Rep. 2019, 4, e776. [Google Scholar] [CrossRef] [PubMed]
- Donlon, N.E.; Mohan, H.; Free, R.; Elbaghir, B.; Soric, I.; Fleming, C.; Balasubramanian, I.; Ivanovski, I.; Schmidt, K.; Mealy, K. Predictive value of CRP/albumin ratio in major abdominal surgery. Ir. J. Med. Sci. 2020, 189, 1465–1470. [Google Scholar] [CrossRef]
- Goulart, A.; Ferreira, C.; Estrada, A.; Nogueira, F.; Martins, S.; Mesquita-Rodrigues, A.; Sousa, N.; Leão, P. Early Inflammatory Biomarkers as Predictive Factors for Freedom from Infection after Colorectal Cancer Surgery: A Prospective Cohort Study. Surg. Infect. 2018, 19, 446–450. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lu, J.; Shen, F.; Xie, H.; Cui, H.; Xu, R. Serum albumin level is associated with mortality and hospital stays: A real-world data analysis. Clin. Nutr. ESPEN 2024, 64, 215–220. [Google Scholar] [CrossRef]
- Allison, S.P.; Lobo, D.N. The clinical significance of hypoalbuminaemia. Clin. Nutr. 2024, 43, 909–914. [Google Scholar] [CrossRef]
- Dharap, S.B.; Barbaniya, P.; Navgale, S. Incidence and Risk Factors of Postoperative Complications in General Surgery Patients. Cureus 2022, 14, e30975. [Google Scholar] [CrossRef] [PubMed]
- Aeschbacher, P.; Nguyen, T.L.; Dorn, P.; Kocher, G.J.; Lutz, J.A. Surgical Site Infections Are Associated With Higher Blood Loss and Open Access in General Thoracic Practice. Front. Surg. 2021, 8, 656249. [Google Scholar] [CrossRef]
- Leylek, M.; Poliquin, V.; Al-Wazzan, A.; Dean, E.; Altman, A.D. Postoperative Infection in the Setting of Massive Intraoperative Blood Loss. J. Obstet. Gynaecol. Can. 2016, 38, 1110–1113. [Google Scholar] [CrossRef]

| Type of Study | ||||||||
|---|---|---|---|---|---|---|---|---|
| Prospective | Retrospective | Total | p | |||||
| N (%) | N (%) | N (%) | ||||||
| Sex | Female | 48 (55.8%) | 38 (44.2%) | 86 (43%) | 0.94 | |||
| Male | 63 (55.3%) | 51 (44.7%) | 114 (57%) | |||||
| Age groups | ≤40 | 8 (66.7%) | 4 (33.3%) | 12 (6%) | 0.68 | |||
| 41–50 | 8 (53.3%) | 7 (46.7%) | 15 (7.5%) | |||||
| 51–60 | 17 (58.6%) | 12 (41.4%) | 29 (14.5%) | |||||
| 61–70 | 38 (61.3%) | 24 (38.7%) | 62 (31%) | |||||
| 71–80 | 27 (49.1%) | 28 (50.9%) | 55 (27.5%) | |||||
| 81+ | 13 (48.1%) | 14 (51.9%) | 27 (13.5%) | |||||
| Elective operation | Yes | 62 (60%) | 40 (39.2%) | 102 (51%) | 0.12 | |||
| No | 49 (40%) | 49 (60.8%) | 98 (49%) | |||||
| Postoperative complications | No | 54 (48.6%) | 27 (30.3%) | 81 (40.5%) | 0.002 | |||
| Yes | 57 (51.4%) | 62 (69.7%) | 119 (59.5%) | |||||
| 1 | 34 (30.6%) | 37 (41.6%) | 71 (35.5%) | |||||
| 2 | 10 (9%) | 21 (23.6%) | 31 (15.5%) | |||||
| 3 | 9 (8.1%) | 4 (4.5%) | 13 (6.5%) | |||||
| 4 | 4 (3.6%) | 0 (0%) | 4 (2%) | |||||
| Wound infection | No | 54 (48.6%) | 27 (30.3%) | 81 (40.5%) | 0.009 | |||
| Yes | 57 (51.4%) | 62 (69.7%) | 119 (59.5%) | |||||
| Wound dehiscence | No | 92 (82.9%) | 72 (80.9%) | 164 (82%) | 0.72 | |||
| Yes | 19 (17.1%) | 17 (19.1%) | 36 (18%) | |||||
| Intrabdominal abscess | No | 99 (89.2%) | 77 (86.5%) | 176 (88%) | 0.56 | |||
| Yes | 12 (10.8%) | 12 (13.5%) | 24 (12%) | |||||
| Anastomotic/stump leak | No | 64 (85.9%) | 61 (100%) | 125 (95.4%) | 0.006 | |||
| Yes | 9 (14.1%) | 0 (0%) | 9 (4.6%) | |||||
| Re-operation | No | 109 (98.2%) | 83 (93.3%) | 192 (96%) | 0.08 | |||
| Yes | 2 (1.8%) | 6 (6.7%) | 8 (4%) | |||||
| Mean | SD | Mean | SD | Mean | SD | |||
| Age (years) | 63.9 | 15 | 66.6 | 14.6 | 65.1 | 14.8 | 0.21 | |
| BMI | 27 | 3.8 | 27.5 | 3.6 | 27.2 | 3.7 | 0.4 | |
| Length of stay (Days) | 8.9 | 4.7 | 13.9 | 8.0 | 11.1 | 6.8 | <0.001 | |
| Charlson comorbidity index (CCI) | 3.4 | 1.2 | 2.9 | 1.3 | 3.1 | 1.2 | 0.23 | |
| Laboratory Test | Day 1 | Day 2 | Day 3 | df1. df2 | F | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||||
| WBCs (103) | Total | 11.8 | 4.5 | 10.9 | 3.9 | 9.7 | 3.3 | 2. 588 | 24.28 | <0.001 |
| N/I | 11.9 | 4.4 | 10.7 | 3.9 | 9.5 | 3. | 3. 585 | 0.86 | 0.46 | |
| Inf | 11.7 | 4.5 | 11 | 4 | 9.9 | 3.4 | ||||
| CRP (mg/dL) | Total | 11.55 | 8.33 | 17.53 | 8.92 | 16.47 | 8.42 | 2. 398 | 66.73 | <0.001 |
| N/I | 7.99 | 6.06 | 11.76 | 7.24 | 10.76 | 6.01 | 2. 396 | 7.24 | 0.001 | |
| Inf | 13.98 | 8.79 | 21.46 | 7.75 | 20.35 | 7.59 | ||||
| ALB (g/dL) | Total | 3.3 | 0.5 | 3.2 | 0.4 | 3.1 | 0.4 | 2. 398 | 99.66 | <0.001 |
| N/I | 3.5 | 0.4 | 3.4 | 0.4 | 3.3 | 0.3 | 2. 396 | 3.13 | 0.052 | |
| Inf | 3.2 | 0.5 | 3.0 | 0.4 | 2.9 | 0.4 | ||||
| Transferrin (mg/dL) | Total | 171.5 | 44.8 | 158.6 | 41.2 | 150.9 | 37.7 | 2. 220 | 76.11 | <0.001 |
| N/I | 182.8 | 43.7 | 172.7 | 41.9 | 163.0 | 37.2 | 2. 218 | 76.05 | 0.27 | |
| Inf | 160.7 | 43.5 | 145.3 | 36.2 | 139.4 | 34.7 | ||||
| CRP/ALB | Total | 3.7 | 3.0 | 5.8 | 3.2 | 5.6 | 3.1 | 2. 398 | 72.19 | <0.001 |
| N/I | 2.4 | 2.0 | 3.6 | 2.3 | 3.3 | 1.9 | 2. 396 | 10.97 | <0.001 | |
| Inf | 4.6 | 3.2 | 7.3 | 2.9 | 7.1 | 2.9 | ||||
| CRP/Trans | Total | 0.07 | 0.06 | 0.11 | 0.07 | 0.11 | 0.07 | 2. 220 | 39.36 | <0.001 |
| N/I | 0.05 | 0.07 | 0.08 | 0.06 | 0.07 | 0.04 | 2. 218 | 15.12 | <0.001 | |
| Inf | 0.08 | 0.06 | 0.15 | 0.07 | 0.15 | 0.07 | ||||
| Postoperative Complications | ||||
|---|---|---|---|---|
| Parameter | No | Yes | p * | |
| N (%) | N (%) | |||
| Sex | Female | 44 (54.3%) | 42 (35.3%) | 0.008 |
| Male | 37 (45.7%) | 77 (64.7%) | ||
| Type of study | Prospective | 54 (66.7%) | 57 (47.9%) | 0.009 |
| Retrospective | 27 (33.3%) | 62 (52.1%) | ||
| Elective operation | No | 52 (64.2%) | 46 (38.7%) | <0.001 |
| Yes | 29 (35.8%) | 73 (61.3%) | ||
| Age (years) | ≤40 | 5 (6.2%) | 7 (5.9%) | 0.34 |
| 41–50 | 3 (3.7%) | 12 (10.1%) | ||
| 51–60 | 10 (12.3%) | 19 (16.0%) | ||
| 61–70 | 25 (30.9%) | 37 (31.1%) | ||
| 71–80 | 23 (28.4%) | 32 (26.9%) | ||
| 81+ | 15 (18.5%) | 12 (10.1%) | ||
| Hypertension | No | 35 (43.2%) | 50 (42.0%) | 0.87 |
| Yes | 46 (56.8%) | 69 (58.0%) | ||
| Diabetes type II | No | 61 (75.3%) | 86 (72.3%) | 0.63 |
| Yes | 20 (24.7%) | 33 (27.7%) | ||
| COPD | No | 67 (82.7%) | 97 (81.5%) | 0.83 |
| Yes | 14 (17.3%) | 22 (18.5%) | ||
| Chronic renal disease | No | 75 (92.6%) | 106 (89.1%) | 0.41 |
| Yes | 6 (7.4%) | 13 (10.9%) | ||
| Cancer | No | 40 (49.4%) | 58 (48.7%) | 0.93 |
| Yes | 41 (50.6%) | 61 (51.3%) | ||
| Metastatic cancer | No | 75 (92.6%) | 111 (93.3%) | 0.95 |
| Yes | 6 (7.4%) | 8 (6.7%) | ||
| Mean (SD) | Mean (SD) | p ** | ||
| Age (years) | 67.16 (14.61) | 63.76 (14.86) | 0.09 | |
| BMI (Kg/m2) | 26.98 (3.4) | 27.37 (3.89) | <0.001 | |
| Post Op CRP DAY2 | 11.76 (7.24) | 21.46 (7.75) | <0.001 | |
| Post Op ALB DAY2 | 3.37 (0.36) | 3 (0.41) | <0.001 | |
| Post Op WBCs DAY2 | 10.54 (3.86) | 10.99 (3.97) | 0.35 | |
| CAR DAY 2 | 3.59 (2.32) | 7.31 (2.89) | <0.001 | |
| CTR DAY 2 | 0.08 (0.06) | 0.15 (0.07) | <0.001 | |
| CTR DAY2 × 100 | 7.56 (6.01) | 14.92 (6.96) | <0.001 | |
| Postoperative Septic Complications = Model Enter | ||||
| Parameter | OR | 95%LL | 95%UL | p |
| Age (years) | 0.98 | 0.94 | 1.01 | 0.17 |
| BMI (Kg/m2) | 0.90 | 0.79 | 1.02 | 0.11 |
| Female sex | 0.23 | 0.08 | 0.68 | 0.008 |
| Elective operation | 0.86 | 0.29 | 2.54 | 0.78 |
| Hypertension | 1.07 | 0.45 | 2.54 | 0.87 |
| Diabetes type II | 1.52 | 0.61 | 3.78 | 0.37 |
| COPD | 1.20 | 0.37 | 3.91 | 0.76 |
| Chronic renal disease | 0.99 | 0.19 | 5.25 | 0.99 |
| Presence of cancer | 1.70 | 0.69 | 4.21 | 0.25 |
| Post-op TRF DAY2 | 1.01 | 1.00 | 1.03 | 0.14 |
| Post-op ALB DAY2 | 0.05 | 0.01 | 0.33 | 0.002 |
| CTR DAY 2 × 100 | 1.23 | 1.10 | 1.39 | 0.000 |
| CTR DAY 3 × 100 | 1.40 | 1.21 | 1.61 | <0.001 |
| CAR DAY 2 | 1.73 | 1.32 | 2.29 | <0.001 |
| CAR DAY 3 | 2.02 | 1.63 | 2.51 | 0.000 |
| Postoperative septic complications = Model Forward | ||||
| Forward | OR | 95%LL | 95%UL | p |
| Female sex | 0.26 | 0.09 | 0.71 | 0.009 |
| Post-op ALB DAY2 | 0.12 | 0.03 | 0.52 | 0.005 |
| CTR DAY 2 × 100 | 1.18 | 1.07 | 1.29 | <0.001 |
| CTR DAY 3 × 100 | 1.42 | 1.24 | 1.63 | <0.001 |
| CAR DAY 2 | 1.68 | 1.31 | 2.17 | <0.001 |
| CAR DAY 3 | 2.02 | 1.65 | 2.48 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolouzakis, T.K.; Alegakis, A.; Niniraki, M.; Kampa, M.; Chrysos, E. Evaluation of the Diagnostic and Predictive Significance of Postoperative C-Reactive Protein to Transferrin or Albumin Ratio in Identifying Septic Events Following Major Abdominal Surgery. J. Clin. Med. 2025, 14, 4341. https://doi.org/10.3390/jcm14124341
Nikolouzakis TK, Alegakis A, Niniraki M, Kampa M, Chrysos E. Evaluation of the Diagnostic and Predictive Significance of Postoperative C-Reactive Protein to Transferrin or Albumin Ratio in Identifying Septic Events Following Major Abdominal Surgery. Journal of Clinical Medicine. 2025; 14(12):4341. https://doi.org/10.3390/jcm14124341
Chicago/Turabian StyleNikolouzakis, Taxiarchis Konstantinos, Athanasios Alegakis, Maria Niniraki, Marilena Kampa, and Emmanouel Chrysos. 2025. "Evaluation of the Diagnostic and Predictive Significance of Postoperative C-Reactive Protein to Transferrin or Albumin Ratio in Identifying Septic Events Following Major Abdominal Surgery" Journal of Clinical Medicine 14, no. 12: 4341. https://doi.org/10.3390/jcm14124341
APA StyleNikolouzakis, T. K., Alegakis, A., Niniraki, M., Kampa, M., & Chrysos, E. (2025). Evaluation of the Diagnostic and Predictive Significance of Postoperative C-Reactive Protein to Transferrin or Albumin Ratio in Identifying Septic Events Following Major Abdominal Surgery. Journal of Clinical Medicine, 14(12), 4341. https://doi.org/10.3390/jcm14124341







