A Narrative Overview of Fatal Myocarditis in Infant with Focus on Sudden Unexpected Death and Forensic Implications
Abstract
1. Introduction
1.1. Introduction to Myocarditis
1.2. Classifications of Myocarditis
1.3. Epidemiology of Myocarditis
1.4. Clinical Characteristics of Myocarditis
1.5. Diagnosis of Myocarditis
1.6. Treatment Options and Infant-Specific Implications
1.7. Sudden Infant Death Syndrome
2. Materials and Methods
3. Results
Review Results
4. Discussion
4.1. Epidemiology of Sudden Infant Death Syndrome (SIDS)
4.2. Multifactorial Causes of Pediatric Sudden Death: From Autopsy Findings to Prevention Strategies
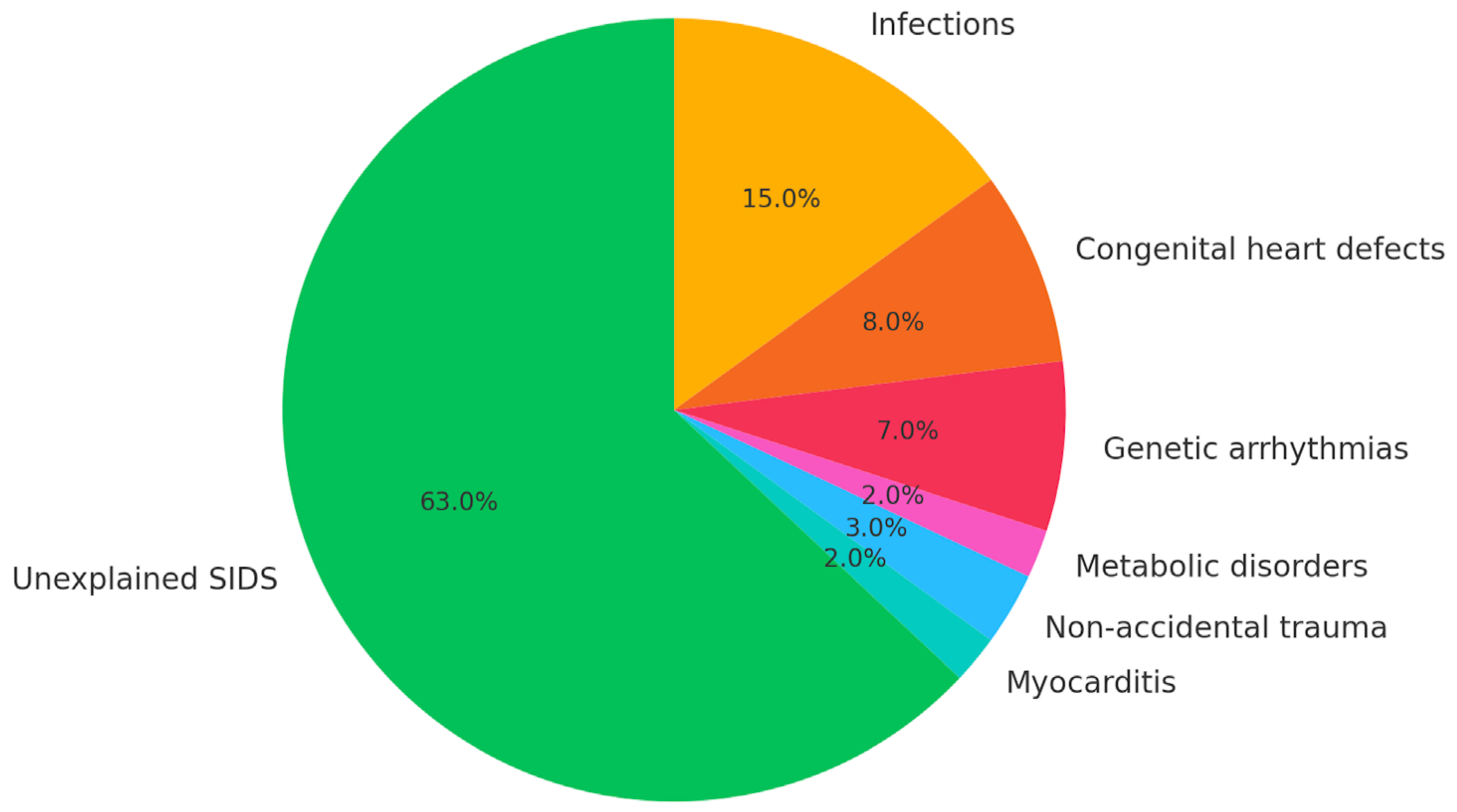
4.3. Role of Myocarditis in SIDS
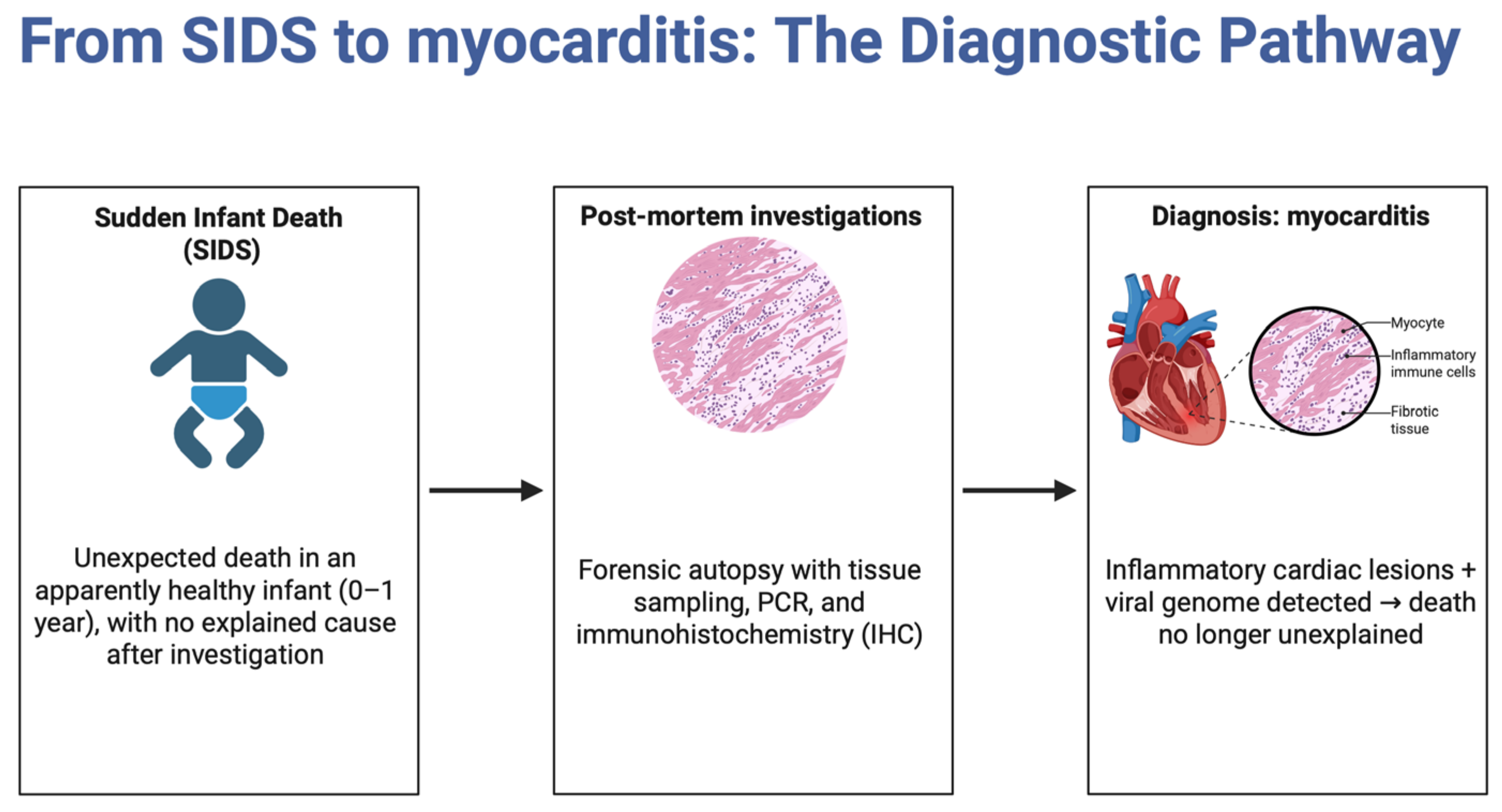
4.4. Autopsy Techniques in SIDS Cases
4.5. External and Internal Signs in SIDS Cases
4.6. Histopathological Identification in SIDS
4.7. Laboratory Techniques for Diagnosing Myocarditis in Infants
4.8. Developing an Operational Protocol for Investigating SIDS
4.9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kühl, U.; Schultheiss, H.-P.; Viral Myocarditis. Swiss Med Wkly [Internet]. 2014. Available online: https://smw.ch/index.php/smw/article/view/1906 (accessed on 2 March 2025).
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A. Myocarditis and Inflammatory Cardiomyopathy: Current Evidence and Future Directions. Available online: https://www.nature.com/articles/s41569-020-00435-x (accessed on 2 March 2025).
- d’Ambrosio, A.; Patti, G.; Manzoli, A.; Sinagra, G. The Fate of Acute Myocarditis Between Spontaneous Improvement and Evolution to Dilated Cardiomyopathy: A Review|Heart. Available online: https://heart.bmj.com/content/85/5/499.short (accessed on 2 March 2025).
- Chan, P.S.; Klimkiewicz, J.J.; Luchetti, W.T.; Esterhai, J.L.; Kneeland, J.B.; Dalinka, M.K.; Heppenstall, R.B. Impact of CT Scan on Treatment Plan and Fracture Classification of Tibial Plateau Fractures. J. Orthop. Trauma. 1997, 11, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Meinhold-Heerlein, I.; Fotopoulou, C.; Harter, P. The New WHO Classification of Ovarian, Fallopian Tube, and Primary Peritoneal Cancer and Its Clinical Implications. Available online: https://link.springer.com/article/10.1007/S00404-016-4035-8 (accessed on 2 March 2025).
- Buoninfante, A.; Andeweg, A.; Genov, G.; Cavaleri, M. Myocarditis Associated with COVID-19 Vaccination. Available online: https://www.nature.com/articles/s41541-024-00893-1 (accessed on 2 March 2025).
- Priyadarshni, S.; Westra, J.; Kuo, Y.; Baillargeon, J. 83633-COVID-19-Infection-and-Incident-of-Myocarditis-a-Multi-Site-Population-Based-Propensity-Score-Matched-Analysis. Available online: https://www.cureus.com (accessed on 2 March 2025).
- Kong, Q.; Xu, X.; Li, M.; Meng, X.; Zhao, C. Global, Regional, and National Burden of Myocarditis in 204 Countries and Territories from 1990 to 2019: Updated Systematic Analysis. Available online: https://publichealth.jmir.org/2024/1/e46635/ (accessed on 2 March 2025).
- Hassan, K.; Kyriakakis, C.; Doubell, A.; Van Zyl, G. Prevalence of Cardiotropic Viruses in Adults with Clinically Suspected Myocarditis in South Africa. Available online: https://openheart.bmj.com/content/9/1/e001942 (accessed on 2 March 2025).
- Lasica, R.; Djukanovic, L.; Savic, L.; Krljanac, G. Update on Myocarditis: From Etiology and Clinical Picture to Modern Diagnostics and Methods of Treatment. Available online: https://www.mdpi.com/2075-4418/13/19/3073 (accessed on 2 March 2025).
- Husby, A.; Hansen, J.; Fosbøl, E.; Thiesson, E. SARS-CoV-2 Vaccination and Myocarditis or Myopericarditis: Population Based Cohort Study. Available online: https://www.bmj.com/content/375/bmj-2021-068665.abstract (accessed on 2 March 2025).
- Gupta, S.; Markham, D.; Drazner, M. Fulminant Myocarditis. Available online: https://www.nature.com/articles/ncpcardio1331 (accessed on 2 March 2025).
- Pankuweit, S.; Klingel, K. Viral Myocarditis: From Experimental Models to Molecular Diagnosis in Patients. Available online: https://link.springer.com/article/10.1007/s10741-012-9357-4 (accessed on 2 March 2025).
- Angelini, A.; Calzolari, V.; Calabrese, F.; Boffa, G. Myocarditis Mimicking Acute Myocardial Infarction: Role of Endomyocardial Biopsy in the Differential Diagnosis|Heart. Available online: https://heart.bmj.com/content/84/3/245.short (accessed on 2 March 2025).
- Liguori, C.; Farina, D.; Vaccher, F.; Ferrandino, G. Myocarditis: Imaging Up to Date. Available online: https://link.springer.com/article/10.1007/s11547-020-01279-8 (accessed on 2 March 2025).
- Łajczak, P.; Jóźwik, K. Artificial Intelligence and Myocarditis—A Systematic Review of Current Applications. Available online: https://link.springer.com/article/10.1007/s10741-024-10431-9 (accessed on 2 March 2025).
- Brociek, E.; Tymińska, A.; Giordani, A.; Caforio, A. Myocarditis: Etiology, Pathogenesis, and Their Implications in Clinical Practice. Available online: https://www.mdpi.com/2079-7737/12/6/874 (accessed on 2 March 2025).
- Ferone, E.; Segev, A.; Tempo, E.; Gentile, P.; Elsanhoury, A.; Baggio, C.; Artico, J.; Bhatti, P.; Scott, P.; Bobbio, E.; et al. Current Treatment and Immunomodulation Strategies in Acute Myocarditis. J. Cardiovasc. Pharmacol. 2024, 83, 364–376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maisch, B. Cardio-Immunology of Myocarditis: Focus on Immune Mechanisms and Treatment Options. Available online: https://www.frontiersin.org/articles/10.3389/fcvm.2019.00048/full (accessed on 2 March 2025).
- Heymans, S.; Eriksson, U.; Lehtonen, J. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. Available online: https://www.jacc.org/doi/abs/10.1016/j.jacc.2016.09.937 (accessed on 2 March 2025).
- Schranz, D.; Recla, S.; Malcic, I.; Kerst, G. Pulmonary Artery Banding in Dilative Cardiomyopathy of Young Children: Review and Protocol Based on the Current Knowledge. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6514280/. (accessed on 2 March 2025).
- Jensen, L.; Marchant, D. Emerging Pharmacologic Targets and Treatments for Myocarditis. Available online: https://www.sciencedirect.com/science/article/pii/S0163725816300146 (accessed on 2 March 2025).
- Bejiqi, R.; Retkoceri, R.; Maloku, A. The Diagnostic and Clinical Approach to Pediatric Myocarditis: A Review of the Current Literature. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6352488/ (accessed on 2 March 2025).
- Clinical Presentation and Early Predictors for Poor Outcomes in Pediatric Myocarditis: A Retrospective Study. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6406197/ (accessed on 2 March 2025).
- Mitchell, E.; Krous, H.F.; Donald, T.; Byard, R.W. Changing Trends in the Diagnosis of Sudden Infant Death. Am. J. Forensic Med. Pathol. 2000, 21, 311–314. [Google Scholar] [CrossRef] [PubMed]
- l’Hoir, M.; Engelberts, A.; van Well, G. Case-Control Study of Current Validity of Previously Described Risk Factors for SIDS in the Netherlands|Archives of Disease in Childhood. Available online: https://adc.bmj.com/content/79/5/386.short (accessed on 2 March 2025).
- Li, D.; Willinger, M.; Petitti, D.; Odouli, R.; Liu, L. Use of a Dummy (Pacifier) During Sleep and Risk of Sudden Infant Death Syndrome (SIDS): Population Based Case-Control Study. Available online: https://www.bmj.com/content/332/7532/18.full-text (accessed on 2 March 2025).
- Jawed, A.; Ehrhardt, C.; Rye, M. Handle with Care: A Narrative Review of Infant Safe Sleep Practices Across Clinical Guidelines and Social Media to Reduce SIDS. Available online: https://www.mdpi.com/2227-9067/10/8/1365 (accessed on 2 March 2025).
- Goldwater, P.; Bettelheim, K. 867520. Available online: https://ibimapublishing.com/articles/PRIJ/2013/867520/867520.pdf (accessed on 2 March 2025).
- Venter, M. Viral Pathogens in Cases of Sudden and Unexpected Death in Infants (SUDI) at the Tygerberg Medico-legal Mortuary and the Role of Myocarditis as a Possible Cause of Death. Available online: https://scholar.sun.ac.za/handle/10019.1/108273 (accessed on 2 March 2025).
- Saayman, J. Enterovirus and Parvovirus B19 in Sudden and Unexpected Death in Infancy (SUDI) at the Tygerberg Medico-legal Mortuary and the Role of Myocarditis as a Possible Cause of Death. Available online: https://scholar.sun.ac.za/handle/10019.1/103439 (accessed on 2 March 2025).
- Tester, D.; Ackerman, M. Cardiomyopathic and Channelopathic Causes of Sudden Unexplained Death in Infants and Children. Available online: https://www.annualreviews.org (accessed on 2 March 2025).
- Bajanowski, T.; Vege, Å.; Byard, R.; Krous, H. Sudden Infant Death Syndrome (SIDS)—Standardised Investigations and Classification: Recommendations. Available online: https://www.sciencedirect.com/science/article/pii/S0379073806003124 (accessed on 2 March 2025).
- Fraile-Martinez, O.; García-Montero, C. Sudden Infant Death Syndrome (SIDS): State of the Art and Future Directions. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11008475/ (accessed on 2 March 2025).
- Grimaldi, F.; Bonasoni, M.; Pelletti, G.; Gabrielli, L. Diagnostic Challenges and Forensic Implications in a Case of Infantile Fatal Myocarditis. Available online: https://link.springer.com/article/10.1007/s12024-023-00659-6 (accessed on 2 March 2025).
- Matturri, L.; Ottaviani, G.; Lavezzi, A. Guidelines for Neuropathologic Diagnostics of Perinatal Unexpected Loss and Sudden Infant Death Syndrome (SIDS)—A Technical Protocol. Available online: https://link.springer.com/article/10.1007/s00428-007-0527-z (accessed on 2 March 2025).
- Grasmeyer, S.; Madea, B. Immunohistochemical Diagnosis of Myocarditis on (infantile) Autopsy Material: Does It Improve the Diagnosis? Available online: https://link.springer.com/article/10.1007/s12024-015-9675-7 (accessed on 2 March 2025).
- Weber, M.; Sebire, N. Post-Mortem Investigation of Sudden Unexpected Death in Infancy: Role of Autopsy in Classification of Death. Available online: https://link.springer.com/chapter/10.1007/978-1-61779-249-6_2 (accessed on 2 March 2025).
- de Lange, C.; Vege, Å.; Stake, G. Radiography After Unexpected Death in Infants and Children Compared to Autopsy. Available online: https://link.springer.com/article/10.1007/s00247-006-0364-2 (accessed on 2 March 2025).
- Byard, R.; Krous, H. Sudden Infant Death Syndrome: Overview and Update. Available online: https://link.springer.com/article/10.1007/s10024-002-0205-8 (accessed on 2 March 2025).
- Rizzo, S.; De Gaspari, M.; Carturan, E.; Paradiso, B. A Standardized Postmortem Protocol to Assess the Real Burden of Sudden Infant Death Syndrome. Available online: https://link.springer.com/article/10.1007/s00428-020-02747-2 (accessed on 2 March 2025).
- Goldwater, P. Sudden Infant Death Syndrome: A Critical Review of Approaches to Research|Archives of Disease in Childhood. Available online: https://adc.bmj.com/content/88/12/1095.short (accessed on 2 March 2025).
- Goldwater, P.; A Perspective on SIDS Pathogenesis. The Hypotheses: Plausibility and Evidence. Available online: https://link.springer.com/article/10.1186/1741-7015-9-64 (accessed on 2 March 2025).
- Hilton, J. The Pathology of the Sudden Infant Death Syndrome. Available online: https://link.springer.com (accessed on 2 March 2025).
- Pérez-Platz, U.; Saeger, W.; Dhom, G. The Pathology of the Adrenal Glands in Sudden Infant Death Syndrome (SIDS). Available online: https://link.springer.com/article/10.1007/BF01225413 (accessed on 2 March 2025).
- Neubauer, J.; Lecca, M.; Russo, G.; Bartsch, C. Post-Mortem Whole-Exome Analysis in a Large Sudden Infant Death Syndrome Cohort with a Focus on Cardiovascular and Metabolic Genetic Diseases. Available online: https://www.nature.com/articles/ejhg2016199 (accessed on 2 March 2025).
- Dettmeyer, R. Letale Lymphozytäre Myokarditis—Eine Unterschätzte Diagnose im Säuglings-und Kindesalter? Available online: https://link.springer.com/article/10.1007/s00292-023-01279-1 (accessed on 2 March 2025).
- Gaaloul, I.; Riabi, S.; Harrath, R.; Evans, M.; Salem, N. Sudden Unexpected Death Related to Enterovirus Myocarditis: Histopathology, Immunohistochemistry and Molecular Pathology Diagnosis at Post-Mortem. Available online: https://link.springer.com/article/10.1186/1471-2334-12-212 (accessed on 2 March 2025).
- Goldwater, P. The Science (or Nonscience) of Research into Sudden Infant Death Syndrome (SIDS). Available online: https://www.frontiersin.org/articles/10.3389/fped.2022.865051/full (accessed on 2 March 2025).
- Calabrese, F.; Rigo, E.; Milanesi, O.; Boffa, G.M.; Angelini, A.; Valente, M.; Thiene, G. Molecular Diagnosis of Myocarditis and Dilated Cardiomyopathy in Children: Clinicopathologic Features and Prognostic Implications. Diagn. Mol. Pathol. 2002, 11, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Sebire, N. Molecular Diagnostic Techniques in the Post-Mortem Investigation of Sudden Unexpected Infant Deaths: Current and Future Applications. Available online: https://benthamopenarchives.com/contents/pdf/TOPATJ/TOPATJ-4-110.pdf (accessed on 3 March 2025).
- D’Arcy, C.; Hazrati, L. Histopathologic Analysis in Sudden Infant and Child Deaths: A Practical Approach. Available online: https://journals.sagepub.com/doi/abs/10.1177/1925362118797727 (accessed on 3 March 2025).
- Bajanowski, T.; Vennemann, M. Sudden and Unexpected Deaths in Infants and Sudden Infant Death Syndrome. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118570654.ch35 (accessed on 3 March 2025).
- Knottnerus, J.; van Weel, C.; Muris, J. Evaluation of Diagnostic Procedures. Available online: https://www.bmj.com (accessed on 3 March 2025).
- Jayamohan, H.; Lambert, C.; Sant, H.; Jafek, A. SARS-CoV-2 Pandemic: A Review of Molecular Diagnostic Tools Including Sample Collection and Commercial Response with Associated Advantages and Limitations. Available online: https://link.springer.com/article/10.1007/s00216-020-02958-1 (accessed on 3 March 2025).
- Tursz, A.; Crost, M.; Gerbouin-Rérolle, P.; Cook, J. Underascertainment of Child Abuse Fatalities in France: Retrospective Analysis of Judicial Data to Assess Underreporting of Infant Homicides in Mortality Statistics—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0145213410001122 (accessed on 3 March 2025).
- Leach, C.; Blair, P.; Fleming, P.; Smith, I. Epidemiology of SIDS and Explained Sudden Infant Deaths. Available online: https://publications.aap.org (accessed on 3 March 2025).
- Weber, M.; Sebire, N. Postmortem Investigation of Sudden Unexpected Death in Infancy: Current Issues and Autopsy Protocol. Available online: https://www.sciencedirect.com/science/article/pii/S1756231709001595 (accessed on 3 March 2025).
- Krous, H.; Chadwick, A.; Crandall, L. Sudden Unexpected Death in Childhood: A Report of 50 Cases. Available online: https://link.springer.com/article/10.1007/s10024-005-1155-8 (accessed on 3 March 2025).
- O’Donnell, D.; Ahern, E.; Davies, C.; De Brún, A. A Realist Process Evaluation of an Intervention to Promote Competencies in Interprofessional Collaboration Among Interdisciplinary Integrated Care Teams for Older People: Study Protocol. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10579852/ (accessed on 3 March 2025).
- Dettmeyer, R.; Baasner, A.; Schlamann, M.; Haag, C. Coxsackie B3 Myocarditis in 4 Cases of Suspected Sudden Infant Death Syndrome: Diagnosis by Immunohistochemical and Molecular-Pathologic Investigations. Available online: https://www.sciencedirect.com/science/article/pii/S0344033804703209 (accessed on 3 March 2025).
- Weber, B.; Paik, J.; Aghayev, A.; Klein, A. Novel Imaging Approaches to Cardiac Manifestations of Systemic Inflammatory Diseases: JACC Scientific Statement. Available online: https://www.jacc.org/doi/abs/10.1016/j.jacc.2023.09.819 (accessed on 3 March 2025).
- Bryant, V.; Sebire, N. Natural Diseases Causing Sudden Death in Infancy and Early Childhood. Available online: https://europepmc.org/article/nbk/nbk513402 (accessed on 3 March 2025).
- Bowles, N.; Ni, J.; Kearney, D.; Pauschinger, M. Detection of Viruses in Myocardial Tissues by Polymerase Chain Reaction: Evidence of Adenovirus as a Common Cause of Myocarditis in Children and Adults. Available online: https://www.jacc.org/doi/abs/10.1016/S0735-1097 (accessed on 3 March 2025).
- La Grange, H. Respiratory Pathogens in Cases of Sudden Unexpected Death in Infancy (SUDI) at Tygerberg Forensic Pathology Service Mortuary. Available online: https://scholar.sun.ac.za/handle/10019.1/86628 (accessed on 3 March 2025).
- Dettmeyer, R.; Dettmeyer, R. Pregnancy-Related Death, Death in Newborns, and Sudden Infant Death Syndrome. Available online: https://link.springer.com/chapter/10.1007/978-3-319-77997-3_17 (accessed on 3 March 2025).
- Wedekind, H.; Schulze-Bahr, E.; Debus, V. Cardiac Arrhythmias and Sudden Death in Infancy: Implication for the Medicolegal Investigation. Available online: https://link.springer.com/article/10.1007/s00414-005-0069-3 (accessed on 3 March 2025).
- Caforio, A.; Malipiero, G.; Marcolongo, R. Myocarditis: A Clinical Overview. Available online: https://link.springer.com/article/10.1007/s11886-017-0870-x (accessed on 3 March 2025).
- Madea, B. Sudden Death, Especially in Infancy–Improvement of Diagnoses by Biochemistry, Immunohistochemistry and Molecular Pathology. Leg. Med. 2009, 11 (Suppl. S1), S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.M.; Albrecht, M.; Verkaik, B.J.; de Jonge, R.C.J.; Buysse, C.M.P.; Blom, N.A.; Rammeloo, L.A.J.; Verhagen, J.M.A.; Riedijk, M.A.; Yap, S.C.; et al. Sudden cardiac arrest in infants and children: Proposal for a diagnostic workup to identify the etiology. An 18-year multicenter evaluation in the Netherlands. Eur. J. Pediatr. 2024, 183, 335–344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vassalini, M.; Verzeletti, A.; Restori, M.; De Ferrari, F. An autopsy study of sudden cardiac death in persons aged 1–40 years in Brescia (Italy). J. Cardiovasc. Med. 2016, 17, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Dauger, S.; Le Goff, J.; Deho, A.; Jones, P. Varicella zoster in sudden infant death. BMJ Case Rep. 2018, 11, e227034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ondruschka, B.; Baier, C.; Siekmeyer, M.; Buschmann, C.; Dreßler, J.; Bernhard, M. Cardiopulmonary resuscitation-associated injuries in still-/newborns, infants and toddlers in a German forensic collective. Forensic Sci. Int. 2017, 279, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Kon, F.C.; Scheimberg, I.; Haini, M.; Cohen, M.C. Cardiovascular-related death in infancy and childhood: A clinicopathological study of two referral institutions in England. Forensic Sci. Med. Pathol. 2024, 20, 423–433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morentin, B.; Suárez-Mier, M.P.; Aguilera, B.; Arrieta, J.; Audicana, C.; Fernández-Rodríguez, A. Clinicopathological features of sudden unexpected infectious death: Population-based study in children and young adults. Forensic Sci. Int. 2012, 220, 80–84. [Google Scholar] [CrossRef] [PubMed]
- deSa, D.J. Isolated myocarditis as a cause of sudden death in the first year of life. Forensic Sci. Int. 1986, 30, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.J.; Loewe, C.; Jackson, A. Death caused by myocarditis in Wayne County, Michigan: A 9-year retrospective study. Am. J. Forensic Med. Pathol. 2006, 27, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Fragkouli, K.; Vougiouklakis, T. Sudden cardiac death: An 11-year postmortem analysis in the region of Epirus, Greece. Pathol. Res. Pract. 2010, 206, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Tavora, F.; Li, L.; Burke, A. Sudden coronary death in children. Cardiovasc. Pathol. 2010, 19, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Yagmur, G.; Ziyade, N.; Elgormus, N.; Das, T.; Sahin, M.F.; Yildirim, M.; Ozgun, A.; Akcay, A.; Karayel, F.; Koc, S. Postmortem diagnosis of cytomegalovirus and accompanying other infection agents by real-time PCR in cases of sudden unexpected death in infancy (SUDI). J. Forensic Leg. Med. 2016, 38, 18–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Byard, R.W. Sudden Infant Death Syndrome: Definitions. In SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future; Duncan, J.R., Byard, R.W., Eds.; Chapter 1; University of Adelaide Press: Adelaide, Australia, 2018. [Google Scholar] [PubMed]
- Ilina, M.V.; Kepron, C.A.; Taylor, G.P.; Perrin, D.G.; Kantor, P.F.; Somers, G.R. Undiagnosed heart disease leading to sudden unexpected death in childhood: A retrospective study. Pediatrics 2011, 128, e513–e520. [Google Scholar] [CrossRef] [PubMed]
- Shatz, A.; Hiss, J.; Arensburg, B. Myocarditis misdiagnosed as sudden infant death syndrome (SIDS). Med. Sci. Law. 1997, 37, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Doolan, A.; Langlois, N.; Semsarian, C. Causes of sudden cardiac death in young Australians. Med. J. Aust. 2004, 180, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Råsten-Almqvist, P.; Eksborg, S.; Rajs, J. Heart weight in infants--a comparison between sudden infant death syndrome and other causes of death. Acta Paediatr. 2000, 89, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.M.; Bourne, A.J.; Clapton, W.K.; Byard, R.W. The spectrum of presentation at autopsy of myocarditis in infancy and childhood. Pathology 1992, 24, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, C.; Rambaud, C.; Cheron, G.; Rouzioux, C.; Lozinski, G.M.; Rao, A.; Stanway, G.; Krous, H.F.; Burns, J.C. Molecular identification of viruses in sudden infant death associated with myocarditis and pericarditis. Pediatr. Infect. Dis. J. 1995, 14, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.R.; Crawford, J.; Smith, W.; Aitken, A.; Heaven, D.; Evans, C.A.; Hayes, I.; Neas, K.R.; Stables, S.; Koelmeyer, T.; et al. Cardiac Inherited Disease Group New Zealand. Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Heart Rhythm. 2011, 8, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Kawai, S.; Kasyuya, H. Nonspecific myocarditis: A statistical and clinicopathological study of autopsy cases. JPN Circ. J. 1989, 53, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Okuni, M.; Yamada, T.; Mochizuki, S.; Sakurai, I. Studies on myocarditis in childhood, with special reference to the possible role of immunological process and the thymus in the chronicity of the disease. JPN Circ. J. 1975, 39, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Pucci, A.; Martino, S.; Tibaldi, M.; Bartoloni, G. Incomplete and atypical Kawasaki disease: A clinicopathologic paradox at high risk of sudden and unexpected infant death. Pediatr. Cardiol. 2012, 33, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Byard, R.W. Significant coincidental findings at autopsy in accidental childhood death. Med. Sci. Law. 1997, 37, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Özdemir-Kara, D.; Pehlivan, S.; Türkkan, D.; Alkan-Alkurt, H.; Akduman, B.; Karapirli, M. Idiopathic giant cell myocarditis in a newborn: Case report. Turk. J. Pediatr. 2016, 58, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Savage, T.R.; Smith, J.F. Polyarteritis nodosa and congenital pyloric hypertrophy in a 3-month-old infant. J. Clin. Pathol. 1960, 13, 291–296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dettmeyer, R.; Kandolf, R.; Schmidt, P.; Schlamann, M.; Madea, B. Lympho-monocytic enteroviral myocarditis: Traditional, immunohistological and molecularpathological methods for diagnosis in a case of suspected sudden infant death syndrome (SIDS). Forensic Sci. Int. 2001, 119, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Jedidi, M.; Tilouche, S.; Masmoudi, T.; Sahnoun, M.; Chkirbène, Y.; Mestiri, S.; Boughamoura, L.; Ben Dhiab, M.; Souguir, M.K. Infant acute myocarditis mimicking acute myocardial infarction. Autops. Case Rep. 2016, 6, 15–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yajima, D.; Shimizu, K.; Oka, K.; Asari, M.; Maseda, C.; Okuda, K.; Shiono, H.; Ohtani, S.; Ogawa, K. A Case of Sudden Infant Death Due to Incomplete Kawasaki Disease. J. Forensic Sci. 2016, 61 (Suppl. S1), S259–S264. [Google Scholar] [CrossRef] [PubMed]
- Puffer, P. Der plötzliche Tod aus kardialer Ursache im Kindes- und Jugendalter [Sudden death of cardiac origin in childhood and adolescence]. Beitr. Gerichtl. Med. 1990, 48, 251–254. (In German) [Google Scholar] [PubMed]
- Weber, M.A.; Ashworth, M.T.; Risdon, R.A.; Malone, M.; Burch, M.; Sebire, N.J. Clinicopathological features of paediatric deaths due to myocarditis: An autopsy series. Arch. Dis. Child. 2008, 93, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.K.; Evans, M.J. Cardiac findings in fetal and pediatric autopsies: A five-year retrospective review. Pediatr. Dev. Pathol. 2009, 12, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Partoune, B.; Malchair, R.; Boniver, J. Mort subite de l’enfant [Sudden death in children]. Rev. Med. Liege 1992, 47, 623–627. (In French) [Google Scholar] [PubMed]
- Dettmeyer, R.; Schlamann, M.; Madea, B. Myokarditisdiagnostik bei SIDS-Verdacht [Suspected SIDS and diagnosis of myocarditis]. Klin. Padiatr. 1999, 211, 456–458. (In German) [Google Scholar] [CrossRef] [PubMed]
- Brady, M.T.; Reiner, C.B.; Singley, C.; Roberts WH3rd Sneddon, J.M. Unexpected death in an infant with AIDS: Disseminated cytomegalovirus infection with pancarditis. Pediatr. Pathol. 1988, 8, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Van Reken, D.E.; Geffen, W.A.; Cramer, S.F. Clinical conference: Sudden congestive heart failure in a 5-month-old infant. J. Pediatr. 1974, 85, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.; Sagy, M.; Gonzalez, R. Reye’s syndrome associated with acute myocarditis and fatal circulatory failure. Pediatr. Emerg. Care 1991, 7, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Mounts, A.W.; Amr, S.; Jamshidi, R.; Groves, C.; Dwyer, D.; Guarner, J.; Dawson, J.E.; Oberste, M.S.; Parashar, U.; Spevak, P.; et al. A cluster of fulminant myocarditis cases in children, Baltimore, Maryland, 1997. Pediatr. Cardiol. 2001, 22, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Sudden Unexplained Infant Death Investigation Reporting Form (SUIDIRF); CDC: Atlanta, GA, USA, 2006. Available online: https://stacks.cdc.gov/view/cdc/25614 (accessed on 3 March 2025).
- Giordani, A.S.; Baritussio, A.; Vicenzetto, C.; Peloso-Cattini, M.G.; Pontara, E.; Bison, E.; Fraccaro, C.; Basso, C.; Iliceto, S.; Marcolongo, R.; et al. Fulminant Myocarditis: When One Size Does Not Fit All—A Critical Review of the Literature. Eur. Cardiol. 2023, 18, e15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, F.; Ammirati, E.; Ponnaiah, M.; Montero, S.; Raimbault, V.; Abrams, D.; Lebreton, G.; Pellegrino, V.; Ihle, J.; Bottiroli, M.; et al. Fulminant myocarditis proven by early biopsy outcomes. Eur. Heart J. 2023, 44, 5110–5124, Erratum in: Eur. Heart J. 2024, 45, 375. https://doi.org/10.1093/eurheartj/ehad813. [Google Scholar] [CrossRef] [PubMed]

| Author(s) | Study Description | Additional Notes | Study Type |
|---|---|---|---|
| Dettmeyer R. [47] | Incidence of lethal lymphocytic myocarditis in neonates and children. | Study highlighting the pathological findings of lymphocytic myocarditis leading to sudden death in pediatric autopsies. | Original research |
| Vassalini M et al. [71] | Sudden cardiac death in individuals aged 1–40 in Brescia. | Epidemiological study of sudden cardiac death in young individuals; emphasizes need for autopsy and histological analysis. | Original research |
| Dauger S et al. [72] | Sudden death in an infant with Varicella Zoster infection. | Case of viral myocarditis leading to sudden death; emphasizes the importance of viral screening postmortem. | Original research |
| Ondruschka B et al. [73] | CPR-related injuries in neonates and children. | Analysis of rib and sternal fractures caused by CPR in infants and their implications in forensic evaluation. | Original research |
| Kon FC et al. [74] | Cardiovascular deaths in childhood and adolescence. | Comprehensive review of cardiovascular causes of death in young patients, with emphasis on undiagnosed heart conditions. | Original research |
| Morentin B et al. [75] | Sudden unexpected infectious deaths in children and young adults. | Evaluation of infectious diseases causing sudden death; key findings from autopsy and histopathology. | Original research |
| deSa DJ. [76] | Three cases of isolated myocarditis in infants. | Three clinical and autopsy cases presenting isolated myocarditis as a cause of death in otherwise healthy infants. | Case report/case series |
| Diaz FJ, Loewe C, Jackson A. [77] | Analysis of 72 myocarditis cases across all age groups. | A multiage study analyzing clinical and histological findings across 72 myocarditis cases. | Original research |
| Fragkouli K, Vougiouklakis T. [78] | 11 years of autopsies for sudden cardiac death in Greece. | Review of forensic autopsies over 11 years focused on identifying causes of pediatric cardiac arrest. | Original research |
| Tavora F, Li L, Burke A. [79] | Coronary anomalies as cause of sudden death in children. | Autopsy findings of coronary artery anomalies in children linked to exercise-induced sudden death. | Original research |
| Yagmur G et al. [80] | Real-time PCR for postmortem diagnosis of infections in infants. | Use of molecular biology tools (RT-PCR) in postmortem detection of pathogens associated with sudden death. | Original research |
| Grimaldi F et al. [35] | Case of fatal infantile myocarditis. | Report of an infant death due to rapidly progressive myocarditis confirmed on histology. | Original research |
| Ilina MV et al. [82] | Undiagnosed heart diseases and sudden death in children. | Retrospective review of missed congenital and acquired cardiac diseases in sudden death cases. | Original research |
| Shatz A, Hiss J, Arensburg B. [83] | Myocarditis misdiagnosed as SIDS. | Postmortem reevaluation showing misdiagnosis of myocarditis as SIDS; implications for cause of death certification. | Original research |
| Doolan A, Langlois N, Semsarian C. [84] | Sudden cardiac death in young Australians. | National study analyzing causes and genetic risks in youth cardiac arrest; promotes screening in schools. | Original research |
| Råsten-Almqvist P et al. [85] | Heart weight in SIDS and other causes of death. | Comparison of heart weights in SIDS and non-SIDS cases; questions overdiagnosis based on organ size. | Original research |
| Smith NM et al. [86] | 32 cases of myocarditis in childhood and adolescence. | Series emphasizing myocarditis patterns across age groups, including cellular infiltrates and viral detection. | Original research |
| Shimizu C et al. [87] | Viruses in SIDS cases with myocarditis and pericarditis. | Demonstrates viral genome presence in myocarditis/pericarditis-related infant deaths. | Original research |
| Skinner JR et al. [88] | Molecular autopsy for long QT gene. | Genetic autopsy identifying mutations linked to long QT syndrome in cases of sudden unexplained death. | Original research |
| Okada R, Kawai S, Kasyuya H. [89] | Autopsy cases of nonspecific myocarditis. | Series of autopsy cases without definitive etiology; suggests need for standardized myocarditis criteria. | Original research |
| Okuni M et al. [90] | Thymus role in chronic pediatric myocarditis. | Histological and clinical correlation of thymic alterations with chronic myocarditis course in children. | Original research |
| Grasmeyer S, Madea B. [37] | Immunohistochemistry in myocarditis diagnosis from autopsy tissue. | Utility of immunohistochemistry markers (CD3, CD68) for confirming myocarditis in autopsy samples. | Original research |
| Pucci A et al. [91] | Incomplete Kawasaki disease and sudden death in infants. | Case linking incomplete Kawasaki syndrome to fatal coronary arteritis in infants. | Original research |
| Byard RW. [92] | Coincidental findings in infant accidental death autopsies. | Highlights accidental findings that can mimic signs of abuse in infant autopsies. | Original research |
| Özdemir-Kara D et al. [93] | Case of giant cell myocarditis in a neonate. | Unique case of giant cell myocarditis in neonate; underscores importance of early diagnosis and biopsy. | Original research |
| Savage TR, Smith JF. [94] | Polyarteritis nodosa and congenital pyloric hypertrophy in an infant. | Combined occurrence of autoimmune vasculitis and gastrointestinal anomaly in a fatal infant case. | Original research |
| Dettmeyer R et al. [95] | Enteroviral myocarditis diagnosis in SIDS. | Evidence of enteroviral infection in SIDS case with myocardial inflammation confirmed by PCR. | Original research |
| Jedidi M et al. [96] | Acute myocarditis mimicking myocardial infarction in a neonate. | Neonatal myocarditis clinically mimicking myocardial infarction; confirmed via autopsy. | Original research |
| Yajima D et al. [97] | Sudden death in an infant due to incomplete Kawasaki disease. | Infant death due to vasculitis-like Kawasaki pathology with cardiac involvement. | Original research |
| Puffer P. [98] | Sudden cardiac death in childhood and adolescence. | Autopsy-based review showing arrhythmogenic mechanisms in pediatric sudden death. | Original research |
| Weber MA et al. [99] | Pediatric myocarditis in autopsy series. | Survey of myocarditis prevalence and features in forensic autopsies of children. | Original research |
| Grant EK, Evans MJ. [100] | Cardiac findings in fetal and pediatric autopsies. | Findings from congenital and acquired cardiac malformations in perinatal and pediatric autopsies. | Original research |
| Partoune B et al. [101] | Analysis of causes of sudden death in children. | Broad overview of sudden death causes in children across a multicenter autopsy dataset. | Original research |
| Dettmeyer R et al. [102] | Myocarditis in suspected SIDS cases. | Postmortem myocardial analysis in suspected SIDS cases reveals inflammatory changes. | Original research |
| Brady MT et al. [103] | Neonatal death with AIDS and CMV pancarditis. | AIDS-related neonatal death associated with CMV pancarditis; confirmed immunohistochemically. | Original research |
| Van Reken DE et al. [104] | Congestive heart failure in infant with echovirus infection. | Case of fatal viral myocarditis due to echovirus with signs of cardiac failure in infant. | Original research |
| Lajoie J et al. [105] | Reye’s syndrome with acute myocarditis in an infant. | Report of Reye’s syndrome associated with myocardial involvement in autopsied infant. | Original research |
| Author(s) | Study Description | Additional Notes | Study Type |
|---|---|---|---|
| Bakker AM et al. [70] | Diagnostic protocol for sudden cardiac arrest in children. | Proposed standardized diagnostic workflow for evaluating sudden cardiac arrest in pediatric emergency settings. | Review/methodological |
| Byard RW. [81] | Definitions of sudden infant death syndrome (SIDS). | Discussion of evolving definitions and diagnostic challenges in SIDS classification and reporting. | Review/methodological |
| Rizzo S et al. [41] | Postmortem protocol for sudden infant death syndrome (SIDS). | Proposal of a thorough postmortem protocol integrating molecular and histological diagnostics in SIDS. | Review/methodological |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, M.A.; Gualtieri, S.; Verrina, M.C.; Aquila, V.R.; Tarda, L.; Tarallo, A.P.; Aquila, I. A Narrative Overview of Fatal Myocarditis in Infant with Focus on Sudden Unexpected Death and Forensic Implications. J. Clin. Med. 2025, 14, 4340. https://doi.org/10.3390/jcm14124340
Sacco MA, Gualtieri S, Verrina MC, Aquila VR, Tarda L, Tarallo AP, Aquila I. A Narrative Overview of Fatal Myocarditis in Infant with Focus on Sudden Unexpected Death and Forensic Implications. Journal of Clinical Medicine. 2025; 14(12):4340. https://doi.org/10.3390/jcm14124340
Chicago/Turabian StyleSacco, Matteo Antonio, Saverio Gualtieri, Maria Cristina Verrina, Valerio Riccardo Aquila, Lucia Tarda, Alessandro Pasquale Tarallo, and Isabella Aquila. 2025. "A Narrative Overview of Fatal Myocarditis in Infant with Focus on Sudden Unexpected Death and Forensic Implications" Journal of Clinical Medicine 14, no. 12: 4340. https://doi.org/10.3390/jcm14124340
APA StyleSacco, M. A., Gualtieri, S., Verrina, M. C., Aquila, V. R., Tarda, L., Tarallo, A. P., & Aquila, I. (2025). A Narrative Overview of Fatal Myocarditis in Infant with Focus on Sudden Unexpected Death and Forensic Implications. Journal of Clinical Medicine, 14(12), 4340. https://doi.org/10.3390/jcm14124340







