Radiomics Models to Predict Tumor Response and Pneumonitis in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy
Abstract
1. Introduction
2. Methods
2.1. Study Cohort
2.2. Clinical Variables of Interest
3. Radiomics Features
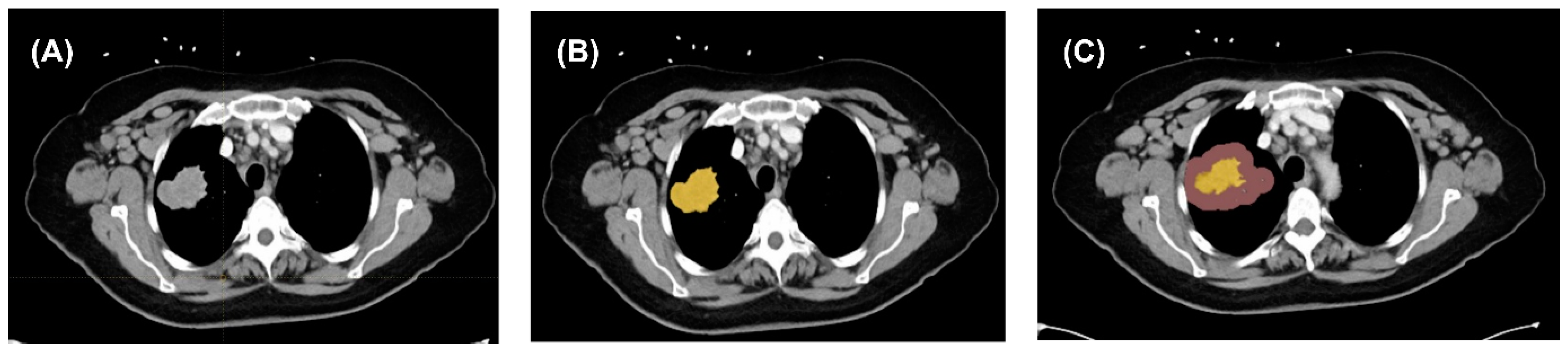
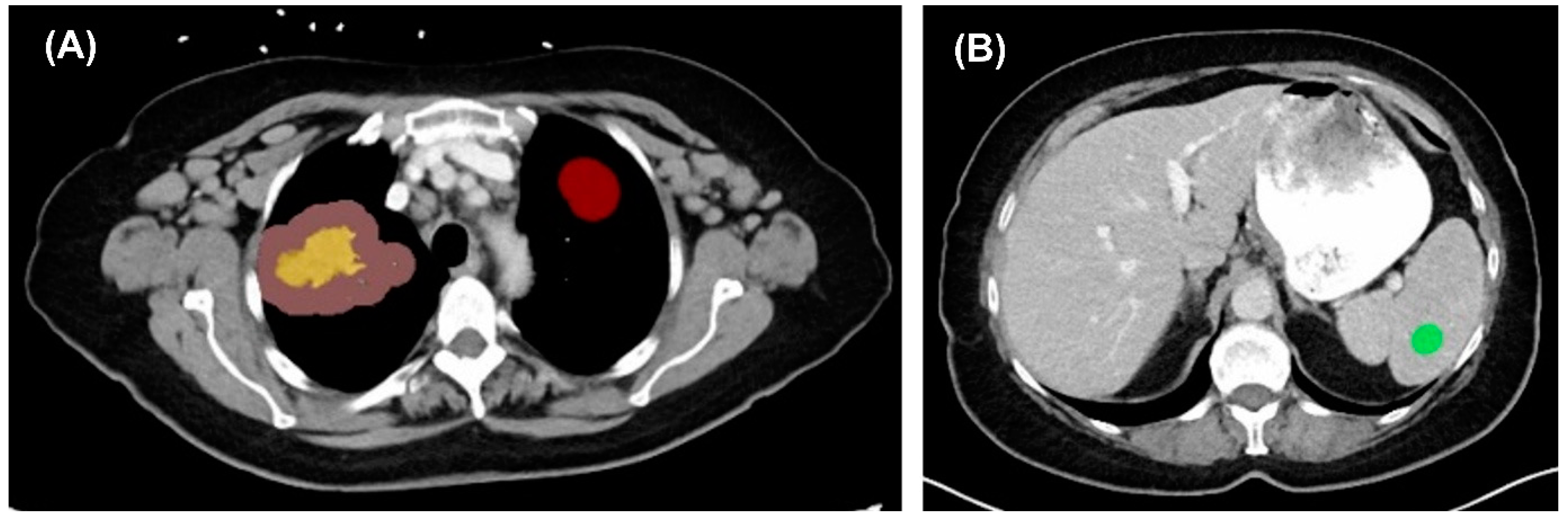
3.1. Image Extraction
3.2. Image Segmentation and Feature Extraction
3.3. Prediction Model Development
4. Outcomes of Interest
5. Statistical Analysis
6. Results
6.1. Study Population and Characteristics
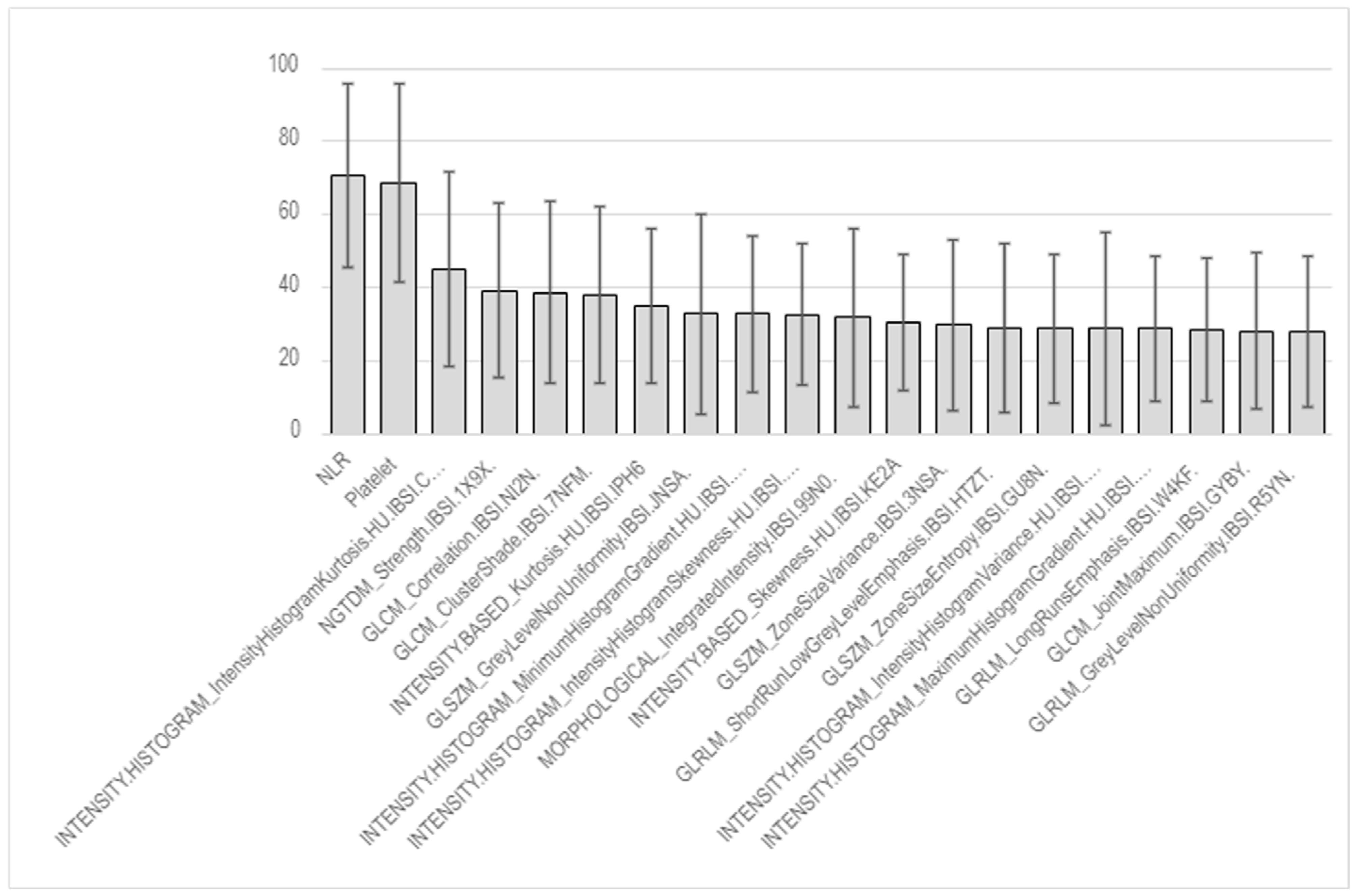
6.2. Pneumonitis Prediction via Radiomics Analysis
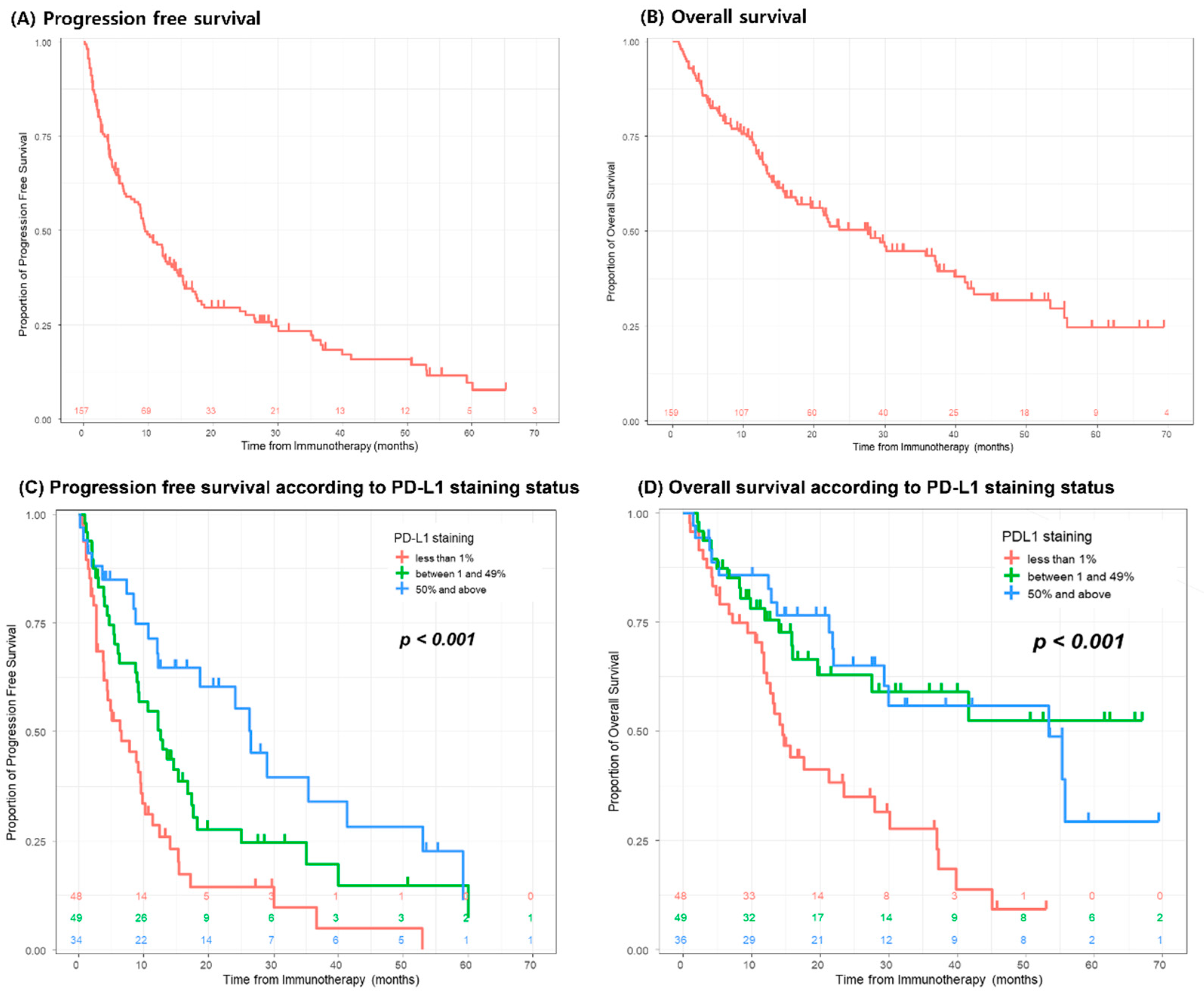
7. Clinical Outcomes
8. Responses to Immunotherapy
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Lung and Bronchus-Cancer Stat Facts. In: SEER. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 28 May 2024).
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Friedes, C.; Mai, N.; Hazell, S.; Fu, W.; Han, P.; Bowers, M.; Levy, B.; Forde, P.M.; Voong, R.; Hales, R.K. Consolidative Radiotherapy in Oligometastatic Lung Cancer: Patient Selection With a Prediction Nomogram. Clin. Lung Cancer 2020, 21, e622–e632. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Sun, J.; Fang, J.; Zhang, M.; Liu, H.; Xia, R.; Zhou, W.; Liu, K.; Chen, X. A Machine Learning Model Based on PET/CT Radiomics and Clinical Characteristics Predicts Tumor Immune Profiles in Non-Small Cell Lung Cancer: A Retrospective Multicohort Study. Front. Immunol. 2022, 13, 859323. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Wu, T.; Kong, S.; Lou, X.; Jiang, Y.; Tan, Z.; Wu, L.; Gao, C. Could the underlying biological basis of prognostic radiomics and deep learning signatures be explored in patients with lung cancer? A systematic review. Eur. J. Radiol. 2024, 171, 111314. [Google Scholar] [CrossRef] [PubMed]
- Trebeschi, S.; Drago, S.G.; Birkbak, N.J.; Kurilova, I.; Cǎlin, A.M.; Delli Pizzi, A.; Lalezari, F.; Lambregts, D.M.J.; Rohaan, M.W.; Parmar, C.; et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann. Oncol. 2019, 30, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- LIFEx-LIFEx. Available online: https://www.lifexsoft.org/ (accessed on 28 May 2024).
- Soliman, M.A.S.; Kelahan, L.C.; Magnetta, M.; Savas, H.; Agrawal, R.; Avery, R.J.; Aouad, P.; Liu, B.; Xue, Y.; Chae, Y.K.; et al. A Framework for Harmonization of Radiomics Data for Multicenter Studies and Clinical Trials. JCO Clin. Cancer Inform. 2022, 6, e2200023. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.P.; Li, M.Y.; Zhao, M.M.; Yu, M.J.; Teng, M.F. Distinguishing immune checkpoint inhibitor-related pneumonitis from radiation pneumonitis by CT radiomics features in non-small cell lung cancer. Int. Immunopharmacol. 2024, 128, 111489. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Naidoo, J.; Zhong, Q.; Xiong, Y.; Mammen, J.; de Flores, M.V.; Cappelli, L.; Balaji, A.; Palmer, T.; Forde, P.M.; et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J. Clin. Investig. 2019, 129, 4305–4315. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Cottrell, T.R.; Lipson, E.J.; Forde, P.M.; Illei, P.B.; Yarmus, L.B.; Voong, K.R.; Feller-Kopman, D.; Lee, H.; Riemer, J.; et al. Chronic immune checkpoint inhibitor pneumonitis. J. Immunother. Cancer 2020, 8, e000840. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, Z.; Tian, P.; Li, W. Safety and tolerability of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer: A meta-analysis of randomized controlled trials. J. Cancer Res. Clin. Oncol. 2018, 144, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, F.; Wang, Y.; Du, Z. Establishment and validation of nomogram for predicting immuno checkpoint inhibitor related pneumonia. BMC Pulm. Med. 2022, 22, 331. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sheikh, K.; Nakajima, E.; Lin, C.T.; Lee, J.; Hu, C.; Hales, R.K.; Forde, P.M.; Naidoo, J.; Voong, K.R. Radiation Versus Immune Checkpoint Inhibitor Associated Pneumonitis: Distinct Radiologic Morphologies. Oncologist 2021, 26, e1822–e1832. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, J.; Zhou, L.; Xia, W.; Zhang, R.; Wei, H.; Feng, J.; Zhao, X.; Jian, J.; Gao, X.; et al. CT-based radiomics signatures can predict the tumor response of non-small cell lung cancer patients treated with first-line chemotherapy and targeted therapy. Eur. Radiol. 2022, 32, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Xu, W.; Su, R.; Ran, P.; Liu, L.; Zhang, Z.; Zhao, J.; Chao, Z.; Fu, G. Clinical applications of radiomics in non-small cell lung cancer patients with immune checkpoint inhibitor-related pneumonitis. Front. Immunol. 2023, 14, 1251645. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, G.H.; Kim, K.W.; Lee, C.W.; Yoon, S.; Chae, Y.K.; Tirumani, S.H.; Ramaiya, N.H. Comparison of RECIST 1.1 and iRECIST in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 120. [Google Scholar] [CrossRef] [PubMed]
| Clinical Variables | Overall | Pneumonitis | |||
|---|---|---|---|---|---|
| No | Yes | p-Value | |||
| N = 159 | N = 128 | N = 31 | |||
| Age, years | 66.0 [59.0, 74.0] | 65.5 [59.0, 74.0] | 68.0 [61.0, 74.5] | 0.303 | |
| Body mass index, kg/m2 | 25.6 [22.3, 30.3] | 25.2 [22.0, 30.5] | 27.2 [23.1, 29.9] | 0.435 | |
| Sex | Female | 90 (56.6) | 79 (61.7) | 11 (35.5) | 0.014 |
| Male | 69 (43.4) | 49 (38.3) | 20 (64.5) | ||
| Smoking history | Never smoked | 33 (20.8) | 29 (22.7) | 4 (12.9) | 0.428 |
| Former smoker | 111 (69.8) | 88 (68.8) | 23 (74.2) | ||
| Current smoker | 15 (9.4) | 11 (8.6) | 4 (12.9) | ||
| ECOG status | ECOG 0 | 81 (50.9) | 67 (52.3) | 14 (45.2) | 0.476 |
| ECOG 1 | 58 (36.5) | 44 (34.4) | 14 (45.2) | ||
| ECOG 2 | 17 (10.7) | 15 (11.7) | 2 (6.5) | ||
| ECOG 3 | 3 (1.9) | 2 (1.6) | 1 (3.2) | ||
| Histology | Adenocarcinoma | 118 (74.2) | 101 (78.9) | 17 (54.8) | 0.032 |
| Squamous cell | 29 (18.2) | 18 (14.1) | 11 (35.5) | ||
| Adenosquamous | 2 (1.3) | 2 (1.6) | 0 (0.0) | ||
| Large cell | 1 (0.6) | 1 (0.8) | 0 (0.0) | ||
| Others | 9 (5.7) | 6 (4.7) | 3 (9.7) | ||
| Staging, 8th TNM | III | 14 (8.8) | 10 (7.8) | 4 (12.9) | 0.477 |
| IV | 145 (91.2) | 118 (92.2) | 27 (87.1) | ||
| PD-L1—tumor staining | <1% | 48 (36.1) | 39 (37.1) | 9 (32.1) | 0.531 |
| 1–49% | 49 (36.8) | 36 (34.3) | 13 (46.4) | ||
| ≥50% | 36 (27.1) | 30 (28.6) | 6 (21.4) | ||
| Baseline laboratory findings | |||||
| Platelet counts | 280 [220, 351] | 288 [221, 353] | 263 [200, 343] | 0.242 | |
| Lymphocyte proportion, % | 16.0 [10.3, 23.5] | 17.0 [11.0, 24.3] | 13.2 [9.0, 20.0] | 0.086 | |
| Neutrophil proportion, % | 71.0 [63.0, 79.0] | 70.0 [61.8, 78.0] | 71.0 [64.0, 81.0] | 0.486 | |
| NLR | 4.41 [2.67, 7.41] | 4.33 [2.54, 6.75] | 5.26 [3.07, 9.06] | 0.142 | |
| Treatment | ICI only | 96 (60.4) | 77 (60.2) | 19 (61.3) | 1 |
| ICI with CTx | 63 (39.6) | 51 (39.8) | 12 (38.7) | ||
| ICI Regimens | Pembrolizumab | 86 (54.1) | 71 (55.5) | 15 (48.4) | 0.267 |
| Durvalumab | 10 (6.3) | 5 (3.9) | 5 (16.1) | ||
| Nivolumab only | 24 (15.1) | 20 (15.6) | 4 (12.9) | ||
| Nivolumab/ipilimumab | 14 (8.8) | 10 (7.8) | 4 (12.9) | ||
| Atezolizumab | 22 (13.8) | 19 (14.8) | 3 (9.7) | ||
| Ramucirumab | 2 (1.3) | 2 (1.6) | 0 (0.0) | ||
| Cemiplimab | 1 (0.6) | 1 (0.8) | 0 (0.0) | ||
| TMB, mutations/Mb | 8.0 [3.8, 11.7] | 6.7 [3.2, 9.8] | 10.8 [6.4, 19.7] | 0.059 | |
| TMB <10 | 43 (68.3) | 37 (75.5) | 6 (42.9) | 0.047 | |
| TMB ≥ 10 | 20 (31.7) | 12 (24.5) | 8 (57.1) | ||
| Microsatellite instability | Negative | 67 (84.8) | 54 (85.7) | 13 (81.2) | 0.701 |
| Positive | 12 (15.2) | 9 (14.3) | 3 (18.8) | ||
| Veristrat | 1. Good | 38 (66.7) | 30 (65.2) | 8 (72.7) | 0.735 |
| 2. Poor | 19 (33.3) | 16 (34.8) | 3 (27.3) | ||
| Identified gene mutations | |||||
| ALK | 11/133 (8.3) | 8/108 (7.4) | 3/25 (12.0) | 0.432 | |
| ARID1A | 15/154 (9.7) | 14/123 (11.4) | 1/31 (3.2) | 0.307 | |
| ATM | 16/154 (10.4) | 14/123 (11.4) | 2/31 (6.5) | 0.529 | |
| BRAF | 21/104 (20.2) | 19/84 (22.6) | 2/20 (10.0) | 0.352 | |
| BRCA1 | 7/154 (4.5) | 3/123 (2.4) | 4/31 (12.9) | 0.031 | |
| BRCA2 | 10/154 (6.5) | 7/123 (5.7) | 3/31 (9.7) | 0.422 | |
| CDKN2A | 21/142 (14.8) | 17/113 (15.0) | 4/29 (13.8) | 1 | |
| CDKN2B | 8/154 (5.2) | 7/123 (5.7) | 1/31 (3.2) | 1 | |
| EGFR | 33/152 (21.7) | 25/122 (20.5) | 8/30 (26.7) | 0.465 | |
| ERBB2 | 9/157 (5.7) | 9/126 (7.1) | 0/31 (0.0) | 0.207 | |
| KRAS | 44/150 (29.3) | 41/121 (33.9) | 3/29 (10.3) | 0.012 | |
| MET | 14/158 (8.9) | 10/127 (7.9) | 4/31 (12.9) | 0.478 | |
| NF1 | 13/155 (8.4) | 11/124 (8.9) | 2/31 (6.5) | 1 | |
| NOTCH1 | 11/157 (7.0) | 7/126 (5.6) | 4/31 (12.9) | 0.229 | |
| PIK3CA | 17/158 (10.8) | 12/127 (9.4) | 5/31 (16.1) | 0.331 | |
| PTEN | 10/158 (6.3) | 7/127 (5.5) | 3/31 (9.7) | 0.413 | |
| RB1 | 7/154 (4.5) | 4/123 (3.3) | 3/31 (9.7) | 0.146 | |
| ROS1 | 10/108 (9.3) | 8/86 (9.3) | 2/22 (9.1) | 1 | |
| STK11 | 13/158 (8.2) | 13/127 (10.2) | 0/31 (0.0) | 0.074 | |
| TP53 | 91/125 (72.8) | 72/99 (72.7) | 19/26 (73.1) | 1 | |
| TTF1 | 85/153 (55.6) | 70/123 (56.9) | 15/30 (50.0) | 0.542 | |
| Follow-up duration, months | 14.5 [6.9, 30.1] | 14.9 [7.4, 30.2] | 12.7 [5.9, 28.9] | 0.419 | |
| Mortality | 87/159 (54.7) | 68/128 (53.1) | 19/31 (61.3) | 0.432 | |
| Tumor response by irRECIST | CR | 1 (0.6) | 1 (0.8) | 0 (0.0) | 1 |
| PD | 42 (26.4) | 34 (26.6) | 8 (25.8) | ||
| PR | 51 (32.1) | 41 (32.0) | 10 (32.3) | ||
| SD | 65 (40.9) | 52 (40.6) | 13 (41.9) | ||
| Tumor response by RECIST1.1 | CR | 1 (0.6) | 1 (0.8) | 0 (0.0) | 0.653 |
| PD | 56 (35.2) | 47 (36.7) | 9 (29.0) | ||
| PR | 40 (25.2) | 30 (23.4) | 10 (32.3) | ||
| SD | 62 (39.0) | 50 (39.1) | 12 (38.7) | ||
| Clinical Variables | Number of Patients | |
|---|---|---|
| Severity by CTCAE V5 | ||
| Grade 1 | 17/31 (54.8) | |
| Grade 2 | 12/31 (38.7) | |
| Grade 3 | 2/31 (6.5) | |
| Types by causes | ||
| ICI-related | 18/29 (62.1) | |
| Mixed | 1/29 (3.4) | |
| Radiation-related | 10/29 (34.5) |
| Clinical Variables | Overall | Tumor Response | |||
|---|---|---|---|---|---|
| Durable Responder | Non-Responder | p-Value | |||
| N = 159 | N = 117 | N = 42 | |||
| Age, years | 66.0 [59.0, 74.0] | 67.0 [59.0, 76.0] | 64.5 [59.0, 71.7] | 0.174 | |
| Body mass index, kg/m2 | 25.6 [22.3, 30.3] | 25.3 [22.0, 30.4] | 25.8 [22.9, 29.8] | 0.467 | |
| Sex | Female | 90 (56.6) | 66 (56.4) | 24 (57.1) | 1 |
| Male | 69 (43.4) | 51 (43.6) | 18 (42.9) | ||
| Smoking history | Never smoked | 33 (20.8) | 23 (19.7) | 10 (23.8) | 0.808 |
| Former smoker | 111 (69.8) | 82 (70.1) | 29 (69.0) | ||
| Current smoker | 15 (9.4) | 12 (10.3) | 3 (7.1) | ||
| ECOG status | ECOG 0 | 81 (50.9) | 55 (47.0) | 26 (61.9) | 0.086 |
| ECOG 1 | 58 (36.5) | 49 (41.9) | 9 (21.4) | ||
| ECOG 2 | 17 (10.7) | 11 (9.4) | 6 (14.3) | ||
| ECOG 3 | 3 (1.9) | 2 (1.7) | 1 (2.4) | ||
| Histology | Adenocarcinoma | 118 (74.2) | 86 (73.5) | 32 (76.2) | 0.722 |
| Squamous cell | 29 (18.2) | 23 (19.7) | 6 (14.3) | ||
| Adenosquamous | 2 (1.3) | 1 (0.9) | 1 (2.4) | ||
| Large cell | 1 (0.6) | 1 (0.9) | 0 (0.0) | ||
| Others | 9 (5.7) | 6 (5.1) | 3 (7.1) | ||
| Staging, 8th TNM | III | 14 (8.8) | 12 (10.3) | 2 (4.8) | 0.358 |
| IV | 145 (91.2) | 105 (89.7) | 40 (95.2) | ||
| PD-L1—Tumor staining | <1% | 48/133 (36.1) | 31/100 (31.0) | 17/33 (51.5) | 0.108 |
| 1–49% | 49/133 (36.8) | 39/100 (39.0) | 10/33 (30.3) | ||
| ≥50% | 36/133 (27.1) | 30/100 (30.0) | 6/33 (18.2) | ||
| Baseline laboratory findings | |||||
| Platelet counts | 280 [220, 351] | 289 [222, 350] | 267 [210, 364] | 0.546 | |
| Lymphocyte proportion, % | 16.0 [10.3, 23.5] | 16.0 [10.6, 24.0] | 17.0 [10.3, 23.0] | 0.778 | |
| Neutrophil proportion, % | 71.0 [63.0, 79.0] | 70.0 [63.0, 79.0] | 71.0 [63.2, 75.8] | 0.973 | |
| NLR | 4.41 [2.67, 7.41] | 4.39 [2.67, 7.36] | 4.49 [2.79, 7.98] | 0.862 | |
| Treatment | ICI only | 96 (60.4) | 66 (56.4) | 30 (71.4) | 0.132 |
| ICI with CTx | 63 (39.6) | 51 (43.6) | 12 (28.6) | ||
| ICI regimens | Pembrolizumab | 86 (54.1) | 65 (55.6) | 21 (50.0) | 0.222 |
| Durvalumab | 10 (6.3) | 10 (8.5) | 0 (0.0) | ||
| Nivolumab only | 24 (15.1) | 16 (13.7) | 8 (19.0) | ||
| Nivolumab/ipilimumab | 14 (8.8) | 10 (8.5) | 4 (9.5) | ||
| Atezolizumab | 22 (13.8) | 13 (11.1) | 9 (21.4) | ||
| Ramucirumab | 2 (1.3) | 2 (1.7) | 0 (0.0) | ||
| Cemiplimab | 1 (0.6) | 1 (0.9) | 0 (0.0) | ||
| TMB, bp/Mb | 8.00 [3.75, 11.72] | 9.15 [4.15, 16.45] | 4.20 [2.11, 7.30] | 0.003 | |
| TMB <10 | 43/63 (68.3) | 28/48 (58.3) | 15/15 (100.0) | 0.001 | |
| TMB ≥ 10 | 20/63 (31.7) | 20/48 (41.7) | 0/15 (0.0) | ||
| Microsatellite instability | Negative | 67 (84.8) | 50 (82.0) | 17 (94.4) | 0.278 |
| Positive | 12 (15.2) | 11 (18.0) | 1 (5.6) | ||
| Veristrat | (1) Good | 38 (66.7) | 31 (64.6) | 7 (77.8) | 0.703 |
| (2) Poor | 19 (33.3) | 17 (35.4) | 2 (22.2) | ||
| Identified gene mutations | |||||
| ALK | 11/133 (8.3) | 8/101 (7.9) | 3/32 (9.4) | 0.725 | |
| ARID1A | 15/154 (9.7) | 10/112 (8.9) | 5/42 (11.9) | 0.555 | |
| ATM | 16/154 (10.4) | 11/112 (9.8) | 5/42 (11.9) | 0.768 | |
| BRAF | 21/104 (20.2) | 14/84 (16.7) | 7/20 (35.0) | 0.117 | |
| BRCA1 | 7/154 (4.5) | 5/112 (4.5) | 2/42 (4.8) | 1 | |
| BRCA2 | 10/154 (6.5) | 7/112 (6.2) | 3/42 (7.1) | 1 | |
| CDKN2A | 21/142 (14.8) | 14/104 (13.5) | 7/38 (18.4) | 0.438 | |
| CDKN2B | 8/154 (5.2) | 5/112 (4.5) | 3/42 (7.1) | 0.684 | |
| EGFR | 33/152 (21.7) | 26/115 (22.6) | 7/37 (18.9) | 0.819 | |
| ERBB2 | 9/157 (5.7) | 7/116 (6.0) | 2/41 (4.9) | 1 | |
| KRAS | 44/150 (29.3) | 32/113 (28.3) | 12/37 (32.4) | 0.679 | |
| MET | 14/158 (8.9) | 9/116 (7.8) | 5/42 (11.9) | 0.526 | |
| NF1 | 13/155 (8.4) | 11/113 (9.7) | 2/42 (4.8) | 0.516 | |
| NOTCH1 | 11/157 (7.0) | 9/115 (7.8) | 2/42 (4.8) | 0.728 | |
| PIK3CA | 17/158 (10.8) | 11/116 (9.5) | 6/42 (14.3) | 0.393 | |
| PTEN | 10/158 (6.3) | 8/116 (6.9) | 2/42 (4.8) | 1 | |
| RB1 | 7/154 (4.5) | 6/112 (5.4) | 1/42 (2.4) | 0.675 | |
| ROS1 | 10/108 (9.3) | 7/85 (8.2) | 3/23 (13.0) | 0.441 | |
| STK11 | 13/158 (8.2) | 8/116 (6.9) | 5/42 (11.9) | 0.333 | |
| TP53 | 91/125 (72.8) | 70/96 (72.9) | 21/29 (72.4) | 1 | |
| TTF1 | 85/153 (55.6) | 63/112 (56.2) | 22/41 (53.7) | 0.855 | |
| Follow-up duration, months | 14.5 [6.9, 30.1] | 16.8 [9.4, 36.6] | 10.7 [4.3, 16.5] | 0.001 | |
| Mortality | 87/159 (54.7) | 55/117 (47.0) | 32/42 (76.2) | 0.432 | |
| Pneumonitis | Yes | 31/159 (19.5) | 23/117 (19.7) | 8/42 (19.0) | 1 |
| Grade 1 | 17/31 (54.8) | 12/23 (52.2) | 5/8 (62.5) | 0.760 | |
| Grade 2 | 12/31 (38.7) | 10/23 (43.5) | 2/8 (25.0) | ||
| Grade 3 | 2/31 (6.5) | 1/23 (4.3) | 1/8 (12.5) | ||
| Pneumonitis | Tumor Response by irRECIST | Tumor Response by RECIST 1.1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | ICI | RTx | PD | SD | PR + CR | PD | SD | PR + CR | |
| AUC | 0.60 (0.55–0.66) | 0.59 (0.56–0.66) | 0.53 (0.46–0.80) | 0.63 (0.59–0.67) | 0.66 (0.61–0.70) | ||||
| Sensitivity | 0.97 (0.95–0.98) | 0.98 (0.97–0.99) | 1.00 (0.99–1.00) | 0.49 (0.45–0.56) | 0.64 (0.60–0.68) | 0.45 (0.38–0.50) | 0.57 (0.52–0.62) | 0.55 (0.52–0.60) | 0.34 (0.28–0.38) |
| Specificity | 0.08 (0.05–0.14) | 0.06 (0.03–0.11) | 0.04 (0.00–0.07) | 0.82 (0.79–0.85) | 0.63 (0.59–0.67) | 0.85 (0.83–0.87) | 0.65 (0.63–0.69) | 0.67 (0.63–0.72) | 0.91 (0.89–0.93) |
| PPV | 0.83 (0.82–0.83) | 0.91 (0.90–0.91) | 0.93 (0.92–0.93) | 0.56 (0.51–0.61) | 0.54 (0.50–0.57) | 0.53 (0.49–0.59) | 0.52 (0.48–0.54) | 0.51 (0.48–0.55) | 0.51 (0.46–0.56) |
| NPV | 0.38 (0.29–0.50) | 0.27 (0.21–0.40) | 0.14 (0.00–0.67) | 0.78 (0.76–0.80) | 0.72 (0.70–0.75) | 0.79 (0.77–0.81) | 0.71 (0.68–0.73) | 0.71 (0.69–0.73) | 0.82 (0.81–0.83) |
| Balanced Accuracy | 0.52 (0.51–0.55) | 0.53 (0.51–0.54) | 0.51 (0.50–0.53) | 0.65 (0.63–0.69) | 0.64 (0.61–0.67) | 0.65 (0.61–0.68) | 0.62 (0.59–0.64) | 0.61 (0.59–0.64) | 0.62 (0.59–0.64) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, M.; Woo, W.; Chae, Y.K.; Lee, J.; Kim, P.H.; Lee, S.; Um, T.; Lee, S.; Chuchuca, M.J.A.; Djunadi, T.A.; et al. Radiomics Models to Predict Tumor Response and Pneumonitis in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy. J. Clin. Med. 2025, 14, 4330. https://doi.org/10.3390/jcm14124330
Yadav M, Woo W, Chae YK, Lee J, Kim PH, Lee S, Um T, Lee S, Chuchuca MJA, Djunadi TA, et al. Radiomics Models to Predict Tumor Response and Pneumonitis in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy. Journal of Clinical Medicine. 2025; 14(12):4330. https://doi.org/10.3390/jcm14124330
Chicago/Turabian StyleYadav, Monica, Wongi Woo, Young Kwang Chae, Jeeyeon Lee, Peter Haseok Kim, Seyoung Lee, Taegyu Um, Salie Lee, Maria Jose Aguilera Chuchuca, Trie Arni Djunadi, and et al. 2025. "Radiomics Models to Predict Tumor Response and Pneumonitis in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy" Journal of Clinical Medicine 14, no. 12: 4330. https://doi.org/10.3390/jcm14124330
APA StyleYadav, M., Woo, W., Chae, Y. K., Lee, J., Kim, P. H., Lee, S., Um, T., Lee, S., Chuchuca, M. J. A., Djunadi, T. A., Chung, L. I.-Y., Yu, J., Gennaro, N., Kim, L., Nam, M., Oh, Y., Yoon, S., Shah, Z., Kim, Y., ... Velichko, Y. S. (2025). Radiomics Models to Predict Tumor Response and Pneumonitis in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy. Journal of Clinical Medicine, 14(12), 4330. https://doi.org/10.3390/jcm14124330






