Predicting Favorable Conditions for the Determination of Initial Use of Janus Kinase Inhibitors in Patients with Moderate to Severe Atopic Dermatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Inclusion Criteria
2.2. Patient Categorization
2.3. Demographic and Laboratory Data Collection
2.4. Classification Criteria for Predictive Value
2.5. Statistical Analyses
3. Results
3.1. Demographics
3.2. Laboratory Test and PP-NRS Results: Baseline vs. Week 16
3.3. Predictive Factors
3.4. Predictive Factors: Subgroup Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Kim, B.E.; Leung, D.Y. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Darsow, U.; Wollenberg, A.; Simon, D.; Taïeb, A.; Werfel, T.; Oranje, A.; Gelmetti, C.; Svensson, A.; Deleuran, M.; Calza, A.-M. Difficult to control atopic dermatitis. World Allergy Organ. J. 2013, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Patruno, C. Aryl hydrocarbon receptor (AhR) a possible target for the treatment of skin disease. Med. Hypotheses 2018, 116, 96–100. [Google Scholar] [CrossRef]
- Kamata, M.; Tada, Y. Optimal use of jak inhibitors and biologics for atopic dermatitis on the basis of the current evidence. JID Innov. 2023, 3, 100195. [Google Scholar] [CrossRef]
- Kim, R.W.; Lam, M.; Abuabara, K.; Simpson, E.L.; Drucker, A.M. Targeted systemic therapies for adults with atopic dermatitis: Selecting from biologics and JAK inhibitors. Am. J. Clin. Dermatol. 2024, 25, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Merola, J.F.; Silverberg, J.I.; Reich, K.; Warren, R.B.; Staumont-Sallé, D.; Girolomoni, G.; Papp, K.; de Bruin-Weller, M.; Thyssen, J.P. Safety of tralokinumab in adult patients with moderate-to-severe atopic dermatitis: Pooled analysis of five randomized, double-blind, placebo-controlled phase II and phase III trials. Br. J. Dermatol. 2022, 187, 888–899. [Google Scholar] [CrossRef]
- Simpson, E.L.; Paller, A.S.; Siegfried, E.C.; Boguniewicz, M.; Sher, L.; Gooderham, M.J.; Beck, L.A.; Guttman-Yassky, E.; Pariser, D.; Blauvelt, A. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: A phase 3 randomized clinical trial. JAMA Dermatol. 2020, 156, 44–56. [Google Scholar] [CrossRef]
- Reich, K.; Kabashima, K.; Peris, K.; Silverberg, J.I.; Eichenfield, L.F.; Bieber, T.; Kaszuba, A.; Kolodsick, J.; Yang, F.E.; Gamalo, M. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020, 156, 1333–1343. [Google Scholar] [CrossRef]
- Simpson, E.L.; Papp, K.A.; Blauvelt, A.; Chu, C.-Y.; Hong, H.C.-h.; Katoh, N.; Calimlim, B.M.; Thyssen, J.P.; Chiou, A.S.; Bissonnette, R. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: Analysis of follow-up data from the measure up 1 and measure up 2 randomized clinical trials. JAMA Dermatol. 2022, 158, 404–413. [Google Scholar] [CrossRef]
- Simpson, E.L.; Sinclair, R.; Forman, S.; Wollenberg, A.; Aschoff, R.; Cork, M.; Bieber, T.; Thyssen, J.P.; Yosipovitch, G.; Flohr, C. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020, 396, 255–266. [Google Scholar] [CrossRef]
- Sedeh, F.B.; Henning, M.A.; Jemec, G.B.; Ibler, K.S. Comparative efficacy and safety of monoclonal antibodies and Janus kinase inhibitors in moderate-to-severe atopic dermatitis: A systematic review and meta-analysis. Acta Derm.-Venereol. 2022, 102, 2075. [Google Scholar] [CrossRef]
- Woo, Y.R.; Kim, H.S. Burden of Disease and Unmet Needs in the Diagnosis and Management of Atopic Dermatitis in Korea. J. Clin. Med. 2023, 12, 3744. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Immaneni, S.; Singam, V.; Rastogi, S.; Silverberg, J.I. Association between atopic dermatitis, depression, and suicidal ideation: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Oh, S.; Noh, H.; Joo, B.; Shim, J.; Park, J.; Lee, D.; Lee, J.H. Appropriate Injection Intervals of Dupilumab in Patients with Adult Atopic Dermatitis: A Step Toward Developing Guidelines for Daily Practice. Ann. Dermatol. 2024, 36, 39. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Quan, G.; Noh, Y.; Hong, S.H. Impact of Incorporating Future Mandatory Price Reductions with Generic Drug Entry on the Cost-Effectiveness of New Drugs: A Policy Simulation Study of Dupilumab in Atopic Dermatitis Treatment. Healthcare 2024, 12, 938. [Google Scholar] [CrossRef]
- Calabrese, L.; D’Onghia, M.; Lazzeri, L.; Rubegni, G.; Cinotti, E. Blocking the IL-4/IL-13 Axis versus the JAK/STAT Pathway in Atopic Dermatitis: How Can We Choose? J. Pers. Med. 2024, 14, 775. [Google Scholar] [CrossRef]
- Haddad, E.-B.; Cyr, S.L.; Arima, K.; McDonald, R.A.; Levit, N.A.; Nestle, F.O. Current and emerging strategies to inhibit type 2 inflammation in atopic dermatitis. Dermatol. Ther. 2022, 12, 1501–1533. [Google Scholar] [CrossRef]
- Grobe, W.; Bieber, T.; Novak, N. Pathophysiology of atopic dermatitis. JDDG J. Dtsch. Dermatol. Ges. 2019, 17, 433–440. [Google Scholar] [CrossRef]
- Dubin, C.; Del Duca, E.; Guttman-Yassky, E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev. Clin. Immunol. 2021, 17, 835–852. [Google Scholar] [CrossRef]
- Nograles, K.E.; Zaba, L.C.; Shemer, A.; Fuentes-Duculan, J.; Cardinale, I.; Kikuchi, T.; Ramon, M.; Bergman, R.; Krueger, J.G.; Guttman-Yassky, E. IL-22–producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17–producing TH17 T cells. J. Allergy Clin. Immunol. 2009, 123, 1244–1252.e2. [Google Scholar] [CrossRef]
- Chen, L.; Martinez, O.; Overbergh, L.; Mathieu, C.; Prabhakar, B.; Chan, L. Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model. Clin. Exp. Immunol. 2004, 138, 375–387. [Google Scholar] [CrossRef]
- Yu, L.; Li, L. Potential biomarkers of atopic dermatitis. Front. Med. 2022, 9, 1028694. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Song, G.; Song, Z. Intrinsic atopic dermatitis and extrinsic atopic dermatitis: Similarities and differences. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2621–2628. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Kabashima, K.; Staumont-Salle, D.; Nahm, W.K.; Pauser, S.; Da Rosa, J.C.; Martel, B.C.; Madsen, D.E.; Røpke, M.; Arlert, P. Targeting IL-13 with tralokinumab normalizes type 2 inflammation in atopic dermatitis both early and at 2 years. Allergy 2024, 79, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, H.; Chan, L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAK-STAT 2013, 2, e24137. [Google Scholar] [CrossRef]
- Tsiogka, A.; Kyriazopoulou, M.; Kontochristopoulos, G.; Nicolaidou, E.; Stratigos, A.; Rigopoulos, D.; Gregoriou, S. The JAK/STAT pathway and its selective inhibition in the treatment of atopic dermatitis: A systematic review. J. Clin. Med. 2022, 11, 4431. [Google Scholar] [CrossRef]
- Yujin, H.; Woo, Y.R.; Cho, S.H.; Lee, J.D.; Kim, H.S. Itch and Janus kinase inhibitors. Acta Derm.-Venereol. 2023, 15, 5346. [Google Scholar]
- Hagino, T.; Saeki, H.; Fujimoto, E.; Kanda, N. Predictive Factors for Long-Term High Responders to Upadacitinib Treatment in Patients with Atopic Dermatitis. Dermatitis 2025, 36, 62–71. [Google Scholar] [CrossRef]
- Gargiulo, L.; Ibba, L.; Bianco, M.; Di Giulio, S.; Alfano, A.; Cascio Ingurgio, R.; Facheris, P.; Perugini, C.; Valenti, M.; Costanzo, A.; et al. Upadacitinib 30 mg for the optimal management of moderate-to-severe atopic dermatitis: A 52-week single-center real-world study. J. Dermatol. Treat. 2024, 35, 2375102. [Google Scholar] [CrossRef]
- Rodriguez-Sanna, A.I.; Montero-Vilchez, C.; Moreno-Suarez, F.G.; Garrido-Colmenero, C.; Aceituno-Madera, P.; Arias-Santiago, S.; Montero-Vilchez, T. Patients with Atopic Dermatitis Treated With Upadacitinib: Factors Related to Achieving Minimal Disease Activity. Dermatitis 2025. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Gawey, L.; Rahman, M.; Ghanshani, R.; Tran, K.A.; Hsiao, J.L.; Shi, V.Y. Postmarketing safety analysis of upadacitinib in atopic dermatitis: A Food and Drug Administration adverse reporting system review of boxed warning-related adverse events. J. Am. Acad. Dermatol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ibba, L.; Gargiulo, L.; Bianco, M.; Di Giulio, S.; Cascio Ingurgio, R.; Alfano, A.; Vignoli, C.A.; Valenti, M.; Facheris, P.; Perugini, C.; et al. Potential Role of Leptin in JAK2 Inhibitor-Associated Weight Gain: A Monocentric Retrospective Study. Clin. Exp. Dermatol. 2025, 2025, llaf144. [Google Scholar] [CrossRef]
| Overall (n = 43) | Group 1 * (n = 11) | Group 2 † (n = 18) | Group 3 ‡ (n = 14) | p-Value | |
|---|---|---|---|---|---|
| Gender, male | 30 (69.77) | 9 (81.82) | 13 (72.22) | 8 (57.15) | 0.393 |
| Age, years | 31.51 (12.57) | 32.27 (13.31) | 26.61 (6.43) | 37.21 (15.84) | 0.155 |
| AD onset | 0.646 | ||||
| Childhood | 23 (53.49) | 7 (63.64) | 10 (55.56) | 6 (42.86) | |
| Adolescence | 8 (18.60) | 2 (18.18) | 4 (22.22) | 2 (14.26) | |
| Adulthood | 12 (27.91) | 2 (18.18) | 4 (22.22) | 6 (42.46) | |
| Weight (kg) | 72.76 (15.33) | 74.41 (11.19) | 72.93 (15.73) | 71.43 (18.14) | 0.908 |
| Height (cm) | 168.57 (9.33) | 172.56 (6.82) | 167.74 (7.52) | 166.77 (12.15) | 0.334 |
| BMI (kg/m2) | 25.72 (4.73) | 24.95 (3.05) | 26.40 (5.64) | 25.47 (4.79) | 0.909 |
| Baseline EASI | 21.25 (10.33) | 26.36 (6.89) | 21.72 (9.62) | 16.65 (11.92) | 0.06 |
| JAK inhibitors | |||||
| Upadacitinib | 37 (86.05) | 11 (100) | 16 (88.89) | 10 (71.43) | |
| Abrocitinib | 3 (6.98) | 0 (0) | 2 (11.11) | 1 (7.14) | |
| Baricitinib | 3 (6.98) | 0 (0) | 0 (0) | 3 (21.43) |
| Group 1 * (n = 11) | Group 2 † (n = 18) | Group 3 ‡ (n = 14) | p-Value | ||
|---|---|---|---|---|---|
| IgE (IU/mL) | Baseline | 2626.14 (2232.47) | 2691.35 (1907.44) | 2953.09 (2101.06) | 0.925 |
| Week 16 | 2700.13 (2539.43) | 2494.44 (2122.14) | 4190.00 (1682.50) | 0.38 | |
| Δ (Baseline–Week 16) | 43.88 (115.55) | −264.97 (849.78) | −188.50 (811.76) | 0.749 | |
| Eosinophil (/μL) | Baseline | 507.09 (487.29) | 860.20 (659.13) | 456.14 (257.53) | 0.214 |
| Week 16 | 248.50 (144.86) | 424.38 (514.04) | 260.00 (103.15) | 0.565 | |
| Δ (Baseline–Week 16) | −462.83 (612.97) | −501.38 (695.63) | −58.33 (141.76) | 0.32 | |
| ECP (μg/L) | Baseline | 108.05 (131.45) | 55.12 (26.63) | 36.37 (20.22) | 0.439 |
| Week 16 | 38.00 (N/A) | 18.30 (6.51) | 8.51 (N/A) | N/A | |
| Δ (Baseline–Week 16) | −163.00 (N/A) | −16.25 (14.07) | −4.99 (N/A) | N/A | |
| LDH (IU/L) | Baseline | 314.17 (153.80) | 258.86 (70.49) | 199.00 (32.16) | 0.097 |
| Week 16 | 261.00 (44.50) | 257.33 (83.10) | 190.56 (108.37) | 0.428 | |
| Δ (Baseline–Week 16) | −124.67 (149.18) | −15.33 (129.16) | 61.50 (24.93) | 0.142 | |
| Uric acid (mg/dL) | Baseline | 5.86 (0.98) | 6.78 (1.44) | 5.83 (1.83) | 0.282 |
| Week 16 | 5.85 (1.33) | 5.90 (0.96) | 6.24 (0.44) | 0.786 | |
| Δ (Baseline–Week 16) | −0.25 (0.72) | −0.87 (1.26) | 0.16 (0.98) | 0.303 | |
| PP-NRS | Baseline | 8.125 (1.46) | 7.625 (1.51) | 6.429 (2.07) | |
| Week 16 | 1.200 (1.23) | 1.643 (1.28) | 2.000 (1.49) |
| Group 1 * (n = 11) | Group 2 † (n = 18) | Group 3 ‡ (n = 14) | p-Value | |
|---|---|---|---|---|
| Baseline EASI, mean (SD) | 26.35 (6.89) | 21.72 (9.62) | 16.65 (11.92) | 0.06 |
| Week 16 EASI, mean (SD) | 1.23 (0.76) | 3.45 (1.68) | 8.86 (8.65) | 0.001 |
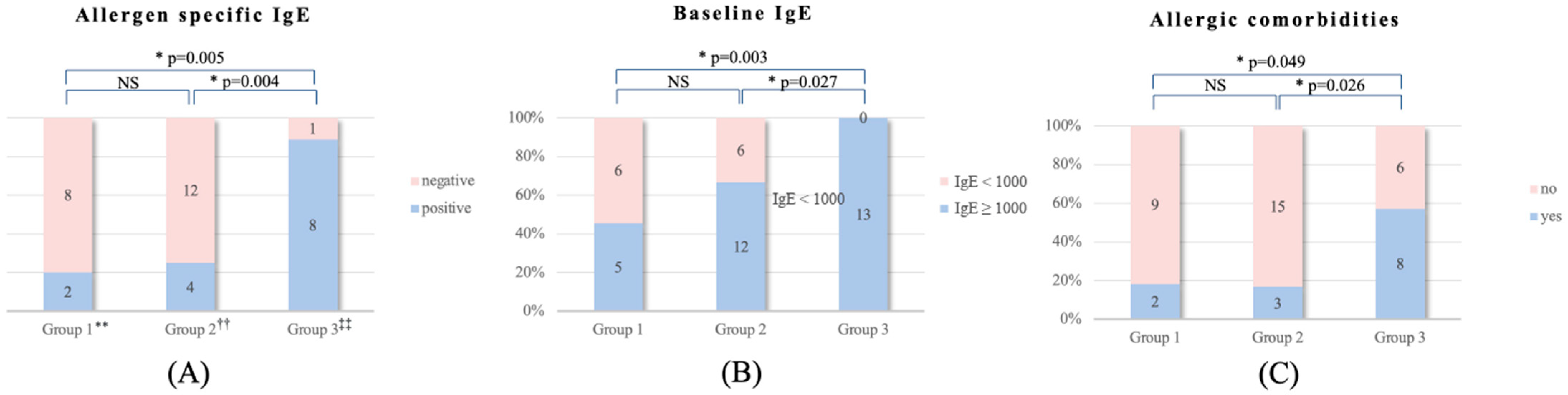
| Allergen-specific IgE (MAST results) | 0.002 | |||
| Positive | 2 | 4 | 8 | |
| Negative | 8 | 12 | 1 | |
| Baseline IgE | 0.011 | |||
| IgE ≥ 1000 | 5 | 12 | 13 | |
| IgE < 1000 | 6 | 6 | 0 | |
| Allergic comorbidity | 0.028 | |||
| Yes | 2 | 3 | 8 | |
| No | 9 | 15 | 6 | |
| Head and neck/EASI ratio (%) median [IQR] | 0.14 [0.10, 0.17] | 0.11 [0.07, 0.16] | 0.14 [0.05, 0.25] | 0.79 |
| Study | Biologics | Janus Kinase Inhibitor |
|---|---|---|
| Kamata et al. [4] 2023 | Risk of malignancy, CVD, DVT, PE, GI perforation Elderly patients (over 50 years, especially over 65 years) History of herpes zoster Repeated skin infections including eczema herpeticum Comorbidities: asthma, eosinophilic esophagitis, and/or chronic rhinosinusitis with nasal polyps | Severe pruritus and/or who wish a rapid onset Fear of needles (trypanophobia) or who prefers oral medicine over injection High risk of developing conjunctivitis during dupilumab treatment (history of conjunctivitis, etc.) Facial redness, alopecia areata, or arthritis/enthesitis caused by biologics |
| Kim et al. [5] 2024 | Higher-risk and elderly patients (considered safer than JAK inhibitors) | Higher doses of abrocitinib and upadacitinib are more effective than biologics |
| Calabrese et al. [16] 2024 | CVD comorbidities Children and elderly Extrinsic AD European American Asthma Recurrent VZV infections Oncological comorbidities Acne | Ocular surface disease Severe AD and itch Intrinsic AD Asian and African American Head and neck AD Vitiligo Inflammatory bowel disease Alopecia areata |
| Present study | Extrinsic AD | Intrinsic AD Multiple allergen positivities Relatively low baseline IgE levels below 1000 Without allergic comorbidities (asthma, allergic rhinitis, allergic conjunctivitis, and food allergy) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Oh, S.; Park, J.; Choi, Y.; Lee, J.H. Predicting Favorable Conditions for the Determination of Initial Use of Janus Kinase Inhibitors in Patients with Moderate to Severe Atopic Dermatitis. J. Clin. Med. 2025, 14, 4312. https://doi.org/10.3390/jcm14124312
Park JH, Oh S, Park J, Choi Y, Lee JH. Predicting Favorable Conditions for the Determination of Initial Use of Janus Kinase Inhibitors in Patients with Moderate to Severe Atopic Dermatitis. Journal of Clinical Medicine. 2025; 14(12):4312. https://doi.org/10.3390/jcm14124312
Chicago/Turabian StylePark, Ju Heon, Sejin Oh, Jihye Park, YoungHwan Choi, and Jong Hee Lee. 2025. "Predicting Favorable Conditions for the Determination of Initial Use of Janus Kinase Inhibitors in Patients with Moderate to Severe Atopic Dermatitis" Journal of Clinical Medicine 14, no. 12: 4312. https://doi.org/10.3390/jcm14124312
APA StylePark, J. H., Oh, S., Park, J., Choi, Y., & Lee, J. H. (2025). Predicting Favorable Conditions for the Determination of Initial Use of Janus Kinase Inhibitors in Patients with Moderate to Severe Atopic Dermatitis. Journal of Clinical Medicine, 14(12), 4312. https://doi.org/10.3390/jcm14124312








