Efficacy and Safety of L-Menthol During Gastrointestinal Endoscopy—A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
2.8. Quality of Evidence
2.9. Synthesis Methods and Statistical Analysis
3. Results
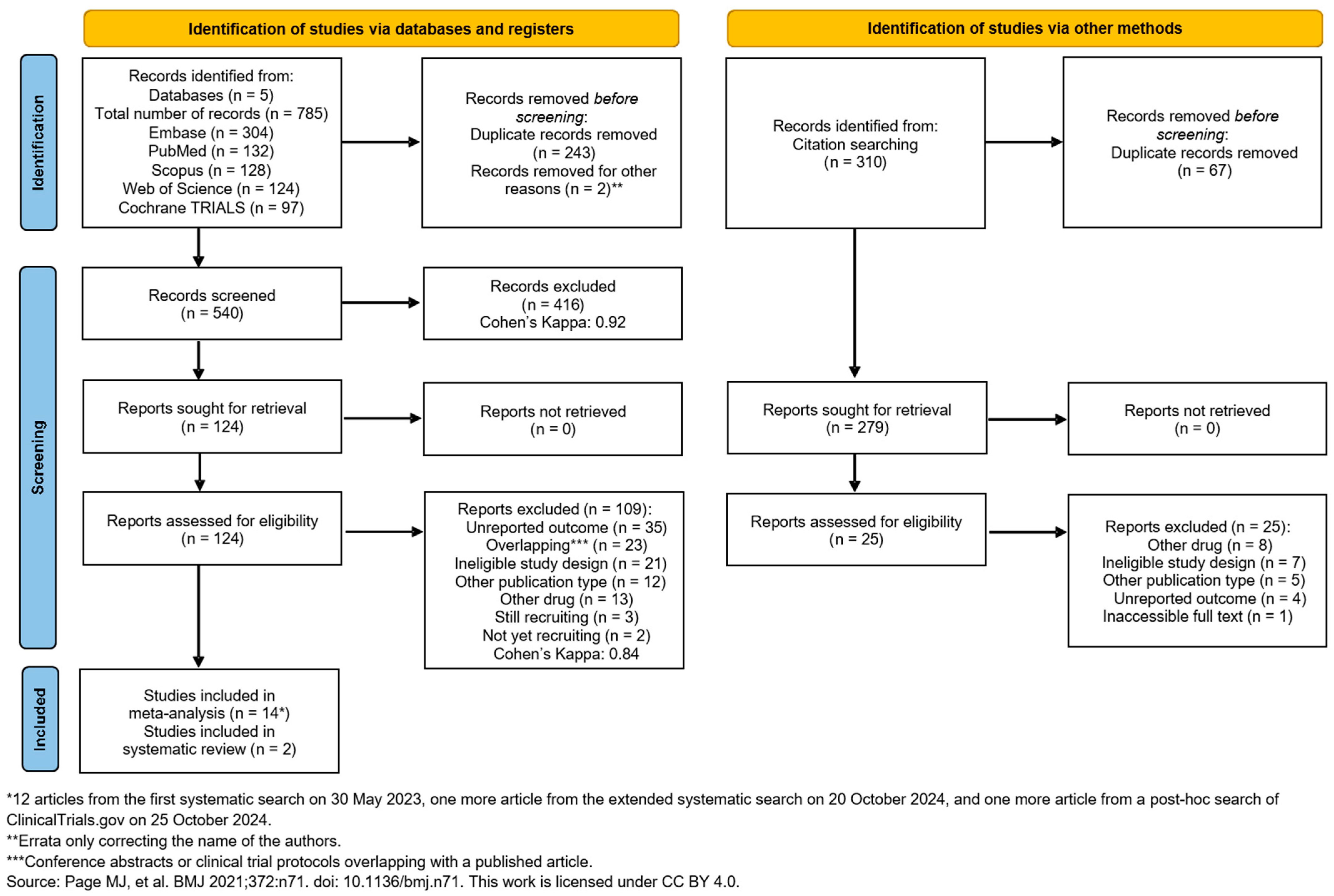
3.1. Search and Selection
3.2. Basic Characteristics of Included Studies
3.3. Meta-Analysis of the Findings
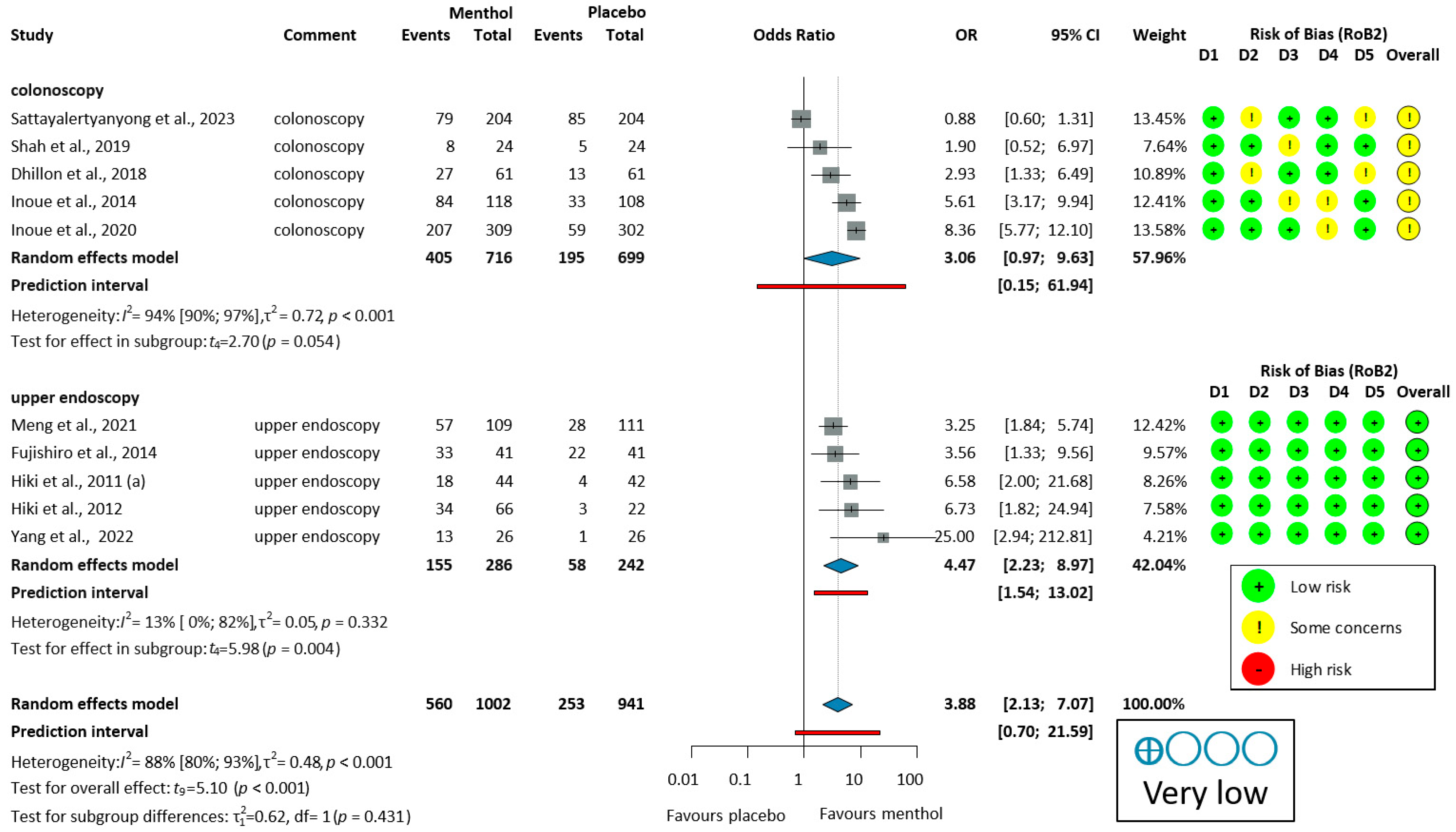
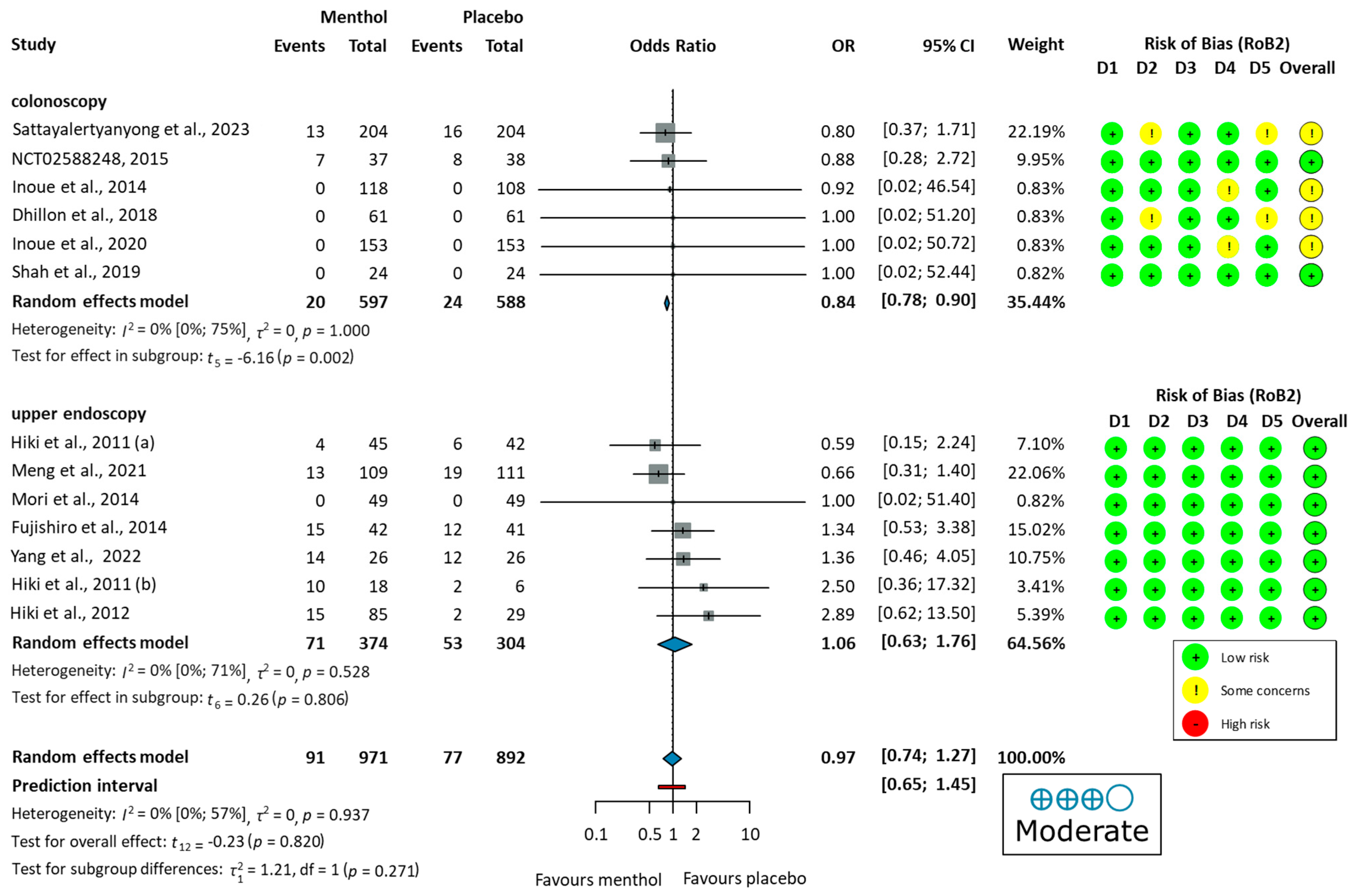
3.3.1. Efficacy of L-Menthol on Adenoma Detection Rate
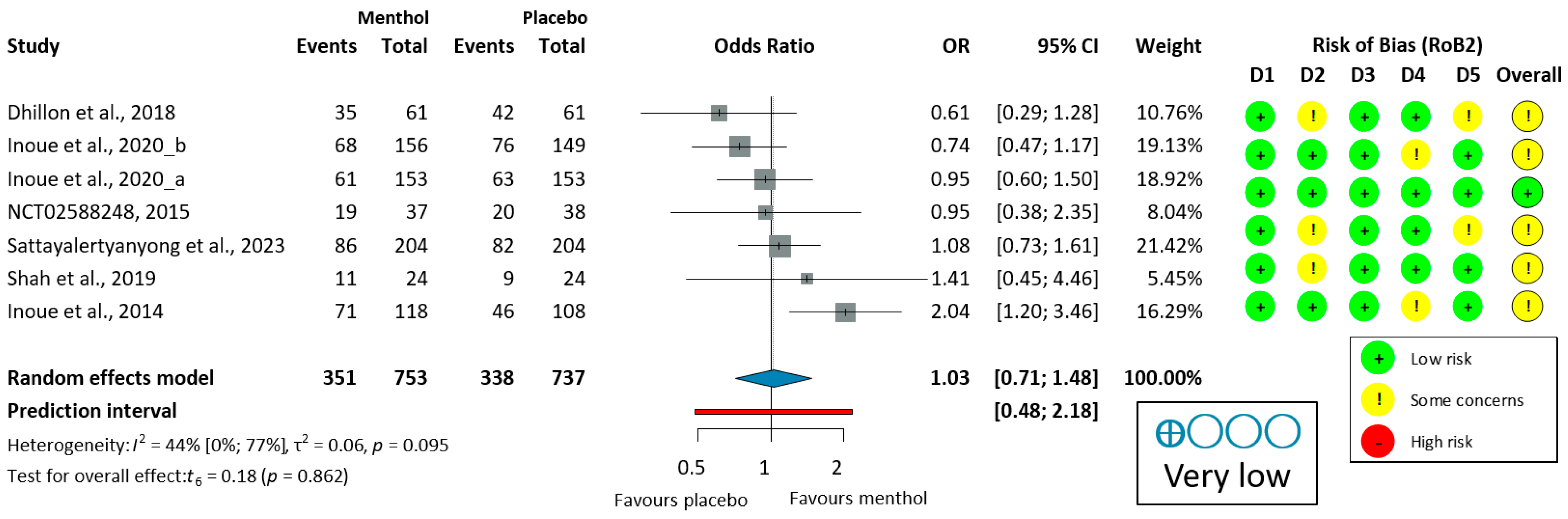
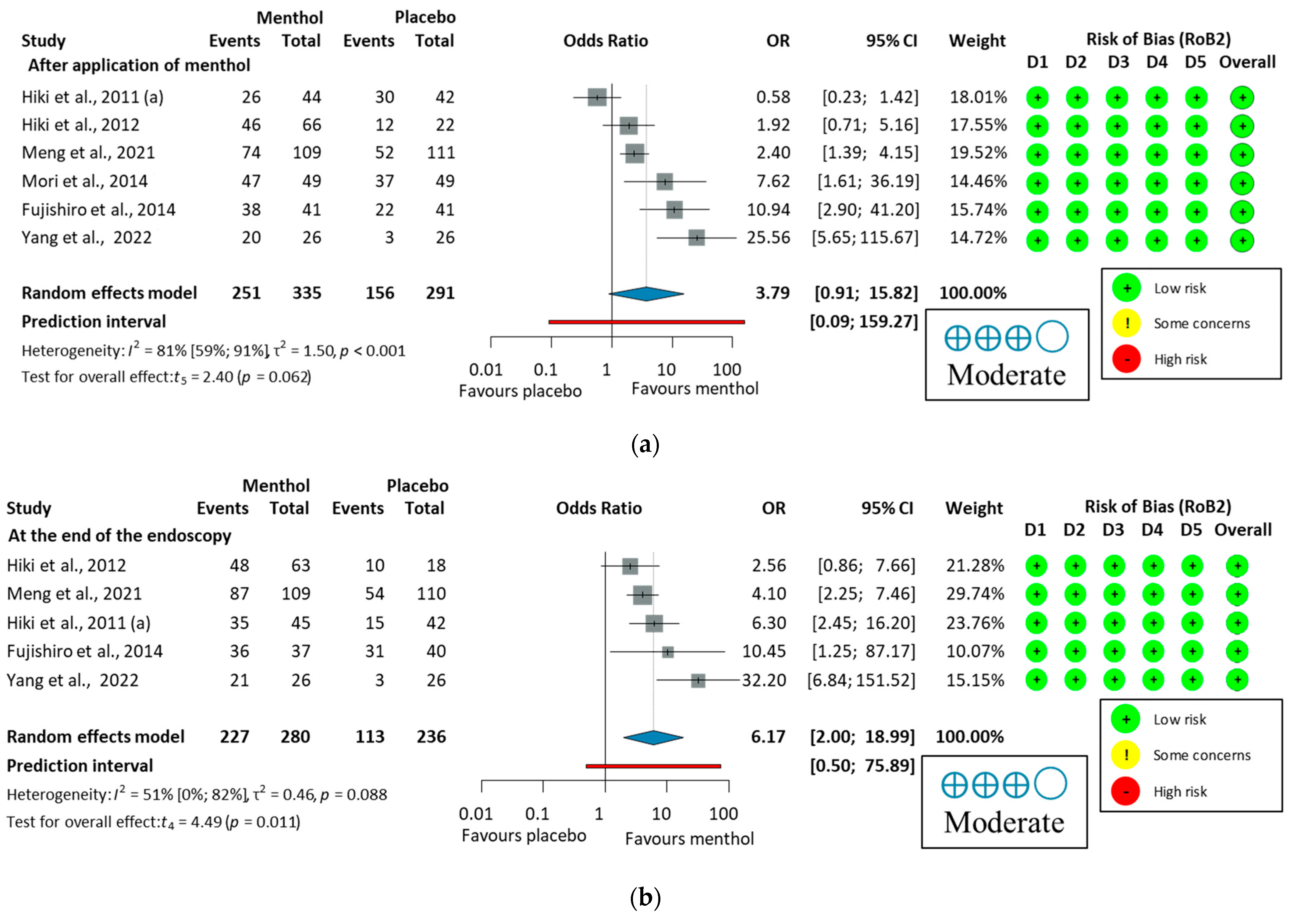
3.3.2. Antiperistaltic Effect of L-Menthol
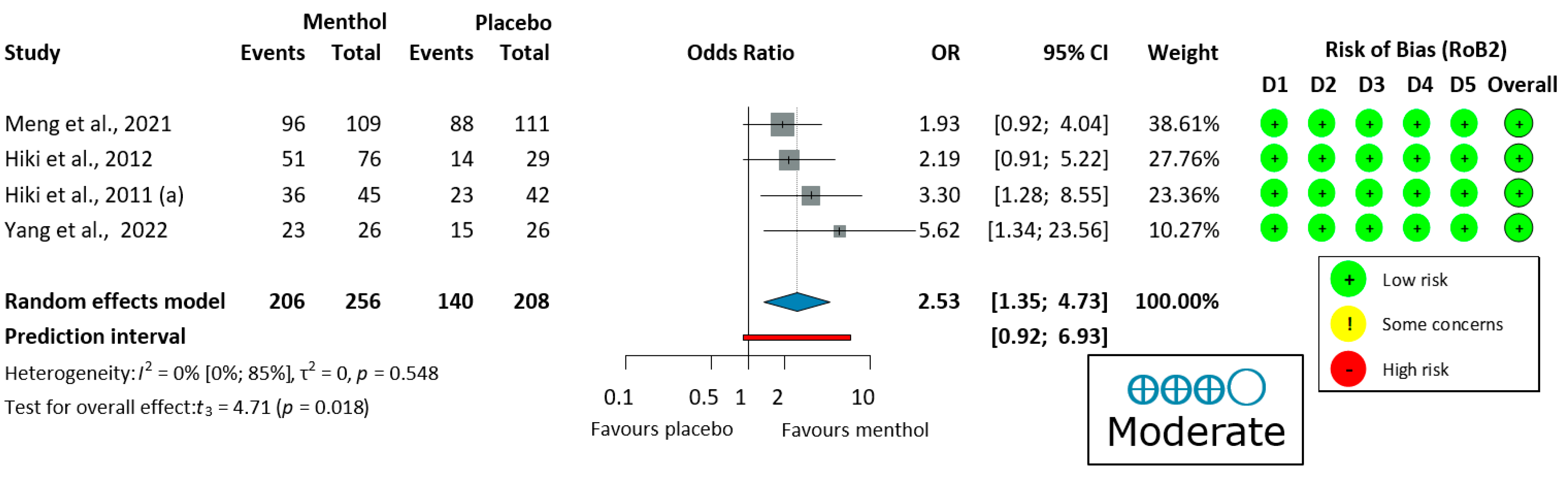
3.3.3. Ease of Examination for the Operator in Upper Endoscopy
3.3.4. Withdrawal Time in Colonoscopy
3.3.5. Total Adverse Events
3.3.6. Total Adverse Drug Reactions
3.4. Studies with Peppermint Capsules
3.5. Risk of Bias and GRADE Assessment
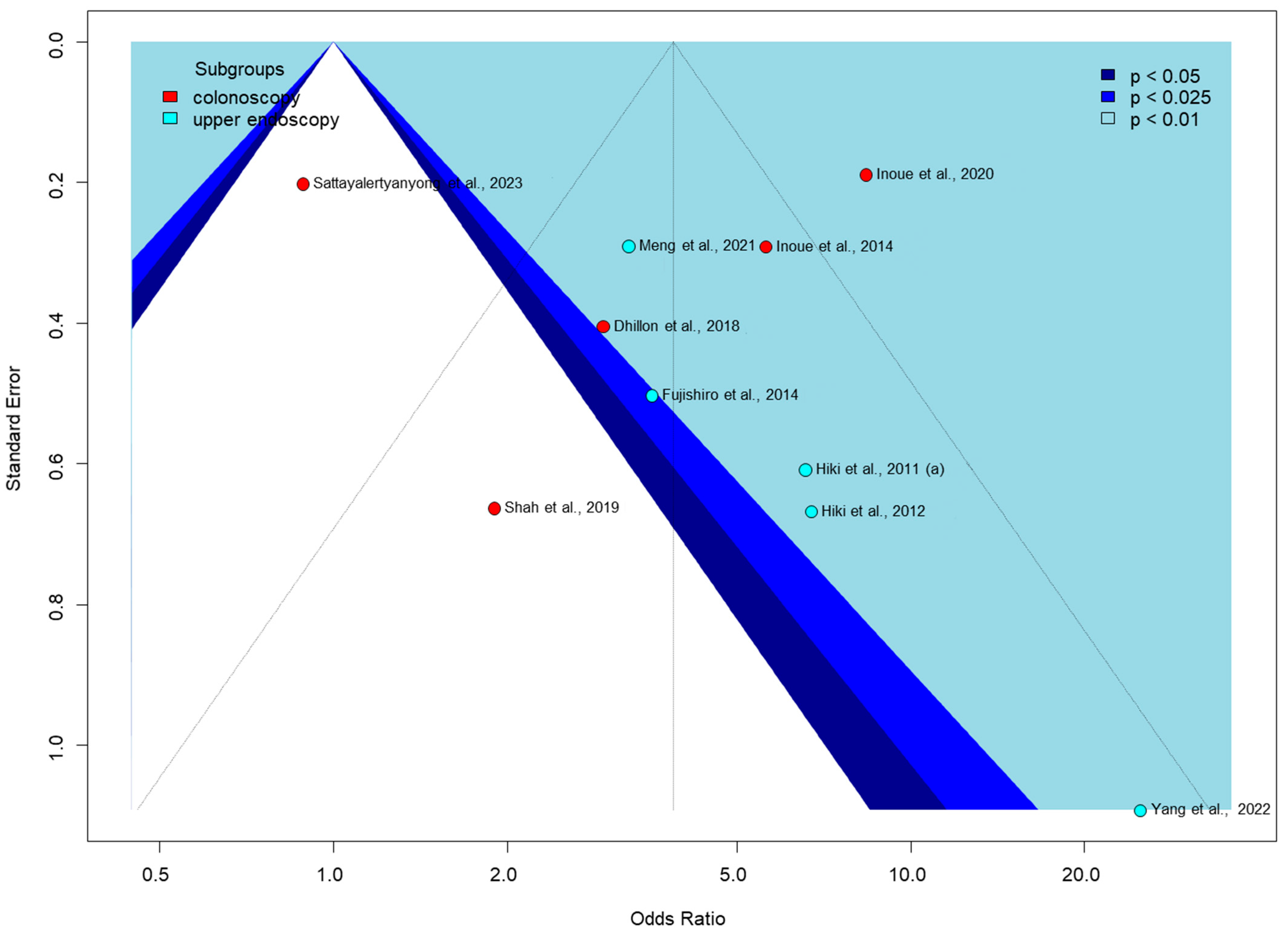
3.6. Publication Bias and Heterogeneity
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Practice and Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADR | adenoma detection rate |
| APC | adenoma per colonoscopy |
| AS | antispasmodic scores |
| C | control |
| CI | confidence interval |
| CR | contraction ratio |
| CRC | colorectal cancer |
| EGC | early gastric cancer |
| EGD | esophagogastroduodenoscopy |
| EIE | ease of the intragastric examination |
| EMR | endoscopic mucosal resection |
| ESD | endoscopic submucosal dissection |
| GI | gastrointestinal |
| GPPM | gastric peristalsis per minute |
| GRADE | Grading of Recommendations, Assessment, Development and Evaluations |
| I | intervention |
| IBD | inflammatory bowel disease |
| LOO | leave-one-out |
| MD | mean difference |
| OR | odds ratio |
| PNMP | proportion of no or mild peristalsis |
| PNP | proportion of no peristalsis |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| PUD | peptic ulcer disease |
| RCT | randomized controlled trial |
| sec | second |
| TADR | total adverse drug reaction |
| TAE | total adverse event |
| WT | withdrawal time |
References
- Steele, S.R.; Johnson, E.K.; Champagne, B.; Davis, B.; Lee, S.; Rivadeneira, D.; Ross, H.; Hayden, D.A.; Maykel, J.A. Endoscopy and polyps-diagnostic and therapeutic advances in management. World J. Gastroenterol. 2013, 19, 4277–4288. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.C.; Mittal, P.K.; Sullivan, P.S.; Rutherford, R.; Staley, C.A.; Cardona, K.; Hawk, N.N.; Dixon, W.T.; Kitajima, H.D.; Kang, J.; et al. Colorectal Cancer Initial Diagnosis: Screening Colonoscopy, Diagnostic Colonoscopy, or Emergent Surgery, and Tumor Stage and Size at Initial Presentation. Clin. Color. Cancer. 2016, 15, 67–73. [Google Scholar] [CrossRef] [PubMed]
- American Society for Gastrointestinal Endoscopy Standards of Practice Committee; Shergill, A.K.; Lightdale, J.R.; Bruining, D.H.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Evans, J.A.; et al. The role of endoscopy in inflammatory bowel disease. Gastrointest. Endosc. 2015, 81, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- ASGE Standards of Practice Committee; Banerjee, S.; Cash, B.D.; Dominitz, J.A.; Baron, T.H.; Anderson, M.A.; Ben-Menachem, T.; Fisher, L.; Fukami, N.; Harrison, M.E.; et al. The role of endoscopy in the management of patients with peptic ulcer disease. Gastrointest. Endosc. 2010, 71, 663–668. [Google Scholar]
- ASGE Standards of Practice Committee; Coelho-Prabhu, N.; Forbes, N.; Thosani, N.C.; Storm, A.C.; Pawa, S.; Kohli, D.R.; Fujii-Lau, L.L.; Elhanafi, S.; Calderwood, A.H.; et al. Adverse events associated with EGD and EGD-related techniques. Gastrointest. Endosc. 2022, 96, 389–401.e1. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef]
- Park, W.G.; Shaheen, N.J.; Cohen, J.; Pike, I.M.; Adler, D.G.; Inadomi, J.M.; Laine, L.A.; Lieb, J.G., 2nd; Rizk, M.K.; Sawhney, M.S.; et al. Quality indicators for EGD. Gastrointest. Endosc. 2015, 81, 17–30. [Google Scholar] [CrossRef]
- Rex, D.K.; Schoenfeld, P.S.; Cohen, J.; Pike, I.M.; Adler, D.G.; Fennerty, M.B.; Lieb, J.G., 2nd; Park, W.G.; Rizk, M.K.; Sawhney, M.S.; et al. Quality indicators for colonoscopy. Gastrointest. Endosc. 2015, 81, 31–53. [Google Scholar] [CrossRef]
- Corte, C.J.; Leong, R.W. Improving the utility of colonoscopy: Recent advances in practice. J. Gastroenterol. Hepatol. 2016, 31, 32–44. [Google Scholar] [CrossRef]
- Bazerbachi, F.; Panganamamula, K.; Nieto, J.M.; Murad, M.H.; Keswani, R.N.; Shaukat, A.; Day, L.W. Interventions to improve the performance of upper GI endoscopy quality indicators. Gastrointest. Endosc. 2022, 96, 184–188.e4. [Google Scholar] [CrossRef]
- Gutzeit, A.; Binkert, C.A.; Koh, D.M.; Hergan, K.; von Weymarn, C.; Graf, N.; Patak, M.A.; Roos, J.E.; Horstmann, M.; Kos, S.; et al. Evaluation of the anti-peristaltic effect of glucagon and hyoscine on the small bowel: Comparison of intravenous and intramuscular drug administration. Eur. Radiol. 2012, 22, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Sanagapalli, S.; Agnihotri, K.; Leong, R.; Corte, C.J. Antispasmodic drugs in colonoscopy: A review of their pharmacology, safety and efficacy in improving polyp detection and related outcomes. Ther. Adv. Gastroenterol. 2017, 10, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Dyde, R.; Chapman, A.H.; Gale, R.; Mackintosh, A.; Tolan, D.J. Precautions to be taken by radiologists and radiographers when prescribing hyoscine-N-butylbromide. Clin. Radiol. 2008, 63, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R. Routine use of hyoscine N butylbromide (Buscopan) in double contrast barium enema examinations. Clin. Radiol. 1982, 33, 273–276. [Google Scholar] [CrossRef]
- Chakraborty, K.; Chakravarti, A.R.; Bhattacharjee, S. Bioactive components of peppermint (Mentha piperita L.), their pharmacological and ameliorative potential and ethnomedicinal benefits: A review. J. Pharmacogn. Phytochem. 2022, 11, 109–114. [Google Scholar] [CrossRef]
- Scarpellini, E.; Broeders, B.; Schol, J.; Santori, P.; Addarii, M.; Boccuto, L.; Carbone, F.; Abenavoli, L.; Tack, J. The Use of Peppermint Oil in Gastroenterology. Curr. Pharm. Des. 2023, 29, 576–583. [Google Scholar] [CrossRef]
- Ingrosso, M.R.; Ianiro, G.; Nee, J.; Lembo, A.J.; Moayyedi, P.; Black, C.J.; Ford, A.C. Systematic review and meta-analysis: Efficacy of peppermint oil in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2022, 56, 932–941. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- Grigoleit, H.G.; Grigoleit, P. Pharmacology and preclinical pharmacokinetics of peppermint oil. Phytomedicine 2005, 12, 612–616. [Google Scholar] [CrossRef]
- Amato, A.; Liotta, R.; Mulè, F. Effects of menthol on circular smooth muscle of human colon: Analysis of the mechanism of action. Eur. J. Pharmacol. 2014, 740, 295–301. [Google Scholar] [CrossRef]
- Hawthorn, M.; Ferrante, J.; Luchowski, E.; Rutledge, A.; Wei, X.Y.; Triggle, D.J. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment. Pharmacol. Ther. 1988, 2, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Hills, J.M.; Aaronson, P.I. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology 1991, 101, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Mahawongkajit, P.; Kanlerd, A. A prospective randomized controlled trial comparing simethicone, N-acetylcysteine, sodium bicarbonate and peppermint for visualization in upper gastrointestinal endoscopy. Surg. Endosc. 2021, 35, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, A.; Namikawa, K.; Tokai, Y.; Yoshimizu, S.; Horiuchi, Y.; Yoshio, T.; Hirasawa, T.; Tsuchida, T.; Itoh, F.; Fujisaki, J. Effect of spraying l-menthol on peristalsis resumption during endoscopic submucosal dissection of gastric tumors. JGH Open 2021, 5, 653–657. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024). Cochrane, 2024. Available online: www.training.cochrane.org/handbook (accessed on 24 October 2024).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. Citationchaser: A tool for transparent and efficient forward and backward citation chasing in systematic searching. Res. Synth. Methods 2022, 13, 533–545. [Google Scholar] [CrossRef]
- Rex, D.K.; Petrini, J.L.; Baron, T.H.; Chak, A.; Cohen, J.; Deal, S.E.; Hoffman, B.; Jacobson, B.C.; Mergener, K.; Petersen, B.T.; et al. Quality indicators for colonoscopy. Am. J. Gastroenterol. 2006, 101, 873–885. [Google Scholar] [CrossRef]
- Asao, T.; Mochiki, E.; Suzuki, H.; Nakamura, J.; Hirayama, I.; Morinaga, N.; Shoji, H.; Shitara, Y.; Kuwano, H. An easy method for the intraluminal administration of peppermint oil before colonoscopy and its effectiveness in reducing colonic spasm. Gastrointest. Endosc. 2001, 53, 172–177. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, J.; Chen, Y.; Sun, H.; Li, B.; Zhang, Q.; Sun, K.; Wang, Z.; Qian, X.; Zhan, T.; et al. Negative Effects of Endoscopists' Fatigue on Colonoscopy Quality on 34,022 Screening Colonoscopies. J. Gastrointestin Liver Dis. 2021, 30, 358–365. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.B.J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 16 January 2025).
- GRADEpro GDT. GRADEpro Guideline Development Tool. McMaster University and Evidence Prime, 2023. Available online: https://www.gradepro.org/ (accessed on 18 January 2025).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D.D. 2019 Dmetar: Companion R Package For The Guide ‘Doing Meta-Analysis in R’. R Package Version 0.1.0, 2019. Available online: http://dmetar.protectlab.org/ (accessed on 9 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 12 November 2024).
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Thompson, S.G.; Turner, R.M.; Warn, D.E. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat. Methods Med. Res. 2001, 10, 375–392. [Google Scholar] [CrossRef]
- Robins, J.; Greenland, S.; Breslow, N.E. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am. J. Epidemiol. 1986, 124, 719–723. [Google Scholar] [CrossRef]
- Knapp, G.; Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003, 22, 2693–2710. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. The Handbook of Research Synthesis and Meta-Analysis, 2nd ed.; Russell Sage Foundation: New York, NY, USA, 2009. [Google Scholar]
- Sweeting, M.J.; Sutton, A.J.; Lambert, P.C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 2004, 23, 1351–1375. [Google Scholar] [CrossRef]
- Paule, R.C.; Mandel, J. Consensus Values and Weighting Factors. J. Res. Natl. Bur. Stand. 1982, 87, 377–385. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W.; Cheung, M.W. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Nakamura, T.; Fujino, M. Endoscopic Observation on Gastric Peristalsis and Pyloric Movement. Gastrointest. Endosc. 1975, 17, 236–242. [Google Scholar]
- Dhillon, A.S.; Alshankiti, S.; Khorasani-zadeh, A.; Sultanian, R.; Sandha, G.S.; Kohansal, A.R.; Montano-Loza, A.J.; Zepeda-Gomez, S. A247 L-menthol during colonoscopy for adenoma detection in an intermediate risk patient population: A double-blind, randomized controlled trial. J. Can. Assoc. Gastroenterol. 2018, 1, 360–361. [Google Scholar] [CrossRef]
- University Hospitals Cleveland Medical Center L-Menthol Injection as a Novel Technique During Colonoscopy (MINT-C) NCT02588248, 2015. Available online: https://clinicaltrials.gov/study/NCT02588248 (accessed on 18 June 2023).
- Inoue, K.; Dohi, O.; Gen, Y.; Jo, M.; Mazaki, T.; Tokita, K.; Yoshida, N.; Okayama, T.; Kamada, K.; Katada, K.; et al. L-menthol improves adenoma detection rate during colonoscopy: A randomized trial. Endoscopy 2014, 46, 196–202. [Google Scholar] [CrossRef]
- Inoue, K.; Okuda, T.; Oka, K.; Sugino, S.; Endo, Y.; Ota, T.; Minagawa, Y.; Yasue, C.; Tsuji, T.; Katayama, T.; et al. Effects of L-Menthol and Carbon Dioxide on the Adenoma Detection Rate during Colonoscopy: L-Menthol and Carbon Dioxide on Colonoscopy. Digestion 2020, 101, 323–331. [Google Scholar] [CrossRef]
- Sattayalertyanyong, O.; Sathirawich, P.; Maipang, K.; Chukaewrungroj, P.; Limsrivilai, J.; Kaosombatwattana, U. Efficacy and safety of intraluminal peppermint oil during colonoscopy on colonic peristalsis and adenoma detection rate: A randomized, double-blinded, placebo-controlled trial. Gastrointest. Endosc. 2023, 97, AB696–AB697. [Google Scholar] [CrossRef]
- Shah, I.; Baffy, N.J.; Horsley-Silva, J.L.; Langlais, B.T.; Ruff, K.C. Peppermint Oil to Improve Visualization in Screening Colonoscopy: A Randomized Controlled Clinical Trial. Gastroenterol. Res. 2019, 12, 141–147. [Google Scholar] [CrossRef]
- Fujishiro, M.; Kaminishi, M.; Hiki, N.; Oda, I.; Fujisaki, J.; Uedo, N.; Kaise, M.; Tanabe, S.; Iguchi, M.; Matsuhashi, N.; et al. Efficacy of spraying l-menthol solution during endoscopic treatment of early gastric cancer: A phase III, multicenter, randomized, double-blind, placebo-controlled study. J. Gastroenterol. 2014, 49, 446–454. [Google Scholar] [CrossRef]
- Hiki, N.; Kaminishi, M.; Yasuda, K.; Uedo, N.; Honjo, H.; Matsuhashi, N.; Hiratsuka, T.; Sekine, C.; Nomura, S.; Yahagi, N.; et al. Antiperistaltic effect and safety of L-menthol sprayed on the gastric mucosa for upper GI endoscopy: A phase III, multicenter, randomized, double-blind, placebo-controlled study. Gastrointest. Endosc. 2011, 73, 932–941. [Google Scholar] [CrossRef]
- Hiki, N.; Kaminishi, M.; Yasuda, K.; Uedo, N.; Kobari, M.; Sakai, T.; Hiratsuka, T.; Ohno, K.; Honjo, H.; Nomura, S.; et al. Multicenter phase II randomized study evaluating dose-response of antiperistaltic effect of L-menthol sprayed onto the gastric mucosa for upper gastrointestinal endoscopy. Dig. Endosc. 2012, 24, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Li, W.; Zhi, F.; Li, Z.; Xue, Z.; He, S.; Chen, W.; Chen, Y.; Xing, X.; Yao, C.; et al. Antiperistaltic effect and safety of l-menthol oral solution on gastric mucosa for upper gastrointestinal endoscopy in Chinese patients: Phase III, multicenter, randomized, double-blind, placebo-controlled study. Dig. Endosc. 2021, 33, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Hachiya, H.; Yumura, T.; Ito, S.; Hayashi, S.; Nozaki, M.; Yoshida, A.; Ohashi, N. l-Menthol sprayed on gastric mucosa causes edematous change. Endosc. Int. Open. 2014, 2, E51–E57. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Chen, P.H.; Hou, M.C.; Peng, L.N.; Lin, M.H.; Chen, L.K.; Huang, Y.H. Antiperistaltic effect and safety of L-menthol for esophagogastroduodenoscopy in the elderly with contraindication to hyoscine-N-butylbromide. Sci. Rep. 2022, 12, 10418. [Google Scholar] [CrossRef]
- Hiki, N.; Kaminishi, M.; Hasunuma, T.; Nakamura, M.; Nomura, S.; Yahagi, N.; Tajiri, H.; Suzuki, H. A phase I study evaluating tolerability, pharmacokinetics, and preliminary efficacy of L-menthol in upper gastrointestinal endoscopy. Clin. Pharmacol. Ther. 2011, 90, 221–228. [Google Scholar] [CrossRef]
- Imagawa, A.; Hata, H.; Nakatsu, M.; Yoshida, Y.; Takeuchi, K.; Inokuchi, T.; Imada, T.; Kohno, Y.; Takahara, M.; Matsumoto, K.; et al. Peppermint oil solution is useful as an antispasmodic drug for esophagogastroduodenoscopy, especially for elderly patients. Dig. Dis. Sci. 2012, 57, 2379–2384. [Google Scholar] [CrossRef]
- Yoshida, N.; Naito, Y.; Hirose, R.; Ogiso, K.; Inada, Y.; Fernandopulle, N.; Kamada, K.; Katada, K.; Uchiyama, K.; Handa, O.; et al. Prevention of colonic spasm using L-menthol in colonoscopic examination. Int. J. Color. Dis. 2014, 29, 579–583. [Google Scholar] [CrossRef]
- Anishchenko, M.; Schapovalyanz, S.; Nazmeev, M.; Budzinskiy, S.; Rogov, A.Z.; Ruslan, Z.; Fedorov, E. Antiperistalsis effect of l-menthol sprayed into duodenum during ERCP: Initial results of prospective two-center randomized placebo-controlled study. Gastrointest. Endosc. 2023, 97, AB611. [Google Scholar] [CrossRef]
- Park, S.; Chun, H.J.; Kim, E.S.; Park, S.C.; Jung, E.S.; Lee, S.D.; Jang, J.S.; Kwon, Y.D.; Keum, B.; Seo, Y.S.; et al. Peppermint Oil Solution Is An Effective Antispasmodics On Esophagogastroduodenoscopy. Gastrointest. Endosc. 2009, 69, AB209. [Google Scholar] [CrossRef]
- Mabuchi, M.; Adachi, T.; Kajiyama, H.; Kuniyoshi, N.; Onda, T.; Matsumoto, K.; Tsunashima, H.; Sekine, K.; Tsujikawa, T.; Kajiyama, Y.; et al. Antispasmodic effect of l-menthol during endoscopic retrograde cholangiopancreatography. J. Gastroenterol. Hepatol. 2017, 32, 272. [Google Scholar]
- Yamamoto, N.; Nakai, Y.; Sasahira, N.; Hirano, K.; Tsujino, T.; Isayama, H.; Komatsu, Y.; Tada, M.; Yoshida, H.; Kawabe, T.; et al. Efficacy of peppermint oil as an antispasmodic during endoscopic retrograde cholangiopancreatography. J. Gastroenterol. Hepatol. 2006, 21, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Liu, H.; Li, W.; Shen, J. Spray of peppermint oil on papilla shortens the cannulation time of endoscopic retrograde cholangiopancreatography (ERCP): A randomized study. Int. J. Clin. Exp. Med. 2019, 12, 2813–2818. [Google Scholar]
- Shavakhi, A.; Ardestani, S.K.; Taki, M.; Goli, M.; Keshteli, A.H. Premedication with peppermint oil capsules in colonoscopy: A double blind placebo-controlled randomized trial study. Acta Gastroenterol. Belg. 2012, 75, 349–353. [Google Scholar] [PubMed]
- Al Moussawi, H.; Al Khatib, M.; El Ahmar, M.; Al Masri, H.; Leddy, A.; Akel, T.; Khalil, A. The effect of premedication with peppermint oil capsules (Colpermin) prior to colonoscopy: A double blind randomized placebo-controlled trial. Arab. J. Gastroenterol. 2017, 18, 220–223. [Google Scholar] [CrossRef]
- Han, J.Y.; Moosvi, Z.; Duh, E.; Park, S.; Albers, G.C.; Samarasena, J.B.; Karnes, W. Oral IBGard™ Before Colonoscopy: A Single-Center Double-Blinded, Randomized, Placebo-Controlled Trial. Dig. Dis. Sci. 2021, 66, 1611–1619. [Google Scholar] [CrossRef]
- IBgard®. Available online: https://www.nestlemedicalhub.com/products/ibgard (accessed on 28 November 2024).
- Colpermin™. Available online: https://www.tillotts.com/products/colpermin/ (accessed on 28 November 2024).
- Merat, S.; Khalili, S.; Mostajabi, P.; Ghorbani, A.; Ansari, R.; Malekzadeh, R. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig. Dis. Sci. 2010, 55, 1385–1390. [Google Scholar] [CrossRef]
- Lucak, S.; Chang, L.; Halpert, A.; Harris, L.A. Current and emergent pharmacologic treatments for irritable bowel syndrome with diarrhea: Evidence-based treatment in practice. Ther. Adv. Gastroenterol. 2017, 10, 253–275. [Google Scholar] [CrossRef]
- Sugino, S.; Inoue, K.; Kobayashi, R.; Hirose, R.; Doi, T.; Harusato, A.; Dohi, O.; Yoshida, N.; Uchiyama, K.; Ishikawa, T.; et al. Association Between the Cool Temperature-dependent Suppression of Colonic Peristalsis and Transient Receptor Potential Melastatin 8 Activation in Both a Randomized Clinical Trial and an Animal Model. J. Neurogastroenterol. Motil. 2022, 28, 693–705. [Google Scholar] [CrossRef]
- Nemoto, D.; Suzuki, S.; Mori, H.; Katsuki, S.; Iwaki, T.; Aizawa, M.; Takeuchi, Y.; Uraoka, T.; Matsuda, T.; Fujita, T.; et al. Inhibitory effect of lidocaine on colonic spasm during colonoscopy: A multicenter double-blind, randomized controlled trial. Dig. Endosc. 2018, 31, 173–179. [Google Scholar] [CrossRef]
- Nemoto, D.; Utano, K.; Isohata, N.; Endo, S.; Kumamoto, K.; Koshimizu, T.A.; Lefor, A.; Togashi, K. Topical lidocaine inhibits spasm during colonoscopy: A double-blind, randomized controlled trial (with video). Endosc. Int. Open. 2017, 5, E402–E407. [Google Scholar] [CrossRef]
- Gubbiotti, A.; Spadaccini, M.; Badalamenti, M.; Hassan, C.; Repici, A. Key factors for improving adenoma detection rate. Expert. Rev. Gastroenterol. Hepatol. 2022, 16, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Rondonotti, E.; Andrealli, A.; Amato, A.; Paggi, S.; Conti, C.B.; Spinzi, G.; Radaelli, F. Technical interventions to increase adenoma detection rate in colonoscopy. Expert. Rev. Gastroenterol. Hepatol. 2016, 10, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Bond, J.H.; Winawer, S.; Levin, T.R.; Burt, R.W.; Johnson, D.A.; Kirk, L.M.; Litlin, S.; Lieberman, D.A.; Waye, J.D.; et al. Quality in the technical performance of colonoscopy the continuous quality improvement process for colonoscopy: Recommendations of the, U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2002, 97, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, R.; Zulfiqar, L.; Gangwani, M.K.; Aziz, M. Adenoma detection rate vs. adenoma per colonoscopy as quality indicators for colon cancer screening. Transl. Gastroenterol. Hepatol. 2023, 8, 24. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.J.; Hyun, J.H.; Han, K.S.; Kim, B.C.; Hong, C.W.; Lee, S.J.; Sohn, D.K. Correlation Between Bowel Preparation and the Adenoma Detection Rate in Screening Colonoscopy. Ann. Coloproctol. 2017, 33, 93–98. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, S.; Huang, W.; Zhang, Y.; Liu, Y.; Yu, X.; Shen, L. The Role and Function of TRPM8 in the Digestive System. Biomolecules 2024, 14, 877. [Google Scholar] [CrossRef]
- Aziz, M.; Sharma, S.; Ghazaleh, S.; Fatima, R.; Acharya, A.; Ghanim, M.; Sheikh, T.; Lee-Smith, W.; Hamdani, S.U.; Nawras, A. The anti-spasmodic effect of peppermint oil during colonoscopy: A systematic review and meta-analysis. Minerva Gastroenterol. Dietol. 2020, 66, 164–171. [Google Scholar] [CrossRef]
- You, Q.; Li, L.; Chen, H.; Lin, C.; Chen, X.; Liu, Y. L-Menthol for Gastrointestinal Endoscopy: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2020, 11, e00252. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; Wiley: Chichester, UK, 2009; Chapter 20. [Google Scholar]
- Hegyi, P.; Petersen, O.H.; Holgate, S.; Erőss, B.; Garami, A.; Szakács, Z.; Dobszai, D.; Balaskó, M.; Kemény, L.; Peng, S.; et al. Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. J. Clin. Med. 2020, 9, e1532. [Google Scholar] [CrossRef]
- Hegyi, P.; Erőss, B.; Izbéki, F.; Párniczky, A.; Szentesi, A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021, 27, 1317–1319. [Google Scholar] [CrossRef]
| Study | (1) Country, (2) Number of Centers, (3) Registration Number | Study Type | Endoscopy Type | Purpose of Endoscopy | Criteria Used to Evaluate Peristalsis | Sample Size (n) | Intervention Type, Dosage | Application Site | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
Al Moussawi et al., 2017 [72]  | Lebanon, 1, NI | double-blind RCT | colonoscopy | diarrhea, bloody diarrhea, iron deficiency anemia, abdominal pain, rectorrhagia | degree of colonic spasm (no movement, minimal, mild, moderate, or marked) | I: 39 C: 39 | I: Colpermin® 374 mg C: placebo comprised of vitamin B12 | orally | PNP, EIE, TAE |
| Asao et al., 2001 [30] | Japan, 1, NI | cohort | colonoscopy | positive test for occult blood in feces, abnormal barium enema, or surveillance after previous polypectomies | NI | I: 409 C: 36 | I: peppermint oil solution C: placebo | cecum | ADR, TAE, |
| Dhillon et al., 2018 [51] ‡ | Canada, 1, NI | double-blind RCT | colonoscopy | screening | NI | I: 61 C: 61 | I: L-menthol solution; C: placebo (water +simethicone) | cecum | ADR, PNP |
| Fujishiro et al., 2014 [57] | Japan, 8, NCT 01411176 | double-blind RCT | upper endoscopy | determine the treatment strategy (EMR or ESD) | the modified version of Niwa’s Classification | I: 42 C: 41 | I: L-menthol solution; 160 mg (0.8%, 20 mL); C: placebo | gastric antrum | PNP, PNMP, TAE, TADR |
Han et al., 2021 [73]  | USA, 1, NA | double-blind RCT | colonoscopy | screening, positive fecal occult blood test or fecal immunochemical test, surveillance for history of colorectal polyps | classification of colonic peristalsis (0–2) | I: 102 C: 90 | I: IBGard™ 180 mg C: placebo containing sucrose | orally | ADR, EIE, WT, TAE |
| Hiki et al., 2011 [58] | Japan, 6, NCT 00742599 | double-blind RCT | upper endoscopy | required treatment or follow-up for confirmed or suspected upper GI disease | the modified version of Niwa’s Classification | I: 45 C: 42 | I: L-menthol solution; 160 mg (0.8%, 20 mL); C: placebo | gastric mucosa/antrum | PNP, PNMP, EIE, TAE, TADR |
| Hiki et al., 2011 [63] | Japan, 6, NI | double-blind RCT | upper endoscopy | assessing the tolerability and pharmacokinetics of the intervention | NI | I1: 6 I2: 6 I3: 6 C: 6 | I: L-Menthol solution; 80 mg (10 mL) 160 mg (20 mL) 320 mg (40 mL) C: placebo | gastric mucosa | GPPM, TAE |
| Hiki et al., 2012 [59] | Japan, multi, NI | double-blind RCT | upper endoscopy | required gastric endoscopy | Niwa’s Classification | I: 87 C: 29 | I: L-menthol solution; 80 mg (0.4%, 20 mL); 160 mg (0.8%, 20 mL); 320 mg (1.6%, 20 mL) C: placebo | gastric antrum | PNP, PNMP, EIE, TAE, TADR |
| Imagawa et al., 2012 [64]  | Japan, 2, UMIN 000004710 | non-randomized prospective study | upper endoscopy | scheduled to undergo EGD were recruited | Niwa’s Classification | I: 1893 C: 156 | I: L-menthol solution; 1.6%, (20 mL) C: placebo | gastric antrum | AS |
| Inoue et al., 2014 [53] | Japan, 1, UMIN 000007972 | single-blind prospective RCT | colonoscopy | screening, positive fecal occult blood test follow-up, or postendoscopic resection surveillance | classification of colonic peristalsis (0–3) | I: 118 C: 108 | I: L-menthol solution; 320 mg (1.6%, 20 mL); C: placebo (water + dimeticone) | cecum | ADR, PNP, PNMP, WT, TAE, TADR |
| Inoue et al., 2020 [54] | Japan, 1, UMIN 000023383 | single-blind prospective RCT | colonoscopy | screening, positive fecal occult blood test follow-up, or postendoscopic resection surveillance | classification of colonic peristalsis (0–3) | I: 309 C: 302 | I: L-menthol solution + CO2/air; 160 mg (0.8%, 20 mL) C: placebo (water + dimeticone) + CO2/air | cecum | ADR, PNP, PNMP, WT, TAE |
| Meng et al., 2021 [60] | China, 5, NCT 03263910 | double-blind RCT | upper endoscopy | advised for UE examination or follow-up for confirmed or suspected upper GI disease | modified version of Niwa’s classification | I: 109 C: 111 | I: L-menthol solution; 160 mg (0.8%, 20 mL); C: placebo | gastric mucosa | PNP, PNMP, EIE, TAE, TADR |
| Mori et al., 2014 [61] | Japan, 1, UMIN 000010859 | prospective open-label RCT | upper endoscopy | scheduled to screening or follow-up for upper gastrointestinal disease | modified version of Niwa’s classification | I: 49 C: 49 | I: L-menthol solution; 160 mg (0.8%, 20 mL); C: placebo (water + dimeticone) | gastric mucosa | PNMP, TAE |
| NCT 02588248, 2015 [52] § | USA, 1, NCT 02588248 | prospective, double-blind RCT | colonoscopy | primary colorectal cancer screening or survillance | NI | I: 37 C: 38 | I: peppermint oil solution (0.8% L-menthol, 20 mL) + simethicone C: simethicone | cecum | ADR, WT, TAE |
| Sattayaler-tyanyong et al., 2023 [55] ‡ | Thailand, 1, NCT 05559814 | double-blind RCT | colonoscopy | screening endoscopy | classification of colonic peristalsis (0–3) | I: 204 C: 204 | I: peppermint oil solution (0.8% L-menthol, 50 mL) + simethicone C: simethicone | cecum | ADR, PNP, PNMP, EIE, WT, TAE |
| Shah et al., 2019 [56] | USA, 1, NCT 03286764 | double-blind RCT | colonoscopy | initial screening colonoscopy | classification of colonic peristalsis (0–3) | I: 24 C: 24 | I: peppermint oil solution (0.8% L-menthol, 50 mL) C: placebo (water + simethicone) | cecum | ADR, PNP, PNMP, WT, TAE |
Shavakhi et al., 2012 [71]  | Iran, 1, IRCT201107056957N1 | prospective, double-blind RCT | colonoscopy | diagnostic or screening colonoscopy | colonic spasm score (0–4) | I: 33 C: 32 | I: Colpermin® 374 mg C: placebo with lactulose | orally | PNP, TAE |
| Yang et al., 2022 [62] | Taiwan, 1, NCT 04593836 | prospective, double-blind RCT | upper endoscopy | screening endoscopy | modified version of Niwa’s classification | I: 26 C: 26 | I: L-menthol solution; 160 mg (0.8%, 20 mL); C: placebo (olive oil) | gastric mucosa | PNP, PNMP, EIE, TAE |
| Yoshida et al., 2014 [65]  | Japan, 1, UMIN 000008317 | retrospective study | colonoscopy | patients with severe colonic spasm | NI | I: 65 C: 27 | I: 0.8% L-menthol solution C: water | cecum, ascending colon, transverse colon, descending colon, sigmoid colon, rectum, | TAE, PNP |
 Study included only in the systematic review. ‡ Conference abstract. § Unpublished study from clinical trial registry with reported results.
Study included only in the systematic review. ‡ Conference abstract. § Unpublished study from clinical trial registry with reported results.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gergő, D.; Tóth-Mészáros, A.; Schulze Wenning, A.; Fehérvári, P.; Do To, U.N.; Hegyi, P.; Erőss, B.; Ványolós, A.; Csupor, D. Efficacy and Safety of L-Menthol During Gastrointestinal Endoscopy—A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Med. 2025, 14, 4296. https://doi.org/10.3390/jcm14124296
Gergő D, Tóth-Mészáros A, Schulze Wenning A, Fehérvári P, Do To UN, Hegyi P, Erőss B, Ványolós A, Csupor D. Efficacy and Safety of L-Menthol During Gastrointestinal Endoscopy—A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Journal of Clinical Medicine. 2025; 14(12):4296. https://doi.org/10.3390/jcm14124296
Chicago/Turabian StyleGergő, Dorottya, Andrea Tóth-Mészáros, Alexander Schulze Wenning, Péter Fehérvári, Uyen Nguyen Do To, Péter Hegyi, Bálint Erőss, Attila Ványolós, and Dezső Csupor. 2025. "Efficacy and Safety of L-Menthol During Gastrointestinal Endoscopy—A Systematic Review and Meta-Analysis of Randomized Clinical Trials" Journal of Clinical Medicine 14, no. 12: 4296. https://doi.org/10.3390/jcm14124296
APA StyleGergő, D., Tóth-Mészáros, A., Schulze Wenning, A., Fehérvári, P., Do To, U. N., Hegyi, P., Erőss, B., Ványolós, A., & Csupor, D. (2025). Efficacy and Safety of L-Menthol During Gastrointestinal Endoscopy—A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Journal of Clinical Medicine, 14(12), 4296. https://doi.org/10.3390/jcm14124296