Investigating Gamma Frequency Band PSD in Alzheimer’s Disease Using qEEG from Eyes-Open and Eyes-Closed Resting States
Abstract
1. Introduction
2. Methods
2.1. Demographics of the Participants
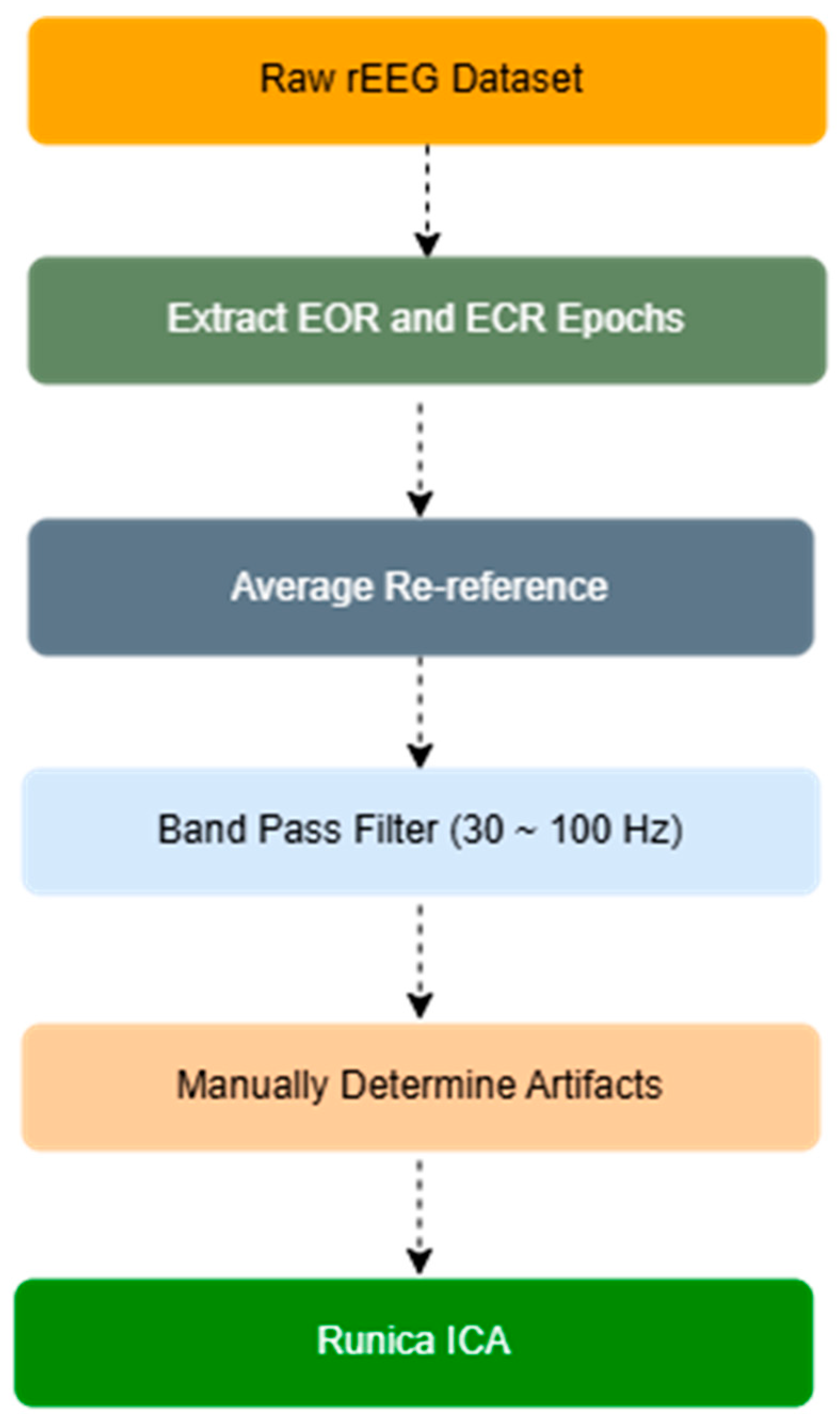
2.2. EEG Signals and Preprocessing
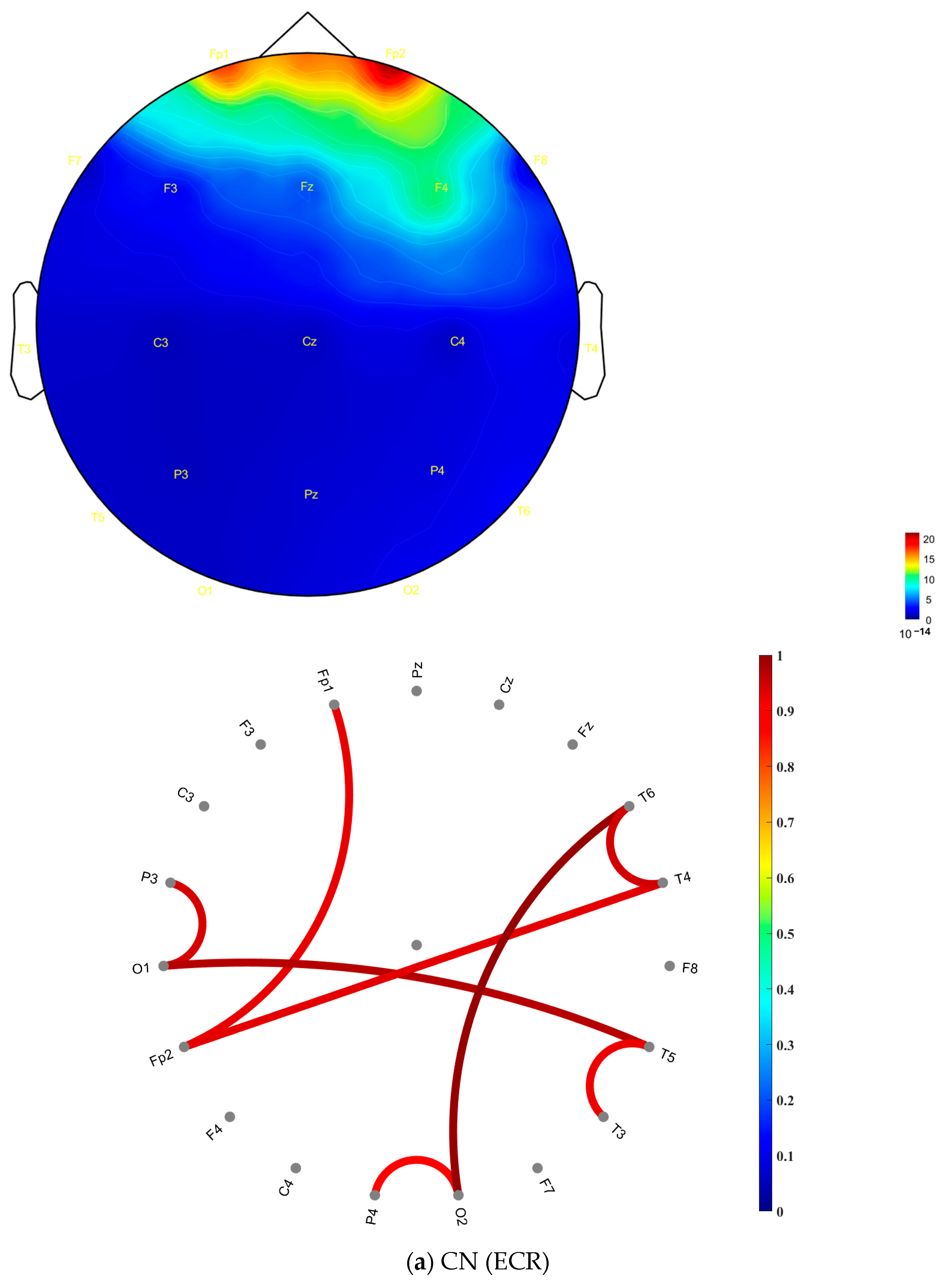
2.3. Spectral and Functional Connectivity Analysis
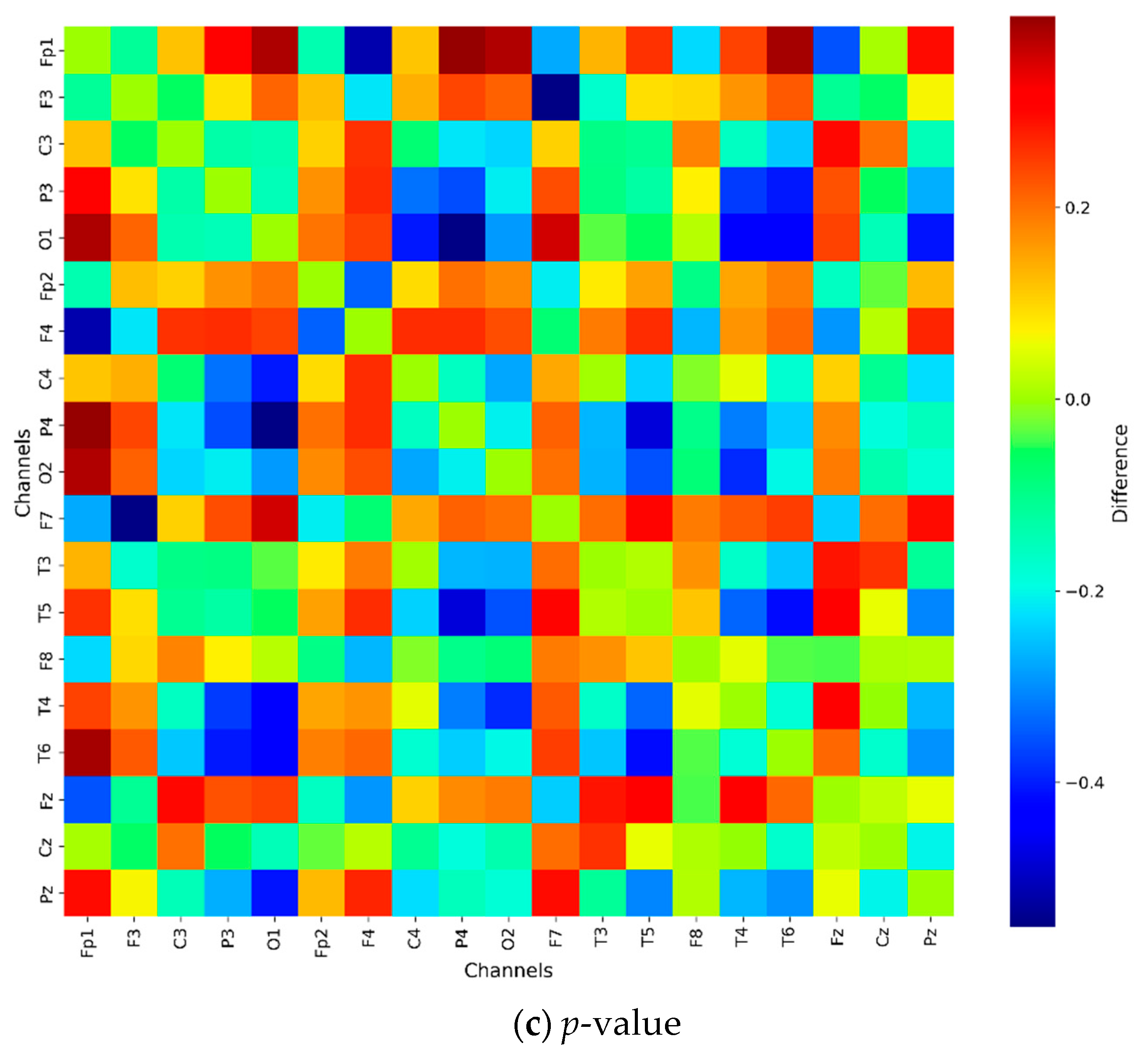
2.4. Statistical Analysis
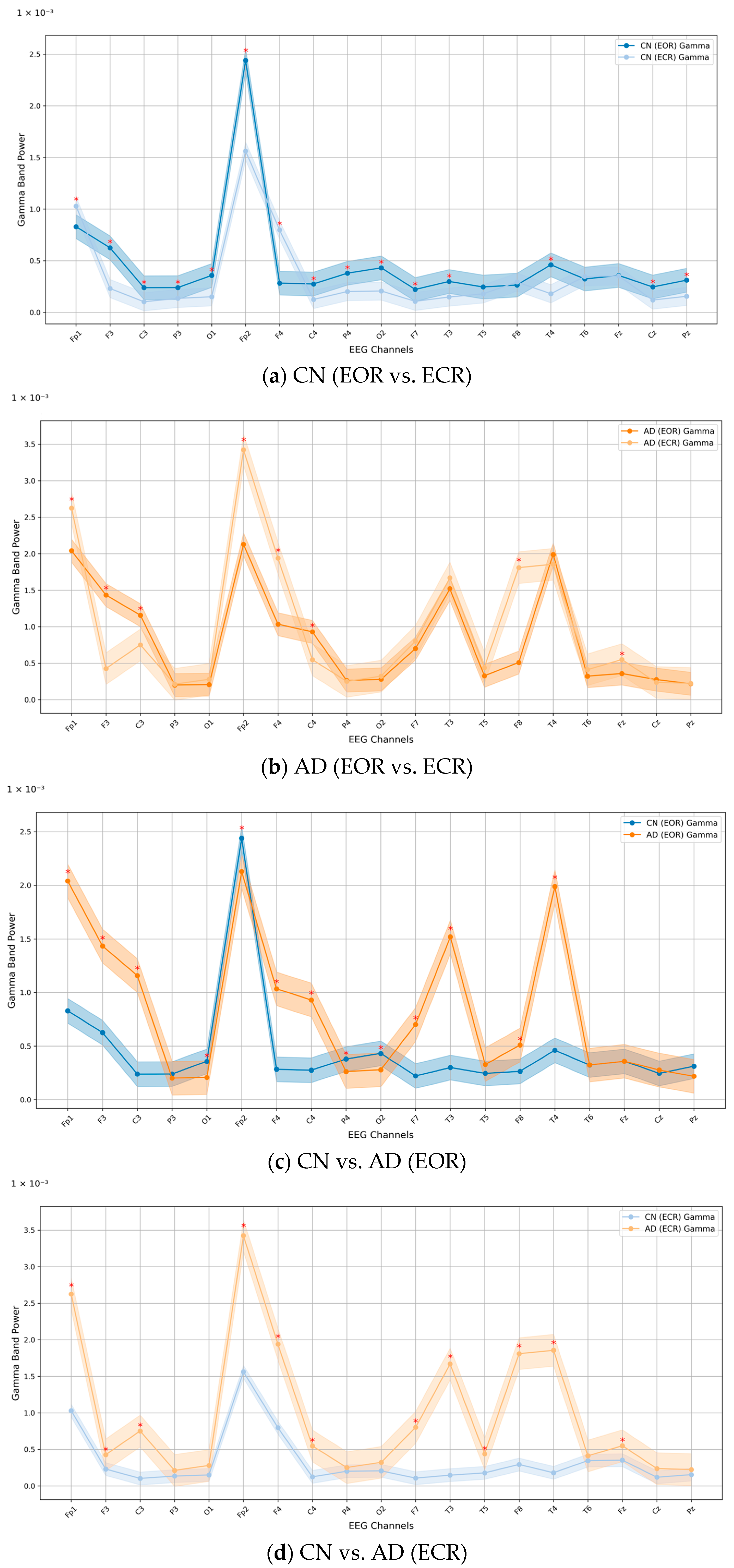
3. Results
Relative PSD Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mably, A.J.; Colgin, L.L. Gamma oscillations in cognitive disorders. Curr. Opin. Neurobiol. 2018, 52, 182–187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fries, P.; Reynolds, J.H.; Rorie, A.E.; Desimone, R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 2001, 291, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Bartos, M.; Vida, I.; Jonas, P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 2007, 8, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Verret, L.; Mann, E.O.; Hang, G.B.; Barth, A.M.; Cobos, I.; Ho, K.; Devidze, N.; Masliah, E.; Kreitzer, A.C.; Mody, I.; et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 2012, 149, 708–721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinez-Losa, M.; Tracy, T.E.; Ma, K.; Verret, L.; Clemente-Perez, A.; Khan, A.S.; Cobos, I.; Ho, K.; Gan, L.; Mucke, L.; et al. Nav1.1-Overexpressing Interneuron Transplants Restore Brain Rhythms and Cognition in a Mouse Model of Alzheimer’s Disease. Neuron 2018, 98, 75–89.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stam, C.J.; Van Cappellen van Walsum, A.M.; Pijnenburg, Y.A.; Berendse, H.W.; de Munck, J.C.; Scheltens, P.; van Dijk, B.W. Generalized synchronization of MEG recordings in Alzheimer’s Disease: Evidence for involvement of the gamma band. J. Clin. Neurophysiol. 2002, 19, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Demiralp, T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophysiol. 2005, 116, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, J.A.; Vuurman, E.F.; Verhey, F.R.; Van Kranen-Mastenbroek, V.H.; Riedel, W.J. Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J. Neural. Transm. 2008, 115, 1301–1311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Fang, Y.; Wang, X.; Yang, H.; Yu, X.; Wang, H. Enhanced Gamma Activity and Cross-Frequency Interaction of Resting-State Electroencephalographic Oscillations in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osipova, D.; Pekkonen, E.; Ahveninen, J. Enhanced magnetic auditory steady-state response in early Alzheimer’s disease. Clin. Neurophysiol. 2006, 117, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Murty, D.V.P.S.; Manikandan, K.; Kumar, W.S.; Ramesh, R.G.; Purokayastha, S.; Javali, M.; Rao, N.P.; Ray, S. Gamma oscillations weaken with age in healthy elderly in human EEG. Neuroimage 2020, 215, 116826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Böttger, D.; Herrmann, C.S.; Von Cramon, D.Y. Amplitude differences of evoked alpha and gamma oscillations in two different age groups. Int. J. Psychophysiol. 2002, 45, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Erdal, F.; Bölükbaş, B.; Hanoğlu, L.; Yener, G.; Duygun, R. Alterations of resting-state Gamma frequency characteristics in aging and Alzheimer’s disease. Cogn. Neurodyn. 2023, 17, 829–844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palop, J.J.; Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016, 17, 777–792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Başar, E.; Femir, B.; Emek-Savaş, D.D.; Güntekin, B.; Yener, G.G. Increased long distance event-related gamma band connectivity in Alzheimer’s disease. Neuroimage Clin. 2017, 14, 580–590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2022, 25, 5789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Pearson, H.A.; Peers, C. Physiological roles for amyloid beta peptides. J. Physiol. 2006, 575 Pt 1, 5–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koenig, T.; Prichep, L.; Dierks, T.; Hubl, D.; Wahlund, L.O.; John, E.R.; Jelic, V. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2005, 26, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J.; Montez, T.; Jones, B.F.; Rombouts, S.A.; Van der Made, Y.; Pijnenburg, Y.A.; Scheltens, P. Disturbed fluctuations of resting state EEG synchronization in Alzheimer’s disease. Clin. Neurophysiol. 2005, 116, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.M.; Hall, J.R.; Cornwell, S.L.; Falk, J.D. Addition of EEG improves accuracy of a logistic model that uses neuropsychological and cardiovascular factors to identify dementia and MCI. Psychiatry Res. 2011, 186, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Babiloni, C.; Lizio, R.; Fallani Fde, V.; Blinowska, K.; Verrienti, G.; Frisoni, G.; Rossini, P.M. Resting state cortical EEG rhythms in Alzheimer’s disease: Toward EEG markers for clinical applications: A review. Suppl. Clin. Neurophysiol. 2013, 62, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Jelic, V.; Johansson, S.E.; Almkvist, O.; Shigeta, M.; Julin, P.; Nordberg, A.; Winblad, B.; Wahlund, L.O. Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol Aging 2000, 21, 533–540. [Google Scholar] [CrossRef]
- Hernán Díaz, M.; Maureira, F.; Cordova, F. Time series of closed and open eyes EEG conditions reveal differential characteristics in the temporality of linear and non-linear analysis domain. Procedia Comput. Sci. 2018, 139, 570–577. [Google Scholar] [CrossRef]
- Barry, R.J.; De Blasio, F.M. EEG differences between eyes-closed and eyes-open resting remain in healthy ageing. Biol. Psychol. 2017, 129, 293–304. [Google Scholar] [CrossRef]
- D’Atri, A.; Scarpelli, S.; Gorgoni, M.; Truglia, I.; Lauri, G.; Cordone, S.; Ferrara, M.; Marra, C.; Rossini, P.M.; De Gennaro, L. EEG alterations during wake and sleep in mild cognitive impairment and Alzheimer’s disease. iScience 2021, 24, 102386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elgendi, M.; Vialatte, F.; Cichocki, A.; Latchoumane, C.; Jeong, J.; Dauwels, J. Optimization of EEG frequency bands for improved diagnosis of Alzheimer disease. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 2011, 6087–6091. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.; Li, R.; Zhou, H.; Qing, K.; Liu, H.; Pan, H.; Lei, Y.; Fu, W.; Wang, X.; Xiao, X.; et al. Neural biomarker diagnosis and prediction to mild cognitive impairment and Alzheimer’s disease using EEG technology. Alzheimers Res. Ther. 2023, 15, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Christensen, K.J.; Multhaup, K.S.; Nordstrom, S.; Voss, K. A cognitive battery for dementia: Development and measurement characteristics. Psycholog. Assess 1991, 3, 168. [Google Scholar] [CrossRef]
- Chin, J.; Park, J.; Yang, S.J.; Yeom, J.; Ahn, Y.; Baek, M.J.; Ryu, H.J.; Lee, B.H.; Han, N.E.; Ryu, K.H.; et al. Re-standardization of the Korean-Instrumental Activities of Daily Living (K-IADL): Clinical Usefulness for Various Neurodegenerative Diseases. Dement Neurocogn. Disord. 2018, 17, 11–22. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kang, J.M.; Lee, H.; Kim, K.; Kim, S.; Yu, T.Y.; Lee, E.M.; Kim, C.T.; Kim, D.K.; Lewis, M.; et al. Subjective cognitive decline and subsequent dementia: A nationwide cohort study of 579,710 people aged 66 years in South Korea. Alzheimers Res. Ther. 2020, 12, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahn, H.J.; Chin, J.; Park, A.; Lee, B.H.; Suh, M.K.; Seo, S.W.; Na, D.L. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): A useful tool for assessing and monitoring cognitive impairments in dementia patients. J. Korean Med. Sci. 2010, 25, 1071–1076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryu, H.J.; Yang, D.W. The Seoul Neuropsychological Screening Battery (SNSB) for Comprehensive Neuropsychological Assessment. Dement. Neurocogn. Disord. 2023, 22, 1–15. [Google Scholar] [CrossRef]
- Dong, L.; Liu, X.; Zhao, L.; Lai, Y.; Gong, D.; Liu, T.; Yao, D. A Comparative Study of Different EEG Reference Choices for Event-Related Potentials Extracted by Independent Component Analysis. Front. Neurosci. 2019, 13, 1068. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Simfukwe, C.; Han, S.H.; Jeong, H.T.; Youn, Y.C. qEEG as Biomarker for Alzheimer’s Disease: Investigating Relative PSD Difference and Coherence Analysis. Neuropsychiatr. Dis. Treat 2023, 19, 2423–2437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, X.; Wang, B.; Liu, H.; Wu, W.; Sun, J.; Fang, W.; Jiang, R.; Hu, Y.; Jin, C.; Wei, X.; et al. Diagnosis of Alzheimer’s disease via resting-state EEG: Integration of spectrum, complexity, and synchronization signal features. Front. Aging Neurosci. 2023, 15, 1288295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zawiślak-Fornagiel, K.; Ledwoń, D.; Bugdol, M.; Grażyńska, A.; Ślot, M.; Tabaka-Pradela, J.; Bieniek, I.; Siuda, J. Quantitative EEG Spectral and Connectivity Analysis for Cognitive Decline in Amnestic Mild Cognitive Impairment. J. Alzheimers Dis. 2024, 97, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhao, Y.; Shan, X.; Blackburn, D.; Wei, J.; Erkoyuncu, J.A.; Chen, L.; Sarrigiannis, P.G. Ultra-high-resolution time-frequency analysis of EEG to characterise brain functional connectivity with the application in Alzheimer’s disease. J. Neural. Eng. 2022, 19, 046034. [Google Scholar] [CrossRef] [PubMed]
- Abuhassan, K.; Coyle, D.; Maguire, L. Compensating for thalamocortical synaptic loss in Alzheimer’s disease. Front. Comput. Neurosci. 2014, 8, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Q.; Li, Y.; Wu, Y.; Lin, X.; Zhao, X. Brain network hierarchy reorganization in Alzheimer’s disease: A resting-state functional magnetic resonance imaging study. Hum. Brain Mapp. 2022, 43, 3498–3507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Powell, F.; Tosun, D.; Raj, A. Alzheimer’s Disease Neuroimaging Initiative. Network-constrained technique to characterize pathology progression rate in Alzheimer’s disease. Brain Commun. 2021, 3, fcab144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jensen, O.; Kaiser, J.; Lachaux, J.P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 2007, 30, 317–324. [Google Scholar] [CrossRef]
- Barnes, J.; Bartlett, J.W.; van de Pol, L.A.; Loy, C.T.; Scahill, R.I.; Frost, C.; Thompson, P.; Fox, N.C. A meta-analysis of hippocampal atrophy rates in Alzheimer’s disease. Neurobiol. Aging 2009, 30, 1711–1723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, Y.; Hu, Y.; Huang, K. Alzheimer’s Disease Neuroimaging Initiative. Atrophy of hippocampal subfields relates to memory decline during the pathological progression of Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1287122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calderon, J.; Perry, R.J.; Erzinclioglu, S.W.; Berrios, G.E.; Dening, T.R.; Hodges, J.R. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2001, 70, 157–164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Traikapi, A.; Konstantinou, N. Gamma Oscillations in Alzheimer’s Disease and Their Potential Therapeutic Role. Front. Syst. Neurosci. 2021, 15, 782399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, K.; Park, Y.; Suh, S.W.; Kim, S.S.; Kim, D.W.; Lee, J.; Park, J.; Yoo, S.; Kim, K.W. Optimal flickering light stimulation for entraining gamma waves in the human brain. Sci. Rep. 2021, 11, 16206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiesman, A.I.; Murman, D.L.; May, P.E.; Schantell, M.; Wolfson, S.L.; Johnson, C.M.; Wilson, T.W. Visuospatial alpha and gamma oscillations scale with the severity of cognitive dysfunction in patients on the Alzheimer’s disease spectrum. Alzheimers Res. Ther. 2021, 13, 139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bi, D.; Wen, L.; Wu, Z.; Shen, Y. GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer’s disease. Alzheimers Dement. 2020, 16, 1312–1329. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Synaptic depression and aberrant excitatory network activity in Alzheimer’s disease: Two faces of the same coin? Neuromolecular. Med. 2010, 12, 48–55. [Google Scholar] [CrossRef]
- Ahmad, J.; Ellis, C.; Leech, R.; Voytek, B.; Garces, P.; Jones, E.; Buitelaar, J.; Loth, E.; Dos Santos, F.P.; Amil, A.F.; et al. From mechanisms to markers: Novel noninvasive EEG proxy markers of the neural excitation and inhibition system in humans. Transl. Psychiatry 2022, 12, 467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naito, N.; Hirosawa, T.; Tsubomoto, M.; Miyagishi, Y.; Kikuchi, M. Prominent gamma band activity during visual motion perception in early-stage Alzheimer’s disease. PLoS ONE 2022, 17, e0266693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manippa, V.; Palmisano, A.; Filardi, M.; Vilella, D.; Nitsche, M.A.; Rivolta, D.; Logroscino, G. An update on the use of gamma (multi)sensory stimulation for Alzheimer’s disease treatment. Front. Aging Neurosci. 2022, 14, 1095081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, W.S.; Manikandan, K.; Murty, D.V.P.S.; Ramesh, R.G.; Purokayastha, S.; Javali, M.; Rao, N.P.; Ray, S. Stimulus-Induced Narrowband Gamma Oscillations are Test-Retest Reliable in Human EEG. Cereb. Cortex Commun. 2022, 3, tgab066. [Google Scholar] [CrossRef]
- Ray, S.; Maunsell, J.H. Do gamma oscillations play a role in cerebral cortex? Trends Cogn. Sci. 2015, 19, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235, Erratum in: Nature 2018, 562, E1. https://doi.org/10.1038/s41586-018-0351-4; Erratum in: Nature 2024, 636, E2. https://doi.org/10.1038/s41586-024-08343-7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martorell, A.J.; Paulson, A.L.; Suk, H.J.; Abdurrob, F.; Drummond, G.T.; Guan, W.; Young, J.Z.; Kim, D.N.; Kritskiy, O.; Barker, S.J.; et al. Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition. Cell 2019, 177, 256–271.e22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garza, K.M.; Zhang, L.; Borron, B.; Wood, L.B.; Singer, A.C. Gamma Visual Stimulation Induces a Neuroimmune Signaling Profile Distinct from Acute Neuroinflammation. J. Neurosci. 2020, 40, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Hansen, A.K.; Parbo, P.; Brændgaard, H.; Gottrup, H.; Brooks, D.J.; Borghammer, P. The Effect of 40-Hz Light Therapy on Amyloid Load in Patients with Prodromal and Clinical Alzheimer’s Disease. Int. J. Alzheimers. Dis. 2018, 30, 6852303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Q.; Colon-Motas, K.M.; Pybus, A.F.; Piendel, L.; Seppa, J.K.; Walker, M.L.; Manzanares, C.M.; Qiu, D.; Miocinovic, S.; Wood, L.B.; et al. A feasibility trial of gamma sensory flicker for patients with prodromal Alzheimer’s disease. Alzheimers Dement. 2021, 7, e12178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, M.; McDermott, B.; Oliveira, B.L.; O’Brien, A.; Coogan, D.; Lang, M.; Moriarty, N.; Dowd, E.; Quinlan, L.; Mc Ginley, B.; et al. Gamma Band Light Stimulation in Human Case Studies: Groundwork for Potential Alzheimer’s Disease Treatment. J. Alzheimers Dis. 2019, 70, 171–185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]






| Group | EOR (Mean ± SD) | ECR (Mean ± SD) | p-Value | Cohen’s d |
|---|---|---|---|---|
| CN (n = 269) | 0.0040 ± 0.0030 | 0.0042 ± 0.0010 | <0.001 | 0.0894 |
| AD (n = 265) | 0.0060 ± 0.0080 | 0.0860 ± 0.0590 | <0.001 | 1.9000 |
| Condition | Model | Group/ Comparison | Gamma PSD (Mean ± SD) | β (Adjusted) | SE | p-Value |
|---|---|---|---|---|---|---|
| EOR | Unadjusted | CN | 0.0040 ± 0.0030 | <0.001 | ||
| AD | 0.0060 ± 0.0080 | <0.001 | ||||
| Adjusted | CN vs. AD | −0.0022 | 0.0033 | 0.504 | ||
| Age | −0.0003 | 0.0002 | 0.076 | |||
| ECR | Unadjusted | CN | 0.0042 ± 0.0010 | <0.001 | ||
| AD | 0.0860 ± 0.0590 | <0.001 | ||||
| Adjusted | CN vs. AD | −0.0047 | 0.0054 | 0.391 | ||
| Age | −0.0008 | 0.0003 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simfukwe, C.; An, S.S.A.; Youn, Y.C. Investigating Gamma Frequency Band PSD in Alzheimer’s Disease Using qEEG from Eyes-Open and Eyes-Closed Resting States. J. Clin. Med. 2025, 14, 4256. https://doi.org/10.3390/jcm14124256
Simfukwe C, An SSA, Youn YC. Investigating Gamma Frequency Band PSD in Alzheimer’s Disease Using qEEG from Eyes-Open and Eyes-Closed Resting States. Journal of Clinical Medicine. 2025; 14(12):4256. https://doi.org/10.3390/jcm14124256
Chicago/Turabian StyleSimfukwe, Chanda, Seong Soo A. An, and Young Chul Youn. 2025. "Investigating Gamma Frequency Band PSD in Alzheimer’s Disease Using qEEG from Eyes-Open and Eyes-Closed Resting States" Journal of Clinical Medicine 14, no. 12: 4256. https://doi.org/10.3390/jcm14124256
APA StyleSimfukwe, C., An, S. S. A., & Youn, Y. C. (2025). Investigating Gamma Frequency Band PSD in Alzheimer’s Disease Using qEEG from Eyes-Open and Eyes-Closed Resting States. Journal of Clinical Medicine, 14(12), 4256. https://doi.org/10.3390/jcm14124256







