Prognostic Impact of Pre-Treatment Modified Glasgow Prognostic Score (mGPS) on Survival in Patients with Advanced-Stage Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- The presence of synchronous malignancies;
- Evidence of acute infection or inflammatory conditions at the time of laboratory testing;
- Incomplete clinical or laboratory data;
- Receipt of neoadjuvant or experimental therapies outside the standard protocol.
2.2. Data Collection
2.3. mGPS Calculation
- mGPS 0: CRP ≤ 10 mg/L and albumin ≥ 35 g/L;
- mGPS 1: CRP > 10 mg/L and albumin ≥ 35 g/L;
- mGPS 2: CRP > 10 mg/L and albumin < 35 g/L.
2.4. Outcome Measures and Statistical Analysis
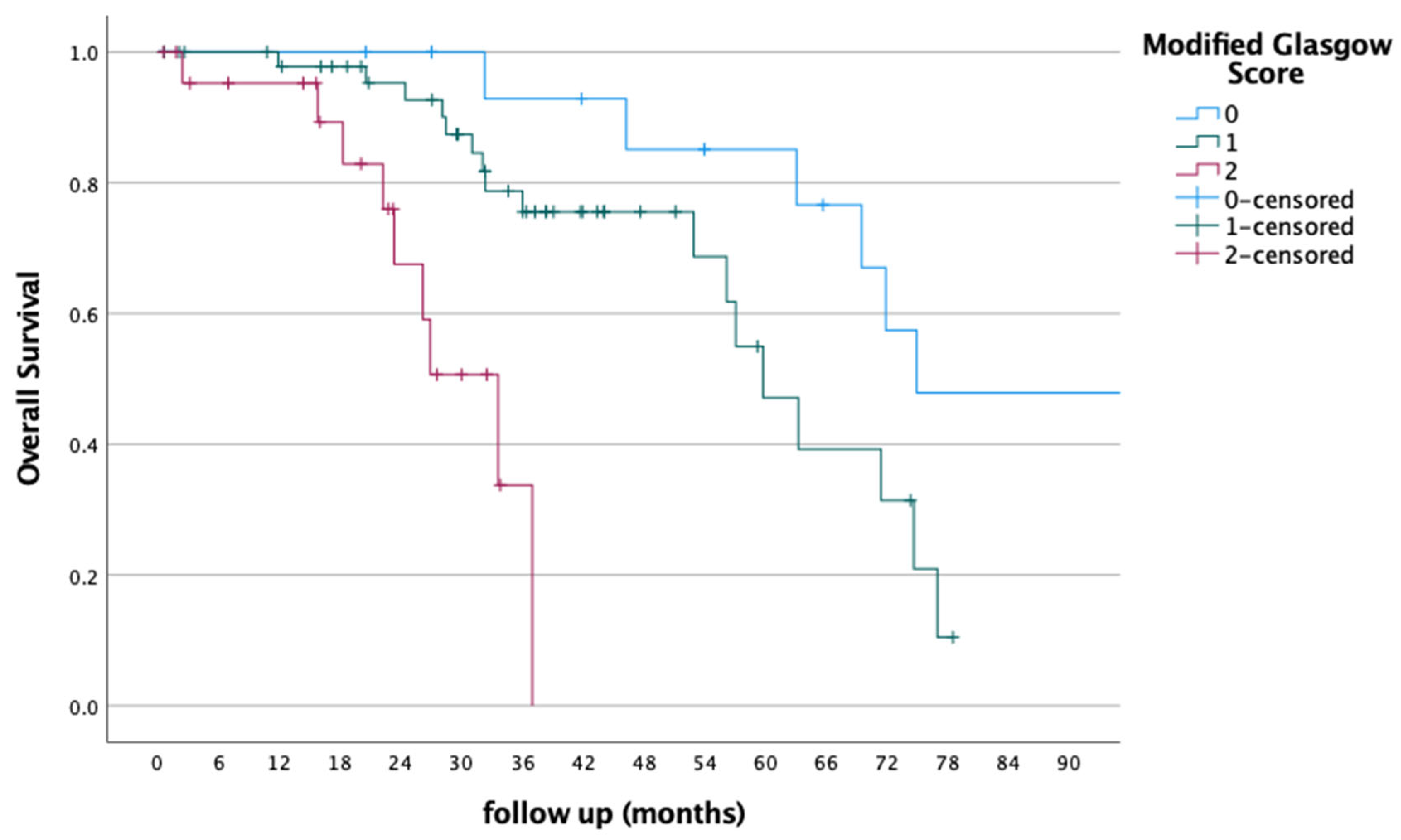
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Momenimovahed, Z.; Tiznobaik; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef]
- Al Rawahi, T.; Lopes, A.D.; E Bristow, R.; Bryant, A.; Elattar, A.; Chattopadhyay, S.; Galaal, K. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst. Rev. 2013, 2013, Cd008765. [Google Scholar] [CrossRef] [PubMed]
- van de Laar, R.; Zusterzeel, P.L.M.; Van Gorp, T.; Buist, M.R.; van Driel, W.J.; Gaarenstroom, K.N.; Arts, H.J.; van Huisseling, J.C.; Hermans, R.H.; Pijnenborg, J.M.; et al. Cytoreductive surgery followed by chemotherapy versus chemotherapy alone for recurrent platinum-sensitive epithelial ovarian cancer (SOCceR trial): A multicenter randomised controlled study. BMC Cancer 2014, 14, 22. [Google Scholar] [CrossRef]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Xue, F.; Pan, C.; Li, L. Integrative prognostic modeling of ovarian cancer: Incorporating genetic, clinical, and immunological markers. Discov. Oncol. 2025, 16, 115. [Google Scholar] [CrossRef]
- Wang, G.; Yang, H.; Wang, Y.; Qin, J. Ovarian cancer targeted therapy: Current landscape and future challenges. Front. Oncol. 2025, 15, 1535235. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, J.; Maass, N.; Bauerschlag, D.O.; Flörkemeier, I. Promising new drugs and therapeutic approaches for treatment of ovarian cancer—Targeting the hallmarks of cancer. BMC Med. 2025, 23, 10. [Google Scholar] [CrossRef]
- Marchetti, C.; Fagotti, A.; Fruscio, R.; Cassani, C.; Incorvaia, L.; Perri, M.T.; Sassu, C.M.; Camnasio, C.A.; Giudice, E.; Minucci, A.; et al. Benefit from maintenance with PARP inhibitor in newly diagnosed ovarian cancer according to BRCA1/2 mutation type and site: A multicenter real-world study. ESMO Open 2025, 10, 104533. [Google Scholar] [CrossRef]
- Roncolato, F.T.; Berton-Rigaud, D.; O’Connell, R.; Lanceley, A.; Sehouli, J.; Buizen, L.; Okamoto, A.; Aotani, E.; Lorusso, D.; Donnellan, P.; et al. Validation of the modified Glasgow Prognostic Score (mGPS) in recurrent ovarian cancer (ROC)—Analysis of patients enrolled in the GCIG Symptom Benefit Study (SBS). Gynecol. Oncol. 2018, 148, 36–41. [Google Scholar] [CrossRef]
- Kus, F.; Guven, D.C.; Yildirim, H.C.; Chalabiyev, E.; Koc, I.; Tatar, O.D.; Sirvan, F.; Sahin, Y.B.; Karaca, E.; Kabukcu, F. Comparative Analysis of Prognostic Potential of Pretreatment Blood-Based Biomarkers in Metastatic Bladder Cancer: Modified Glasgow Prognostic Score. J. Clin. Med. 2025, 14, 1954. [Google Scholar] [CrossRef]
- Hacker, U.T.; Hasenclever, D.; Baber, R.; Linder, N.; Busse, H.; Obermannova, R.; Zdrazilova-Dubska, L.; Valik, D.; Lordick, F. Modified Glasgow prognostic score (mGPS) is correlated with sarcopenia and dominates the prognostic role of baseline body composition parameters in advanced gastric and esophagogastric junction cancer patients undergoing first-line treatment from the phase III EXPAND trial. Ann. Oncol. 2022, 33, 685–692. [Google Scholar] [PubMed]
- Tan, D.; Li, J.; Lin, T.; Tan, P.; Zhang, J.; Xiong, Q.; Jiang, J.; Li, Y.; Zhang, P.; Wei, Q. Prognostic Utility of the Modified Glasgow Prognostic Score in Urothelial Carcinoma: Outcomes from a Pooled Analysis. J. Clin. Med. 2022, 11, 6261. [Google Scholar] [CrossRef]
- Jomrich, G.; Hollenstein, M.; John, M.; Baierl, A.; Paireder, M.; Kristo, I.; Ilhan-Mutlu, A.; Asari, R.; Preusser, M.; Schoppmann, S.F. The modified glasgow prognostic score is an independent prognostic indicator in neoadjuvantly treated adenocarcinoma of the esophagogastric junction. Oncotarget 2018, 9, 6968–6976. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Matsuda, T.; Sawada, R.; Hasegawa, H.; Yamashita, K.; Harada, H.; Urakawa, N.; Goto, H.; Kanaji, S.; Oshikiri, T.; et al. The modified Glasgow prognostic score is a reliable predictor of oncological outcomes in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy. Sci. Rep. 2023, 13, 17111. [Google Scholar] [CrossRef]
- Terazawa, K.; Ohashi, T.; Shibata, H.; Ishihara, T.; Ogawa, T. Immune-modified Glasgow prognostic score: A new prognostic marker for head and neck cancer. Head Neck 2022, 44, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Kus, F.; Guven, D.C.; Yildirim, H.C.; Chalabiyev, E.; Akyildiz, A.; Tatar, O.D.; Sahin, Y.B.; Ileri, S.; Karaca, E.; Kertmen, N.; et al. KELIM score predicts outcome in patients with platinum-resistant/refractory recurrent ovarian cancer. Biomark. Med. 2023, 17, 379–389. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Liu, J.; Kong, P.; Chen, S.; Zhan, Y.; Xu, D. Preoperative C-Reactive Protein/Albumin Ratio Predicts Prognosis of Patients after Curative Resection for Gastric Cancer. Transl. Oncol. 2015, 8, 339–345. [Google Scholar] [CrossRef]
- Saal, J.; Eckstein, M.; Ritter, M.; Brossart, P.; Hölzel, M.; Grünwald, V.; Klümper, N. The modified Glasgow Prognostic Score (mGPS) can guide decisions for immunotherapy treatment beyond progression. Eur. J. Cancer 2025, 215, 115163. [Google Scholar] [CrossRef]
- Tripathi, S.; Sharma, Y.; Kumar, D. Unveiling the link between chronic inflammation and cancer. Metab. Open 2025, 25, 100347. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Rozich, N.S.; Jones, C.E.; Morris, K.T. Malnutrition, frailty, and sarcopenia in pancreatic cancer patients: Assessments and interventions for the pancreatic surgeon. Ann. Pancreat. Cancer 2019, 2, 3. [Google Scholar] [CrossRef]
- Bilgin, B.; Kuralay, Y.; Yucel, S. Prognostic importance of prognostic nutritional index and modified Glasgow prognostic score in advanced lung cancer with targetable mutation. J. Cancer Res. Clin. Oncol. 2024, 150, 215. [Google Scholar] [CrossRef]
- Bacalbasa, N.; Petrea, S.; Gaspar, B.; Pop, L.; Varlas, V.; Hasegan, A.; Gorecki, G.; Martac, C.; Stoian, M.; Zgura, A.; et al. The Influence of Inflammatory and Nutritional Status on the Long-Term Outcomes in Advanced Stage Ovarian Cancer. Cancers 2024, 16, 2504. [Google Scholar] [CrossRef] [PubMed]
- Antasouras, G.; Papadopoulou, S.K.; Tolia, M.; Pandi, A.-L.; Spanoudaki, M.; Tsoukalas, N.; Tsourouflis, G.; Psara, E.; Mentzelou, M.; Giaginis, C. May Nutritional Status Positively Affect Disease Progression and Prognosis in Patients with Esophageal and Pharyngeal Cancers? A Scoping Review of the Current Clinical Studies. Med. Sci. 2023, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Guo, W.; Xu, W.; Zhang, X.; Shi, Z.; Zheng, L.; Zhao, W. Prognostic value of the Glasgow prognostic score in colorectal cancer: A meta-analysis of 9839 patients. Cancer Manag. Res. 2019, 11, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-R.; Kim, A.S.; Choi, H.-I.; Jung, J.-H.; Park, J.Y.; Ko, H.-J.; Coelho-Filho, O.R. Inflammatory markers for predicting overall survival in gastric cancer patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236445. [Google Scholar] [CrossRef]
- Guliyev, M.; Özkan, A.; Safarov, S.; Şen, G.A.; Günaltili, M.; FidAn, M.C.; DemirCi, N.S. Prognostic Significance of Inflammatory and Nutritional Biomarkers in Patients with Metastatic Gastric Cancer. J. Oncol. Sci. 2025, 11, 14–21. [Google Scholar] [CrossRef]
- Takamizawa, Y.; Shida, D.; Boku, N.; Nakamura, Y.; Ahiko, Y.; Yoshida, T.; Tanabe, T.; Takashima, A.; Kanemitsu, Y. Nutritional and inflammatory measures predict survival of patients with stage IV colorectal cancer. BMC Cancer 2020, 20, 1092. [Google Scholar] [CrossRef]
- Lu, L.; Lin, K.; Zheng, J.; Wu, H.; Li, D. Glasgow Prognostic Score and modified Glasgow Prognostic Score and survival in patients with hepatocellular carcinoma: A meta-analysis. BMJ Open 2021, 11, e053061. [Google Scholar] [CrossRef]
- Chu, B.; Chen, Y.; Pan, J. Prognostic significance of systemic immune inflammation index for ovarian cancer: An updated systematic review and meta-analysis. J. Ovarian Res. 2025, 18, 41. [Google Scholar] [CrossRef]
| Variable | n (%) or Value |
|---|---|
| Age (median [range], years) | 56.1 (24.79–84.3) |
| ECOG performance status | |
| 0–1 | 77 (86.5%) |
| 2–3 | 12 (13.5%) |
| FIGO stage | |
| Stage III | 61 (68.8%) |
| Stage IV | 28 (31.2%) |
| Albumin (g/dL, mean ± SD) | 3.6 ± 0.65 |
| CRP (mg/L, mean ± SD) | 21.86 ± 20.47 |
| Modified Glasgow prognostic score | |
| Score 0 | 17 (19.1%) |
| Score 1 | 49 (55.1%) |
| Score 2 | 23 (25.8%) |
| Overall survival (months, median, range) | 32.3 (178.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kus, F.; Sirvan, F.; Yildirim, H.C.; Koc, I.; Guduk, N.; Arik, Z. Prognostic Impact of Pre-Treatment Modified Glasgow Prognostic Score (mGPS) on Survival in Patients with Advanced-Stage Ovarian Cancer. J. Clin. Med. 2025, 14, 4239. https://doi.org/10.3390/jcm14124239
Kus F, Sirvan F, Yildirim HC, Koc I, Guduk N, Arik Z. Prognostic Impact of Pre-Treatment Modified Glasgow Prognostic Score (mGPS) on Survival in Patients with Advanced-Stage Ovarian Cancer. Journal of Clinical Medicine. 2025; 14(12):4239. https://doi.org/10.3390/jcm14124239
Chicago/Turabian StyleKus, Fatih, Firat Sirvan, Hasan Cagri Yildirim, Ilgin Koc, Naciye Guduk, and Zafer Arik. 2025. "Prognostic Impact of Pre-Treatment Modified Glasgow Prognostic Score (mGPS) on Survival in Patients with Advanced-Stage Ovarian Cancer" Journal of Clinical Medicine 14, no. 12: 4239. https://doi.org/10.3390/jcm14124239
APA StyleKus, F., Sirvan, F., Yildirim, H. C., Koc, I., Guduk, N., & Arik, Z. (2025). Prognostic Impact of Pre-Treatment Modified Glasgow Prognostic Score (mGPS) on Survival in Patients with Advanced-Stage Ovarian Cancer. Journal of Clinical Medicine, 14(12), 4239. https://doi.org/10.3390/jcm14124239





