The Development of an Ultrasound-Based Scoring System for the Prediction of Interstitial Pregnancy
Abstract
1. Introduction
2. Materials and Methods
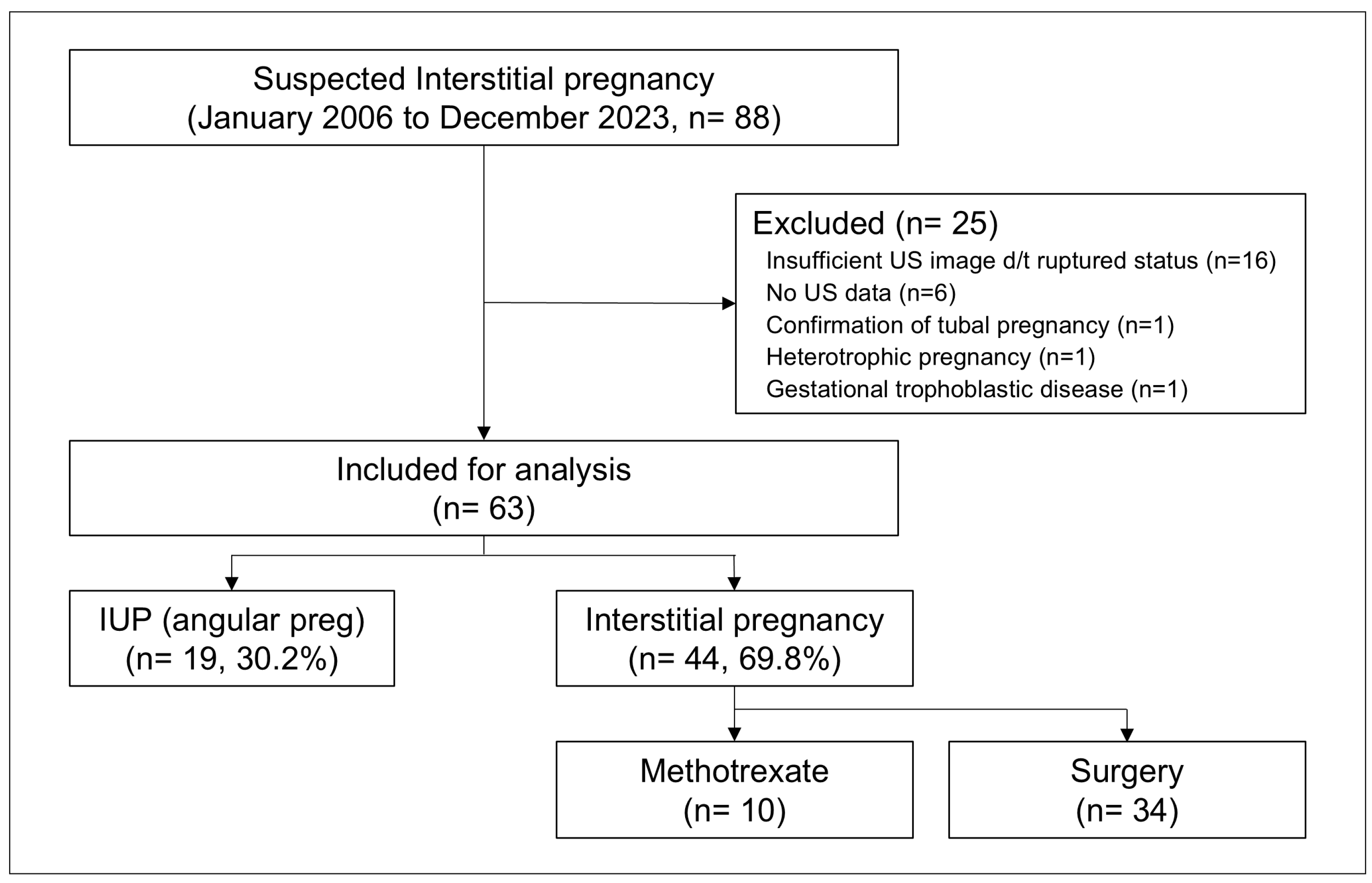
2.1. Patients
2.2. Data Collection and US Examinations
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics and US Findings
3.2. Univariable Analysis
3.3. Selection of the Important Predictors of IP
3.4. Development and Evaluation of the IP Prediction Model
3.5. Subgroup Analysis (Surgically Confirmed IP Versus IUP)
4. Discussion
4.1. Principal Findings
4.2. Clinical Implications
4.3. Research Implications
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LASSO | Least absolute shrinkage selection operator |
| AIC | Akaike Information Criterion |
| IP | Interstitial pregnancy |
| IUP | Intrauterine pregnancy |
| β-hCG | β human chorionic gonadotropin |
| US | Ultrasound |
| TVUS | 2D transvaginal ultrasound |
| PPV | Positive predictive value |
| AUC | Area under the curve |
| NPV | Negative predictive value |
| ROC | Receiver operating characteristic |
References
- Bouyer, J.; Coste, J.; Fernandez, H.; Pouly, J.L.; Job-Spira, N. Sites of ectopic pregnancy: A 10 year population-based study of 1800 cases. Hum. Reprod. 2002, 17, 3224–3230. [Google Scholar] [CrossRef]
- Bahall, V.; Cozier, W.; Latchman, P.; Elias, S.A.; Sankar, S. Interstitial ectopic pregnancy rupture at 17 weeks of gestation: A case report and literature review. Case Rep. Womens Health 2022, 36, e00464. [Google Scholar] [CrossRef]
- Pisarska, M.D.; Carson, S.A. Incidence and risk factors for ectopic pregnancy. Clin. Obstet. Gynecol. 1999, 42, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Tulandi, T.; Saleh, A. Surgical management of ectopic pregnancy. Clin. Obstet. Gynecol. 1999, 42, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Advincula, A.P.; Senapati, S. Interstitial pregnancy. Fertil. Steril. 2004, 82, 1660–1661. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, A.; Bates, S.K. Semantics and pitfalls in the diagnosis of cornual/interstitial pregnancy. Fertil. Steril. 2006, 86, 1764.e11–1764.e14. [Google Scholar] [CrossRef]
- Poon, L.C.; Emmanuel, E.; Ross, J.A.; Johns, J. How feasible is expectant management of interstitial ectopic pregnancy? Ultrasound Obstet. Gynecol. 2014, 43, 317–321. [Google Scholar] [CrossRef][Green Version]
- Moawad, N.S.; Mahajan, S.T.; Moniz, M.H.; Taylor, S.E.; Hurd, W.W. Current diagnosis and treatment of interstitial pregnancy. Am. J. Obstet. Gynecol. 2010, 202, 15–29. [Google Scholar] [CrossRef]
- Devi, L.T.; Kumar, S. Surgically assisted medical management of interstitial ectopic pregnancy. Int. J. Reprod. Contracept. Obstet. Gynecol. 2021, 10, 1210–1215. [Google Scholar] [CrossRef]
- Hiersch, L.; Krissi, H.; Ashwal, E.; From, A.; Wiznitzer, A.; Peled, Y. Effectiveness of medical treatment with methotrexate for interstitial pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 576–580. [Google Scholar] [CrossRef]
- Jansen, R.; Elliott, P. Angular and interstitial pregnancies should not be called ‘cornual’. Aust. N. Z. J. Obstet. Gynaecol. 1983, 23, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.P.; Elliott, P.M. Angular intrauterine pregnancy. Obstet. Gynecol. 1981, 58, 167–175. [Google Scholar] [PubMed]
- Duong, D.; Baker, W.E.; Adedipe, A. Clinician-performed ultrasound diagnosis of ruptured interstitial pregnancy. Am. J. Emerg. Med. 2009, 27, 1170.e1–1170.e2. [Google Scholar] [CrossRef]
- Jafri, S.Z.H.; Loginsky, S.J.; Bouffard, J.A.; Selis, J.E. Sonographic detection of interstitial pregnancy. J. Clin. Ultrasound 1987, 15, 253–257. [Google Scholar] [CrossRef]
- Timor-Tritsch, I.E.; Monteagudo, A.; Matera, C.; Veit, C.R. Sonographic evolution of cornual pregnancies treated without surgery. Obstet. Gynecol. 1992, 79, 1044–1049. [Google Scholar]
- Ackerman, T.E.; Levi, C.S.; Dashefsky, S.M.; Holt, S.C.; Lindsay, D.J. Interstitial line: Sonographic finding in interstitial (cornual) ectopic pregnancy. Radiology 1993, 189, 83–87. [Google Scholar] [CrossRef]
- Grant, A.; Murji, A.; Atri, M. Can the presence of a surrounding endometrium differentiate eccentrically located intrauterine pregnancy from interstitial ectopic pregnancy? J. Obstet. Gynaecol. Can. 2017, 39, 627–634. [Google Scholar] [CrossRef]
- Condous, G.; Van Calster, B.; Kirk, E.; Haider, Z.; Timmerman, D.; Van Huffel, S.; Bourne, T. Prediction of ectopic pregnancy in women with a pregnancy of unknown location. Ultrasound Obstet. Gynecol. 2007, 29, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Rueangket, P.; Rittiluechai, K.; Prayote, A. Predictive analytical model for ectopic pregnancy diagnosis: Statistics vs. machine learning. Front. Med. 2022, 9, 976829. [Google Scholar] [CrossRef]
- Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Krstajic, D.; Buturovic, L.J.; Leahy, D.E.; Thomas, S. Cross-validation pitfalls when selecting and assessing regression and classification models. J. Cheminform 2014, 6, 10. [Google Scholar] [CrossRef]
- Dealberti, D.; Franzò, S.; Bosoni, D.; Pisani, C.; Morales, V.; Gallesio, I.; Bruno, M.; Ricci, G.; Carlucci, S.; Stabile, G. The Use of Methotrexate and Mifepristone for Treatment of Interstitial Pregnancies: An Overview of Effectiveness and Complications. J. Clin. Med. 2023, 12, 7396. [Google Scholar] [CrossRef]
- Hou, L.; Liang, X.; Zeng, L.; Wang, Q.; Chen, Z. Conventional and modern markers of pregnancy of unknown location: Update and narrative review. Int. J. Gynaecol. Obstet. 2024, 167, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Bobdiwala, S.; Guha, S.; Van Calster, B.; Ayim, F.; Mitchell-Jones, N.; Al-Memar, M.; Mitchell, H.; Stalder, C.; Bottomley, C.; Kothari, A.; et al. The clinical performance of the M4 decision support model to triage women with a pregnancy of unknown location as at low or high risk of complications. Hum. Reprod. 2016, 31, 1425–1435. [Google Scholar] [CrossRef]
- Bobdiwala, S.; Christodoulou, E.; Farren, J.; Mitchell-Jones, N.; Kyriacou, C.; Al-Memar, M.; Ayim, F.; Chohan, B.; Kirk, E.; Abughazza, O.; et al. Triaging women with pregnancy of unknown location using two-step protocol including M6 model: Clinical implementation study. Ultrasound Obstet. Gynecol. 2020, 55, 105–114. [Google Scholar] [CrossRef]
- Izquierdo, L.A.; Nicholas, M.C. Three-dimensional transvaginal sonography of interstitial pregnancy. J. Clin. Ultrasound 2003, 31, 484–487. [Google Scholar] [CrossRef]
- Durand, Y.G.; Capoccia-Brugger, R.; Vial, Y.; Balaya, V. Diagnostic dilemma between angular and interstitial ectopic pregnancy: 3D ultrasound features. J. Ultrasound 2022, 25, 989–994. [Google Scholar] [CrossRef]
- Valsky, D.V.; Hamani, Y.; Verstandig, A.; Yagel, S. The use of 3D rendering, VCI-C, 3D power Doppler and B-flow in the evaluation of interstitial pregnancy with arteriovenous malformation treated by selective uterine artery embolization. Ultrasound Obstet. Gynecol. 2007, 29, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Tripathi, R.; Mala, Y.; Batra, A. Diagnostic dilemma in cornual pregnancy-3D ultrasonography may Aid!! J. Clin. Diagn. Res. 2015, 9, QD12–QD13. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Mimura, K.; Kanagawa, T.; Kajimoto, E.; Takahashi, K.; Kakigano, A.; Fujita, S.; Kinugasa-Taniguchi, Y.; Endo, M.; Kimura, T. Three-dimensional sonography in the differential diagnosis of interstitial, angular, and intrauterine pregnancies in a septate uterus. J. Ultrasound Med. 2014, 33, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Cerviño, E.; Ramón, Y.; Cajal, C.L.; Pérez, P.; Couceiro, E. Ultrasound-guided transcervical evacuation of interstitial twin pregnancy. Fertil. Steril. 2011, 96, 927–930. [Google Scholar] [CrossRef]
- Stone, B.S.; Muruganandan, K.M.; Tonelli, M.M.; Dugas, J.N.; Verriet, I.E.; Pare, J.R. Impact of point-of-care ultrasound on treatment time for ectopic pregnancy. Am. J. Emerg. Med. 2021, 49, 226–232. [Google Scholar] [CrossRef]
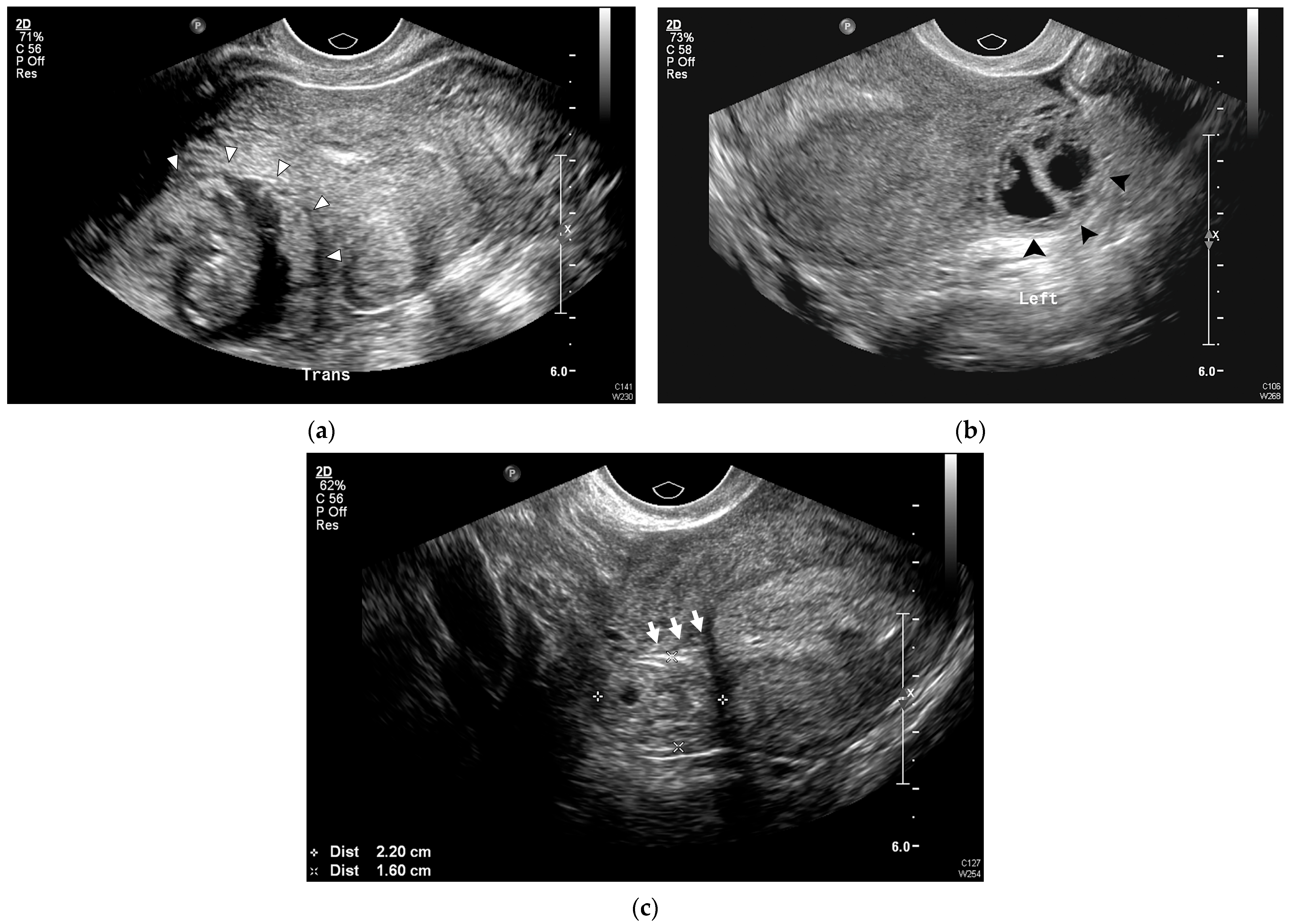

| Variables | IUP (n = 19) | IP (n = 44) | p-Value |
|---|---|---|---|
| Maternal age (years) | 31.2 ± 7.0 | 32.1 ± 4.6 | 0.628 |
| Gravida | 1.7 ± 0.9 | 2.7 ± 1.4 | 0.004 |
| Nullipara | 16 (84.2) | 23 (52.3) | 0.042 |
| Previous history of induced abortion | 5 (26.3) | 25 (56.8) | 0.051 |
| Previous history of ectopic pregnancy | 2 (10.5) | 10 (22.7) | 0.434 |
| Previous history of tubal surgery | 0 | 3 (6.8) | 0.602 |
| In vitro fertilization | 0 | 6 (13.6) | 0.240 |
| Clinical manifestations | |||
| Vaginal bleeding | 0 | 13 (29.5) | 0.020 |
| Abdominal pain | 1 (5.3) | 11 (25.0) | 0.138 |
| Serum β-hCG (mIU/mL) | 35610.9 ± 37610.4 | 31237.2 ± 36365.8 | 0.759 |
| Ultrasound Findings | IUP (n = 19) | IP (n = 44) | p-Value |
|---|---|---|---|
| Mean sac diameter at diagnosis (mm) | 22.9 ± 8.6 | 24.5 ± 8.8 | 0.516 |
| Eccentrically located GS | 15 (78.9) | 44 (100.0) | 0.010 |
| Absent surrounding endometrium | 0 (0.0) | 40 (90.9) | <0.001 |
| Myometrial thickness | |||
| Myometrial thickness (mm) | 6.7 ± 2.5 | 2.6 ± 1.2 | <0.001 |
| Myometrial thinning (<5 mm) | 3 (15.8) | 41 (93.2) | <0.001 |
| Interstitial line sign | 0 (0.0) | 14 (31.8) | 0.014 |
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| (Intercept) | 8.23 (1.29–4.74) | 0.157 |
| Surrounding endometrium | 0.004 (0.000–0.05) | <0.001 |
| Myometrial thinning (<5 mm) | 9.54 (0.45–1547) | 0.146 |
| Vaginal bleeding | 88.5 (3.61–39775) | 0.004 |
| Variable Conditions | Predicted Probability | 5-Point Scale | Risk Group | |||
|---|---|---|---|---|---|---|
| Surrounding Endometrium | Vaginal Bleeding | Myometrial Thinning | N | |||
| Yes | No | No | 15 | 0.030 | 0 | Low |
| Yes | No | Yes | 5 | 0.228 | 1 | |
| Yes | Yes | No | 1 | 0.733 | 3.5 | Indeterminate |
| Yes | Yes | Yes | 2 | 0.963 | 5 | High |
| No | No | No | 2 | 0.892 | 5 | |
| No | No | Yes | 28 | 0.987 | 5 | |
| No | Yes | Yes | 9 | 1.000 | 5 | |
| (a) IP = Yes/No cross-tabulations based on three-tier risk groups | |||||
| Risk Groups | IP = No | IP = Yes | |||
| Low-risk | 19 | 1 | |||
| Indeterminate | 0 | 1 | |||
| High-risk | 0 | 42 | |||
| (b) Diagnostic performance for IP prediction based on three-tier risk groups | |||||
| Risk Groups | Sensitivity | Specificity | PPV | NPV | AUC (95% CI) |
| Low-risk | 97.7% | 100% | 100% | 95.0% | 0.998 (0.992–1.000) |
| Indeterminate | - | - | - | - | |
| High-risk | 95.5% | 100% | 100% | 90.5% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.J.; Zhang, H.-S.; Lee, E.J.; Kwon, H.; Kwon, J.-Y.; Kim, Y.-H.; Lee, J. The Development of an Ultrasound-Based Scoring System for the Prediction of Interstitial Pregnancy. J. Clin. Med. 2025, 14, 4238. https://doi.org/10.3390/jcm14124238
Jung YJ, Zhang H-S, Lee EJ, Kwon H, Kwon J-Y, Kim Y-H, Lee J. The Development of an Ultrasound-Based Scoring System for the Prediction of Interstitial Pregnancy. Journal of Clinical Medicine. 2025; 14(12):4238. https://doi.org/10.3390/jcm14124238
Chicago/Turabian StyleJung, Yun Ji, Hyun-Soo Zhang, Eun Jin Lee, Hayan Kwon, Ja-Young Kwon, Young-Han Kim, and JoonHo Lee. 2025. "The Development of an Ultrasound-Based Scoring System for the Prediction of Interstitial Pregnancy" Journal of Clinical Medicine 14, no. 12: 4238. https://doi.org/10.3390/jcm14124238
APA StyleJung, Y. J., Zhang, H.-S., Lee, E. J., Kwon, H., Kwon, J.-Y., Kim, Y.-H., & Lee, J. (2025). The Development of an Ultrasound-Based Scoring System for the Prediction of Interstitial Pregnancy. Journal of Clinical Medicine, 14(12), 4238. https://doi.org/10.3390/jcm14124238








