The Effect of Attentional Manipulation on Cough Reflex Sensitivity in Individuals with Refractory Chronic Cough and Healthy Controls
Abstract
1. Introduction
2. Methods
2.1. Cough Challenge Testing (CCT) with Capsaicin
2.2. Distraction Task: 2-Back
2.3. Outcome Measures
2.4. Statistical Analyses
3. Results
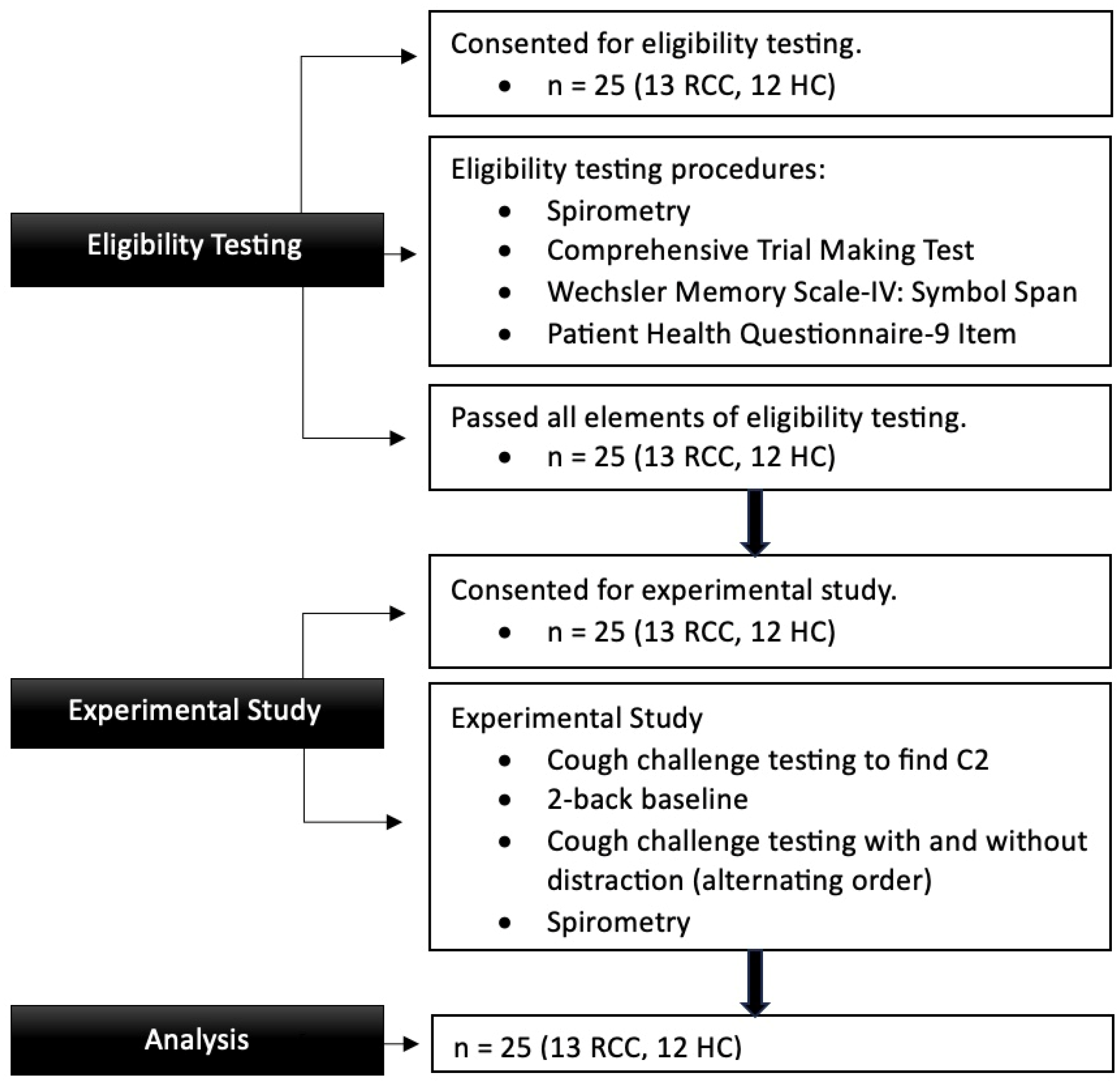
3.1. Participants
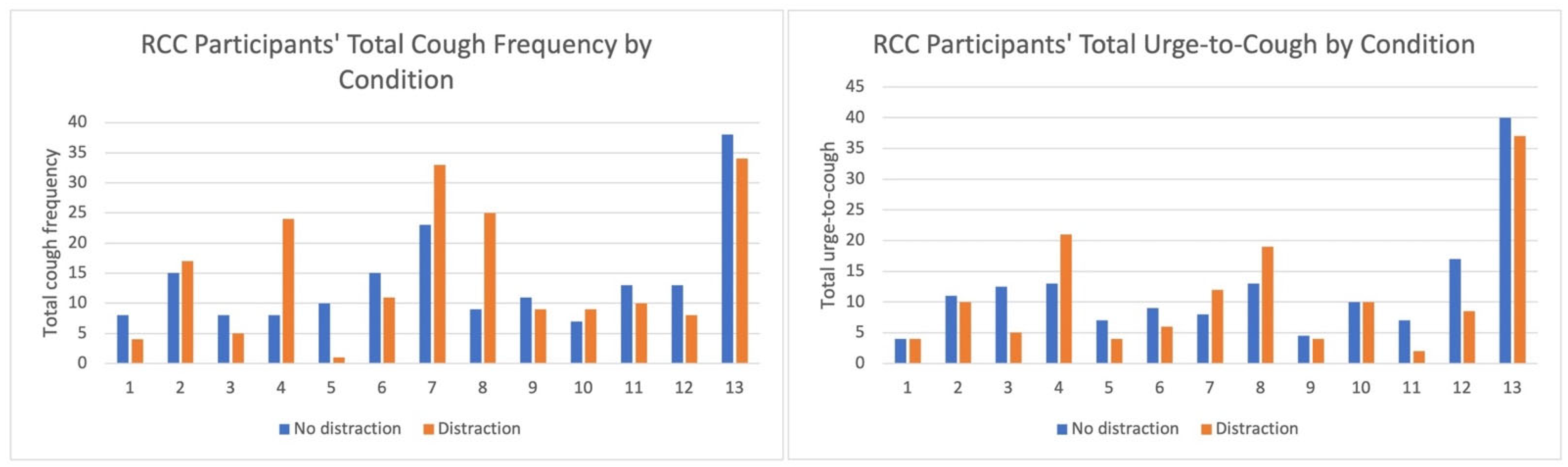
3.2. Cough Frequency and UTC
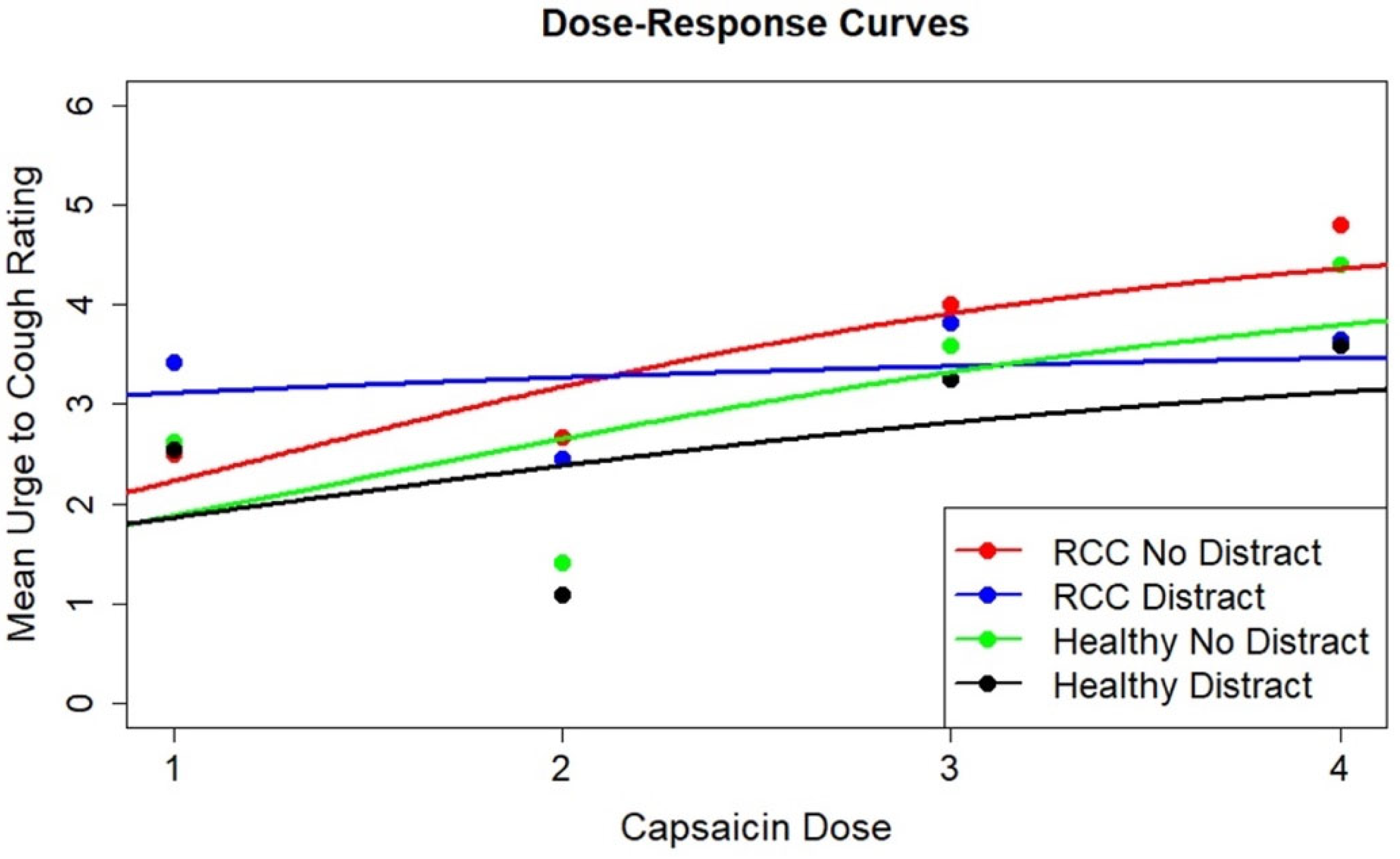
3.3. Dose–Response Modeling
| y | = | cough frequency/urge to cough. |
| x | = | dose level (1, 2, 3, 4). |
| = | 0 if the case is in “RCC with no distraction”, 1 for case where for “RCC with distraction”, “healthy controls with no distraction”, and “healthy controls with distraction”, respectively. | |
| Em | = | mean cough frequency at dose level 4 for the “RCC with no distraction” group. |
| ΔEmi | = | change in cough frequency from the “RCC with no distraction” to group for “RCC with distraction”, “healthy controls with no distraction”, and “healthy controls with distraction”, respectively. |
| E0 | = | mean cough frequency at dose level 0 for the “RCC with no distraction” group. |
| ΔE0i | = | mean cough frequency at dose level 0 for group for “RCC with distraction”, “healthy controls with no distraction”, and “healthy controls with distraction”, respectively. |
| δ | = | dose–response rate parameter for “RCC with no distraction” condition. |
| Δ | = | mean change in the dose–response rate parameter from the “RCC with no distraction” group to group for “RCC with distraction”, “healthy controls with no distraction”, and “healthy controls with distraction”, respectively. |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irwin, R.S.; French, C.L.; Chang, A.B.; Altman, K.W.; CHEST Expert Cough Panel. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest 2018, 153, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Chang, Y.S.; Faruqi, S.; Kim, J.Y.; Kang, M.G.; Kim, S.; Plevkova, J.; Park, H.W.; Cho, S.H.; Morice, A.H. The global epidemiology of chronic cough in adults: A systematic review and meta-analysis. Eur. Respir. J. 2015, 45, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.S. Assessing cough severity and efficacy of therapy in clinical research—ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 232S–237S. [Google Scholar] [CrossRef] [PubMed]
- Dicpinigaitis, P.V.; Tso, R.; Banauch, G. Prevalence of depressive symptoms among patients with chronic cough. Chest 2006, 130, 1839–1843. [Google Scholar] [CrossRef]
- McGarvey, L.P.A.; Carton, C.; Gamble, L.A.; Heaney, L.G.; Shepherd, R.; Ennis, M.; MacMahon, J. Prevalence of psychomorbidity among patients with chronic cough. Cough 2006, 2, 4. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V. Prevalence of stress urinary incontinence in women presenting for evaluation of chronic cough. ERJ Open Res. 2021, 7, 12. [Google Scholar] [CrossRef]
- Chung, K.F. Chronic ‘cough hypersensitivity syndrome’: A more precise label for chronic cough. Pulm. Pharmacol. Ther. 2011, 24, 267–271. [Google Scholar] [CrossRef]
- Chung, K.F.; McGarvey, L.; Mazzone, S. Chronic cough and cough hypersensitivity syndrome. Lancet Respir. Med. 2016, 4, 934–935. [Google Scholar] [CrossRef]
- Driessen, A.K.; McGovern, A.E.; Narula, M.; Yang, S.K.; Keller, J.A.; Farrell, M.J.; Mazzone, S.B. Central mechanisms of airway sensation and cough hypersensitivity. Pulm. Pharmacol. Ther. 2017, 47, 9–15. [Google Scholar] [CrossRef]
- Morice, A.H.; Faruqi, S.; Wright, C.E.; Thompson, R.; Bland, J.M. Cough hypersensitivity syndrome: A distinct clinical entity. Lung 2011, 189, 73–79. [Google Scholar] [CrossRef]
- Singh, N.; Driessen, A.K.; McGovern, A.E.; Moe, A.A.K.; Farrell, M.J.; Mazzone, S.B. Peripheral and central mechanisms of cough hypersensitivity. J. Thorac. Dis. 2020, 12, 5179–5193. [Google Scholar] [CrossRef] [PubMed]
- Ando, A.; Smallwood, D.; McMahon, M.; Irving, L.; Mazzone, S.B.; Farrell, M.J. Neural correlates of cough hypersensitivity in humans: Evidence for central sensitisation and dysfunctional inhibitory control. Thorax 2016, 71, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Moe, A.A.K.; Singh, N.; Dimmock, M.; Cox, K.; McGarvey, L.; Chung, K.F.; McGovern, A.E.; McMahon, M.; Richards, A.L.; Farrell, M.J.; et al. Brainstem processing of cough sensory inputs in chronic cough hypersensitivity. EBioMedicine 2024, 100, 104976. [Google Scholar] [CrossRef]
- Mazzone, S.B.; McLennan, L.; McGovern, A.E.; Egan, G.F.; Farrell, M.J. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am. J. Respir. Crit. Care Med. 2007, 176, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Athwal, B.S.; Berkley, K.J.; Hussain, I.; Brennan, A.; Craggs, M.; Sakakibara, R.; Frackowiak, R.S.; Fowler, C.J. Brain responses to changes in bladder volume and urge to void in healthy men. Brain 2001, 124 Pt 2, 369–377. [Google Scholar] [CrossRef]
- Hanamori, T.; Kunitake, T.; Kato, K.; Kannan, H. Convergence of afferent inputs from the chorda tympani, lingual-tonsillar and pharyngeal branches of the glossopharyngeal nerve, and superior laryngeal nerve on the neurons in the insular cortex in rats. Brain Res. 1997, 763, 267–270. [Google Scholar] [CrossRef]
- Farrell, M.J.; Cole, L.J.; Chiapoco, D.; Egan, G.F.; Mazzone, S.B. Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. NeuroImage 2012, 61, 1324–1335. [Google Scholar] [CrossRef]
- Hegland, K.W.; Bolser, D.C.; Davenport, P.W. Volitional control of reflex cough. J. Appl. Physiol. (1985) 2012, 113, 39–46. [Google Scholar] [CrossRef]
- Hutchings, H.A.; Morris, S.; Eccles, R.; Jawad, M.S. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir. Med. 1993, 87, 379–382. [Google Scholar] [CrossRef]
- Gracely, R.H.; Undem, B.J.; Banzett, R.B. Cough, pain and dyspnoea: Similarities and differences. Pulm. Pharmacol. Ther. 2007, 20, 433–437. [Google Scholar] [CrossRef]
- Van den Bergh, O.; Van Diest, I.; Dupont, L.; Davenport, P. On the psychology of cough. Lung 2012, 190, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pennebaker, J.W. Perceptual and Environmental Determinants of Coughing. Basic Appl. Soc. Psychol. 1980, 1, 83–91. [Google Scholar] [CrossRef]
- Rietveld, S.; Van Beest, I.; Everaerd, W. Psychological confounds in medical research: The example of excessive cough in asthma. Behav. Res. Ther. 2000, 38, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Eccles, R. The powerful placebo effect in cough: Relevance to treatment and clinical trials. Lung 2020, 198, 13–21. [Google Scholar] [CrossRef]
- Slovarp, L.J.; Reynolds, J.E.; Gillespie, A.I.; Jetté, M.E. Reframing refractory chronic cough: The role of interoception. Lung 2025, 203, 32. [Google Scholar] [CrossRef]
- Janssens, T.; Silva, M.; Davenport, P.W.; Van Diest, I.; Dupont, L.J.; Van den Bergh, O. Attentional modulation of reflex cough. Chest 2014, 146, 135–141. [Google Scholar] [CrossRef]
- Morice, A.H.; Higgins, K.S.; Yeo, W.W. Adaptation of cough reflex with different types of stimulation. Eur. Respir. J. 1992, 5, 841–847. [Google Scholar] [CrossRef]
- Perry, S.E.; Troche, M.S. Dual Tasking Influences Cough Sensorimotor Outcomes in Healthy Young Adults. J. Speech Lang. Hear. Res. 2019, 62, 3596–3606. [Google Scholar] [CrossRef]
- Perry, S.E.; Troche, M.S. Dual Tasking Influences Cough Reflex Outcomes in Adults with Parkinson’s Disease: A Controlled Study. Dysphagia 2021, 36, 959–973. [Google Scholar] [CrossRef]
- Pashler, H. Dual-task interference in simple tasks: Data and theory. Psychol. Bull. 1994, 116, 220–244. [Google Scholar] [CrossRef]
- Tombu, M.; Jolicoeur, P. A central capacity sharing model of dual-task performance. J. Exp. Psychol. Hum. Percept. Perform. 2003, 29, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Khoury, N.M.; Lutz, J.; Schuman-Olivier, Z. Interoception in psychiatric disorders: A review of randomized, controlled trials with interoception-based interventions. Harv. Rev. Psychiatry 2018, 26, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of spirometry 2019 update. an official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Reynolds, C. Comprehensive Trail Making Test; PRO-ED, Inc.: Austin, TX, USA, 2002. [Google Scholar]
- Wechsler, D. Wechsler Memory Scale, 4th ed.; Pearson: San Antonio, TX, USA, 2009. [Google Scholar]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr. Ann. 2002, 32, 509–515. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V. Short- and long-term reproducibility of capsaicin cough challenge testing. Pulm. Pharmacol. Ther. 2003, 16, 61–65. [Google Scholar] [CrossRef]
- Morice, A.H.; Kastelik, J.A.; Thompson, R. Cough challenge in the assessment of cough reflex. Br. J. Clin. Pharmacol. 2001, 52, 365–375. [Google Scholar] [CrossRef]
- Morice, A.H.; Fontana, G.A.; Belvisi, M.G.; Birring, S.S.; Chung, K.F.; Dicpinigaitis, P.V.; Kastelik, J.A.; McGarvey, L.P.; Smith, J.A.; Tatar, M.; et al. ERS guidelines on the assessment of cough. Eur. Respir. J. 2007, 29, 1256–1276. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V.; Alva, R.V. Safety of capsaicin cough challenge testing. Chest 2005, 128, 196–202. [Google Scholar] [CrossRef]
- Slovarp, L.; Reynolds, J.; Bozarth-Dailey, E.; Popp, S.; Cambpell, S.; Morkrid, P. Cough Desensitization Therapy: A randomized, sham-controlled pilot trial for patients with refractory chronic cough. Respir. Med. 2022, 193, 106739. [Google Scholar] [CrossRef] [PubMed]
- Prudon, B.; Birring, S.S.; Vara, D.D.; Hall, A.P.; Thompson, J.P.; Pavord, I.D. Cough and glottic-stop reflex sensitivity in health and disease. Chest 2005, 127, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Davenport, P.W.; Bolser, D.C.; Vickroy, T.; Berry, R.B.; Martin, A.D.; Hey, J.A.; Danzig, M. The effect of codeine on the Urge-to-Cough response to inhaled capsaicin. Pulm. Pharmacol. Ther. 2007, 20, 338–346. [Google Scholar] [CrossRef]
- Hilton, E.C.; Baverel, P.G.; Woodcock, A.; Van Der Graaf, P.H.; Smith, J.A. Pharmacodynamic modeling of cough responses to capsaicin inhalation calls into question the utility of the C5 end point. J. Allergy Clin. Immunol. 2013, 132, e841–e845. [Google Scholar] [CrossRef]
- Chung, K.F.; Chaccour, C.; Jover, L.; Galvosas, M.; Song, W.J.; Rudd, M.; Small, P. Longitudinal cough frequency monitoring in persistent coughers: Daily variability and predictability. Lung 2024, 202, 561–568. [Google Scholar] [CrossRef]
- Conway, A.R.; Kane, M.J.; Bunting, M.F.; Hambrick, D.Z.; Wilhelm, O.; Engle, R.W. Working memory span tasks: A methodological review and user’s guide. Psychon. Bull. Rev. 2005, 12, 769–786. [Google Scholar] [CrossRef]
- Kane, M.J.; Conway, A.R.A.; Miura, T.K.; Colflesh, G.J.H. Working Memory, Attention Control, and the N-Back Task: A Question of Construct Validity. J. Exp. Psychol. Learn. Mem. Cogn. 2007, 33, 615–622. [Google Scholar] [CrossRef]
- Jaeggi, S.M.; Schmid, C.; Buschkuehl, M.; Perrig, W.J. Differential age effects in load-dependent memory processing. Neuropsychol. Dev. Cogn. Sect. B Aging Neuropsychol. Cogn. 2009, 16, 80–102. [Google Scholar] [CrossRef]
- Nystrom, L.E.; Braver, T.S.; Sabb, F.W.; Delgado, M.R.; Noll, D.C.; Cohen, J.D. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. NeuroImage 2000, 11, 424–446. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Jaeggi, S.M.; Buschkuehl, M.; Perrig, W.J.; Meier, B. The concurrent validity of the N-back task as a working memory measure. Memory 2010, 18, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, T.; Fernell, E.; Olesen, P.J.; Johnson, M.; Gustafsson, P.; Dahlström, K.; Gillberg, C.G.; Forssberg, H.; Westerberg, H. Computerized training of working memory in children with ADHD—A randomized, controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Des Roches, C.A.; Balachandran, I.; Ascenso, E.M.; Tripodis, Y.; Kiran, S. Effectiveness of an impairment-based individualized rehabilitation program using an iPad-based software platform. Front. Hum. Neurosci. 2015, 8, 1015. [Google Scholar] [CrossRef] [PubMed]
- Constant Therapy Health. The Science and Clinical Application Behind the Remember Pictures in Order (N-Back). 2022. Available online:https://constanttherapyhealth.com/constant-therapy/ (accessed on 1 November 2021).
- Hansson, L.; Wollmer, P.; Dahlbäck, M.; Karlsson, J.A. Regional sensitivity of human airways to capsaicin-induced cough. Am. Rev. Respir. Dis. 1992, 145, 1191–1195. [Google Scholar] [CrossRef]
- Stankewitz, A.; Sorg, C.; von Kalckreuth, A.; Schulz, E.; Valet, M.; Neufang, S.; Zimmer, C.; Henningsen, P.; Gundel, H.; Wohlschlager, A.M.; et al. Fronto-Insular Connectivity during Pain Distraction Is Impaired in Patients with Somatoform Pain. J. Neuroimaging 2018, 28, 621–628. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V. Angiotensin-Converting Enzyme Inhibitor-Induced Cough. Chest 2006, 129, 169S–173S. [Google Scholar] [CrossRef]
- Chincholkar, M. Gabapentinoids: Pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br. J. Pain 2020, 14, 104–114. [Google Scholar] [CrossRef]
- Reynolds, J.E. The Effect of Attentional Manipulation on Cough Reflex Sensitivity in Patients with Cough Hypersensitivity Syndrome. Ph.D. Thesis, University of Montana, Missoula, MT, USA, 2022. [Google Scholar]


| Participant ID | Age | Sex | C2 | Baseline Two-Back % Accuracy | Two-Back % Accuracy During CCT |
|---|---|---|---|---|---|
| RCC-1 | 75 | F | 0.49 µM | 61% | 69% |
| RCC-2 | 73 | F | 15.63 µM | 78% | 69% |
| RCC-3 | 57 | F | 31.25 µM | 60% | 85% |
| RCC-4 | 65 | F | 3.91 µM | 55% | 54% |
| RCC-5 | 54 | M | 1.95 µM | 95% | 94% |
| RCC-6 | 72 | F | 1.95 µM | 73% | 73% |
| RCC-7 | 67 | F | 7.81 µM | 60% | 56% |
| RCC-8 | 44 | F | 0.98 µM | 100% | 89% |
| RCC-9 | 58 | F | 0.49 µM | 100% | 96% |
| RCC-10 | 44 | F | 3.91 µM | 100% | 100% |
| RCC-11 | 67 | F | 0.49 µM | 78% | 97% |
| RCC-12 | 52 | F | 7.81 µM | 97% | 92% |
| RCC-13 | 63 | F | 15.63 µM | 56% | 55% |
| Group mean (SD): | 60.9 (10.4) | 78% (18.4%) | 79% (17.2%) | ||
| HC-1 | 56 | F | 0.98 µM | 100% | 97% |
| HC-2 | 77 | F | 31.25 µM | 56% | 53% |
| HC-3 | 81 | M | 3.91 µM | 84% | 64% |
| HC-4 | 59 | F | 3.91 µM | 29% | 77% |
| HC-5 | 49 | F | 0.49 µM | 74% | 75% |
| HC-6 | 53 | F | 7.81 µM | 92% | 93% |
| HC-7 | 62 | F | 1.95 µM | 88% | 76% |
| HC-8 | 57 | F | 7.81 µM | 17% | 71% |
| HC-9 | 58 | F | 0.98 µM | 100% | 75% |
| HC-10 | 42 | F | 3.91 µM | 53% | 69% |
| HC-11 | 70 | F | 0.98 µM | 81% | 95% |
| HC-12 | 62 | F | 1.95 µM | 100% | 97% |
| Group mean (SD): | 60.5 (11.1) | 73% (28.2%) | 79% (14.2%) | ||
| Dose | UTC Mean (SD) | Cough Frequency Mean (SD) | |||
|---|---|---|---|---|---|
| No Distraction | Distraction | No Distraction | Distraction | ||
| RCC | 1 | 2.46 (3.77) | 2.65 (3.27) | 2.38 (3.31) | 3.69 (4.57) |
| 2 | 2.67 (2.80) | 2.46 (2.97) | 3.50 (3.45) | 4.00 (4.73) | |
| 3 | 4.00 (3.61) | 4.08 (3.60) | 4.82 (5.17) | 3.91 (3.45) | |
| 4 | 4.80 (2.94) | 3.65 (2.89) | 5.20 (3.61) | 5.10 (4.20) | |
| Healthy control | 1 | 2.63 (1.65) | 2.54 (2.18) | 1.50 (1.31) | 1.42 (1.44) |
| 2 | 1.42 (1.81) | 1.08 (1.46) | 0.42 (0.90) | 0.67 (1.23) | |
| 3 | 3.58 (3.08) | 3.25 (2.80) | 2.00 (2.09) | 2.83 (2.76) | |
| 4 | 4.41 (2.73) | 3.59 (1.39) | 3.36 (2.01) | 2.91 (2.07) | |
| Parameter | Estimate | SE | t-Value | p-Value | 95% (Bootstrap) Confidence Intervals |
|---|---|---|---|---|---|
| E0 | 1.01 | 1.00 | 1.009 | 0.314 | (0.04, 3.62) |
| δ | 1.18 | 0.74 | 1.594 | 0.113 | (0.13, 2.86) |
| ΔE02 | 1.02 | 1.70 | 0.600 | 0.550 | (−0.81, 3.41) |
| Δδ2 | −0.44 | 0.96 | −0.459 | 0.647 | (−2.25, 1.15) |
| ΔE03 | −0.80 | 1.08 | −0.742 | 0.459 | (−3.25, 0.66) |
| Δδ3 | −0.11 | 1.08 | −0.100 | 0.921 | (−2.11, 2.73) |
| ΔE04 | −0.69 | 1.18 | −0.584 | 0.560 | (−3.14, 0.94) |
| Δδ4 | −0.05 | 1.25 | −0.038 | 0.970 | (−2.16, 3.36) |
| Parameter | Estimate | ||||
|---|---|---|---|---|---|
| E0 | 1.34 | ||||
| δ | 0.81 | SE | t-value | p-value | 95% (bootstrap) confidence intervals |
| ΔE02 | 1.58 | 1.03 | 1.293 | 0.198 | (0.08, 3.52) |
| Δδ2 | −0.42 | 0.55 | 1.480 | 0.141 | (0.06, 2.00) |
| ΔE03 | −0.15 | 1.93 | 0.817 | 0.415 | (−1.00, 3.11) |
| Δδ3 | −0.11 | 1.53 | −0.276 | 0.783 | (−1.94, 0.84) |
| ΔE04 | −0.01 | 1.41 | −0.107 | 0.915 | (−2.23, 1.66) |
| Δδ4 | −0.20 | 0.74 | −0.143 | 0.887 | (−1.40, 0.80) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salois, J.R.; Slovarp, L.J.; Spinti, I.; Graham, J.; Thorne, J.; Glaspey, A.; Off, C.; Jetté, M. The Effect of Attentional Manipulation on Cough Reflex Sensitivity in Individuals with Refractory Chronic Cough and Healthy Controls. J. Clin. Med. 2025, 14, 4199. https://doi.org/10.3390/jcm14124199
Salois JR, Slovarp LJ, Spinti I, Graham J, Thorne J, Glaspey A, Off C, Jetté M. The Effect of Attentional Manipulation on Cough Reflex Sensitivity in Individuals with Refractory Chronic Cough and Healthy Controls. Journal of Clinical Medicine. 2025; 14(12):4199. https://doi.org/10.3390/jcm14124199
Chicago/Turabian StyleSalois, Jane R., Laurie J. Slovarp, Isabel Spinti, Jon Graham, Jethro Thorne, Amy Glaspey, Catherine Off, and Marie Jetté. 2025. "The Effect of Attentional Manipulation on Cough Reflex Sensitivity in Individuals with Refractory Chronic Cough and Healthy Controls" Journal of Clinical Medicine 14, no. 12: 4199. https://doi.org/10.3390/jcm14124199
APA StyleSalois, J. R., Slovarp, L. J., Spinti, I., Graham, J., Thorne, J., Glaspey, A., Off, C., & Jetté, M. (2025). The Effect of Attentional Manipulation on Cough Reflex Sensitivity in Individuals with Refractory Chronic Cough and Healthy Controls. Journal of Clinical Medicine, 14(12), 4199. https://doi.org/10.3390/jcm14124199






