Improved Cholesteatoma Removal with CADISS: A Quantitative Ultrastructural Comparison Using VP-SEM
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Objectives
2.2. Sample Collection and Grouping
2.3. Inclusion and Exclusion Criteria and Study Approval
2.4. Surgical Procedures
2.4.1. Manual Dissection Procedure
2.4.2. CADISS-Assisted Dissection Procedure
2.5. Sample Processing for VP-SEM
2.6. Qualitative Analysis
2.7. Quantitative Image Analysis
2.8. Statistical Analysis
2.9. Radiological Follow-Up
3. Results
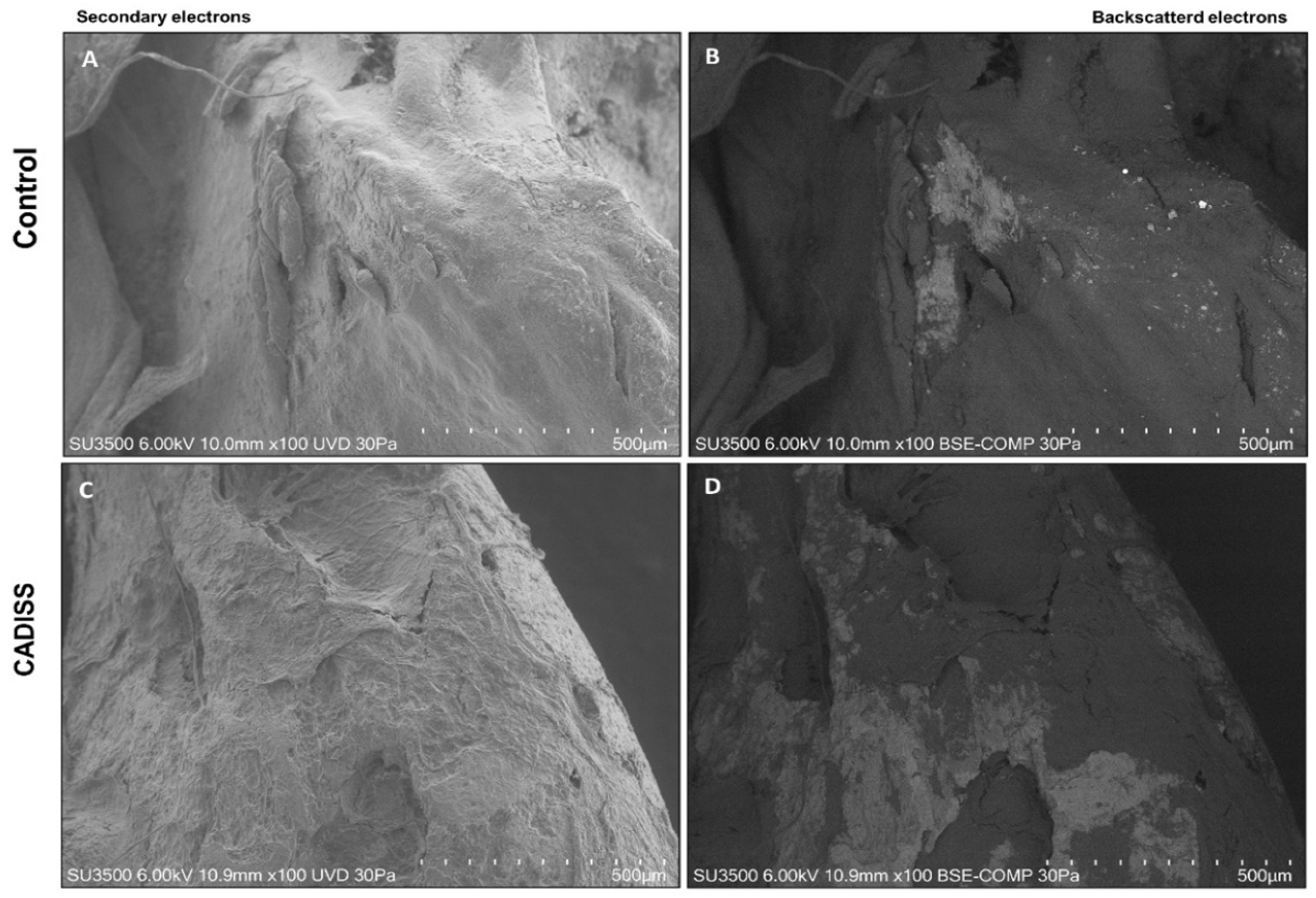
3.1. Macroscopic and Qualitative Findings
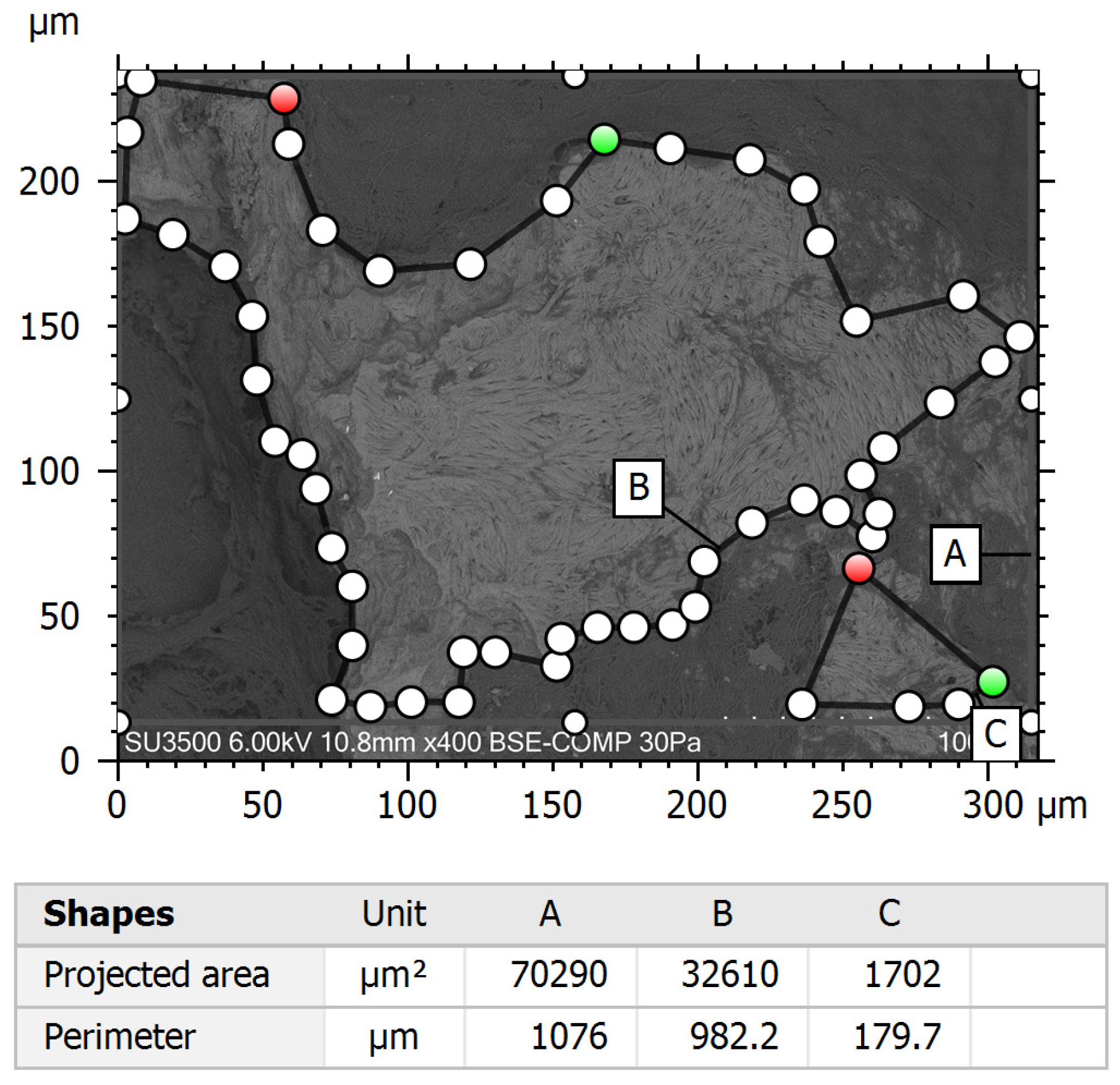
3.2. Quantitative Surface Analysis
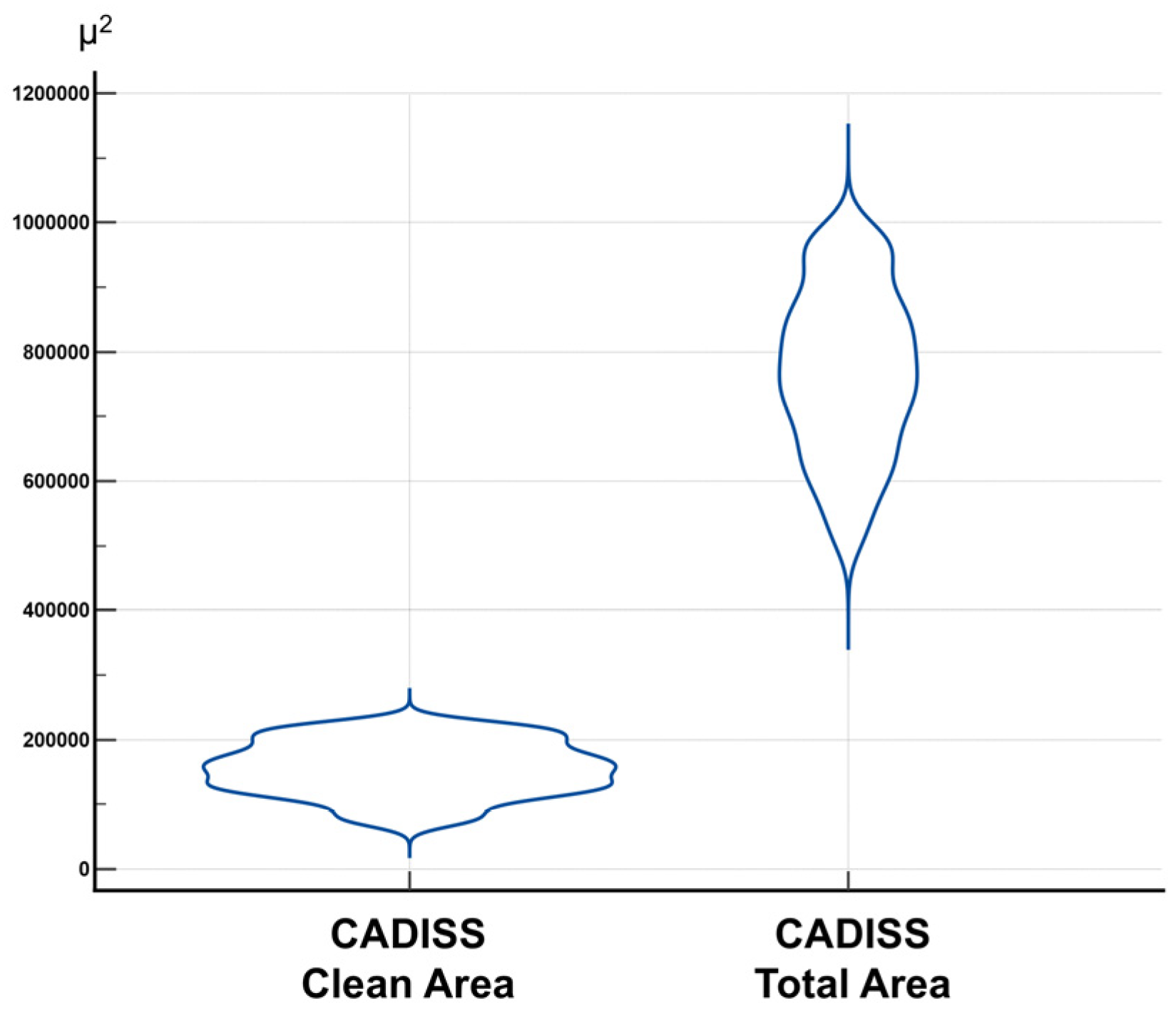
3.3. Clean Area/Total Area Ratio and Statistical Distribution
3.4. Comparative Statistical Evaluation
3.5. Considerations on Sample Size Adequacy
3.6. Radiological Validation
4. Discussion
4.1. Key Mechanisms of CADISS® System in Enhancing Cholesteatoma Surgery
4.2. Radiological Validation and Clinical Relevance
4.3. Mechanistic Insight and Histological Perspective
4.4. Implications for Surgical Practice
4.5. Study Strengths and Limitations
4.6. Future Directions and Research Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yung, M.; Tono, T.; Olszewska, E.; Yamamoto, Y.; Sudhoff, H.; Sakagami, M.; Mulder, J.; Kojima, H.; İncesulu, A.; Trabalzini, F.; et al. EAONO/JOS joint consensus statements on the definitions, classification and staging of middle ear cholesteatoma. J. Int. Adv. Otol. 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, J.; Özgirgin, N.; Olszewska, E. Cholesteatoma definition and classification: A literature review. J. Int. Adv. Otol. 2017, 13, 266–271. [Google Scholar] [CrossRef]
- Castle, J. Cholesteatoma pearls: Practical points and update. Head Neck Pathol. 2018, 12, 419–429. [Google Scholar] [CrossRef]
- Vangrinsven, J.; Van Rompaey, V.; Casselman, J.W. Beyond the otoscope: An imaging review of congenital cholesteatoma. Insights Imaging 2024, 15, 123. [Google Scholar] [CrossRef]
- Beckmann, S.; Mantokoudis, G.; Weder, S.; Borner, U.; Caversaccio, M.; Anschuetz, L. Endoscopic cholesteatoma surgery. J. Vis. Exp. 2022, 179, e63315. [Google Scholar] [CrossRef]
- Bessa, R.G.; Tsuji, R.K. Inside-Out Transcanal Endoscopic Mastoidectomy: Literature Revision. Int. Arch. Otorhinolaryngol. 2023, 27, e370–e376. [Google Scholar] [CrossRef]
- Early, S.; Saad, M.A.; Mallidi, S.; Mansour, A.; Seist, R.; Hasan, T.; Stankovic, K.M. A fluorescent photoimmunoconjugate for imaging of cholesteatoma. Sci. Rep. 2022, 12, 19905. [Google Scholar] [CrossRef]
- Basonbul, R.A.; Ronner, E.A.; Kozin, E.D.; Lee, D.J.; Cohen, M.S. Systematic Review of Endoscopic Ear Surgery Outcomes for Pediatric Cholesteatoma. Otol. Neurotol. 2021, 42, 108–115. [Google Scholar] [CrossRef]
- Hunter, J.B.; Zuniga, M.G.; Sweeney, A.D.; Bertrand, N.; Wanna, G.B.; Haynes, D.S. Pediatric endoscopic cholesteatoma surgery. Otolaryngol. Head Neck Surg. 2016, 154, 1121–1127. [Google Scholar] [CrossRef]
- Tsuchida, K.; Takahashi, M.; Nakazawa, T.; Kurihara, S.; Yamamoto, K.; Yamamoto, Y. Augmented reality-assisted transcanal endoscopic ear surgery for middle ear cholesteatoma. J. Clin. Med. 2024, 13, 1780. [Google Scholar] [CrossRef]
- Iannella, G.; Savastano, E.; Pasquariello, B.; Re, M.; Magliulo, G. Giant petrous bone cholesteatoma: Combined microscopic surgery and an adjuvant endoscopic approach. J. Neurol. Surg. Rep. 2016, 77, e46–e49. [Google Scholar] [CrossRef]
- Kanzara, T.; Virk, J.; Chawda, S.; Owa, A. Wholly endoscopic permeatal removal of a petrous apex cholesteatoma. Case Rep. Otolaryngol. 2014, 2014, 184230. [Google Scholar] [CrossRef]
- Vincenti, V.; Magnan, J.; Saccardi, M.; Zini, C. Chemically assisted dissection by means of Mesna in cholesteatoma surgery. Otol. Neurotol. 2014, 35, 1819–1824. [Google Scholar] [CrossRef]
- Baklacı, D.; Kum, R.; Kulaçoğlu, S.; Yılmaz, Y.; Özcan, M. The effects of Mesna on the facial nerve, an experimental animal study. J. Int. Adv. Otol. 2018, 14, 63–67. [Google Scholar] [CrossRef]
- Yılmaz, M.; Göksu, N.; Bayramoğlu, İ.; Bayazit, Y. Practical use of Mesna in atelectatic ears and adhesive otitis media. ORL 2006, 68, 195–198. [Google Scholar] [CrossRef]
- Ant, A.; Karamert, R.; Kulduk, G.; Ekinci, Ö.; Tutar, H.; Göksu, N. The effects of sodium-2-mercaptoethanesulfonate application on the neural and neurovascular tissues: An experimental animal study. Surg. Neurol. Int. 2015, 6, 150. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Reitzel, R.; Viola, G.; Vargas-Cruz, N.; Selber, J.; Raad, I. Sodium mercaptoethane sulfonate reduces collagenolytic degradation and synergistically enhances antimicrobial durability in an antibiotic-loaded biopolymer film for prevention of surgical-site infections. BioMed Res. Int. 2017, 2017, 3149536. [Google Scholar] [CrossRef]
- Denaro, V.; Di Martino, A.; Longo, U.G.; Costa, V.; Papalia, R.; Forriol, F.; Denaro, L. Effectiveness of a mucolythic agent as a local adjuvant in revision lumbar spine surgery. Eur. Spine J. 2008, 17, 1752–1756. [Google Scholar] [CrossRef]
- Casale, M.; Martino, A.; Salvinelli, F.; Trombetta, M.; Denaro, V. Mesna for chemically assisted tissue dissection. Expert Opin. Investig. Drugs 2010, 19, 699–707. [Google Scholar] [CrossRef]
- Çınar, Z.; Yiğit, Ö.; Turanoğlu, F.; Koca, S. A clinical and histopathological comparison of saline, adrenaline and 2-mercaptoethanesulfonate (Mesna) in mucoperichondrial elevation: Which is superior? Acta Otorhinolaryngol. Ital. 2021, 41, 51–58. [Google Scholar] [CrossRef]
- Barbara, M.; Covelli, E.; Monini, S.; Bandiera, G.; Filippi, C.; Margani, V.; Volpini, L.; Salerno, G.; Romano, A.; Bozzao, A. Early non-EPI DW-MRI after cholesteatoma surgery. Ear Nose Throat J. 2024, 103, 435–441. [Google Scholar] [CrossRef]
- Covelli, E.; Margani, V.; Filippi, C.; Elfarargy, H.H.; Volpini, L.; Romano, A.; Bozzao, A.; Barbara, M. Proposal of a magnetic resonance imaging follow-up protocol after cholesteatoma surgery: A prospective study. Acta Oto-Laryngol. 2022, 142, 484–490. [Google Scholar] [CrossRef]
- Relucenti, M.; Miglietta, S.; Bove, G.; Donfrancesco, O.; Battaglione, E.; Familiari, P.; Barbaranelli, C.; Covelli, E.; Barbara, M.; Familiari, G. SEM BSE 3D Image Analysis of Human Incus Bone Affected by Cholesteatoma Ascribes to Osteoclasts the Bone Erosion and VP-SEM dEDX Analysis Reveals New Bone Formation. Scanning 2020, 2020, 9371516. [Google Scholar] [CrossRef]
- Correr, S.; Makabe, S.; Heyn, R.; Relucenti, M.; Naguro, T.; Familiari, G. Microplicae-like structures of the fallopian tube in postmenopausal women as shown by electron microscopy. Histol. Histopathol. 2006, 21, 219–226. [Google Scholar] [CrossRef]
- Relucenti, M.; Heyn, R.; Correr, S.; Familiari, G. Cumulus oophorus extracellular matrix in the human oocyte: A role for adhesive proteins. Ital. J. Anat. Embryol. 2005, 110 (Suppl. S1), 219–224. [Google Scholar]
- Meissel, K.; Yao, E.S. Using Cliff’s Delta as a non-parametric effect size measure: An accessible web app and R tutorial. Pract. Assess. Res. Eval. 2024, 29, 2. [Google Scholar] [CrossRef]
- Sadé, J. Surgical planning of the treatment of cholesteatoma and postoperative follow-up. Ann. Otol. Rhinol. Laryngol. 2000, 109, 372–376. [Google Scholar] [CrossRef]
- Cohen, M.; Basonbul, R.; Kozin, E.; Lee, D. Residual cholesteatoma during second-look procedures following primary pediatric endoscopic ear surgery. Otolaryngology 2017, 157, 1034–1040. [Google Scholar] [CrossRef]
- Manzoor, N.F.; Totten, D.J.; McLeod, M.E.; Sherry, A.D.; Perkins, E.L.; Haynes, D.S.; Rivas, A. Comparative analysis of recidivism after endoscopic and microscopic-based cholesteatoma resection. Otol. Neurotol. 2022, 43, 466–471. [Google Scholar] [CrossRef]
- Kerckhoffs, K.G.; Kommer, M.B.; van Strien, T.H.; Visscher, S.J.; Bruijnzeel, H.; Smit, A.L.; Grolman, W. The disease recurrence rate after the canal wall up or canal wall down technique in adults. Laryngoscope 2015, 126, 980–987. [Google Scholar] [CrossRef]
- Karamert, R.; Eravci, F.C.; Cebeci, S.; Düzlü, M.; Zorlu, M.E.; Yaşar, N.G.; Tutar, H.; Uğur, M.B.; İriz, A.; Bayazit, Y.A. Canal wall down versus canal wall up surgeries in the treatment of middle ear cholesteatoma. Turk. J. Med. Sci. 2019, 49, 1426–1432. [Google Scholar] [CrossRef]
- Bennett, M.; Wanna, G.; Francis, D.; Murfee, J.; O’Connell, B.; Haynes, D. Clinical and cost utility of an intraoperative endoscopic second look in cholesteatoma surgery. Laryngoscope 2018, 128, 2867–2871. [Google Scholar] [CrossRef]
- Galli, J.; Calò, L.; Giuliani, M.; Sergi, B.; Lucidi, D.; Meucci, D.; Bassotti, E.; Sanguinetti, M.; Paludetti, G. Biofilm’s role in chronic cholesteatomatous otitis media. Otolaryngology 2016, 154, 914–916. [Google Scholar] [CrossRef]
- Popescu, C.; Văruț, R.M.; Puticiu, M.; Belghiru, V.I.; Banicioiu, M.; Rotaru, L.T.; Popescu, M.; Cosmin, A.C.; Popescu, A.I.S. Comprehensive management of cholesteatoma in otitis media: Diagnostic challenges, imaging advances, and surgical outcome. J. Clin. Med. 2024, 13, 6791. [Google Scholar] [CrossRef]
- Louw, L. Acquired cholesteatoma pathogenesis: Stepwise explanations. J. Laryngol. Otol. 2010, 124, 587–593. [Google Scholar] [CrossRef]
- Kozin, E.D.; Gulati, S.; Kaplan, A.B.; Lehmann, A.E.; Remenschneider, A.K.; Landegger, L.D.; Cohen, M.S.; Lee, D.J. Systematic review of outcomes following observational and operative endoscopic middle ear surgery. Laryngoscope 2015, 125, 1205–1214. [Google Scholar] [CrossRef]
- Makhchoune, M.; Collard, X.; Triffaux, M.; DEWitte, O.; Lubansu, A. The utility of the CADISS® system in the dissection of epidural fibrosis in revision lumbar spine surgery (A case series). Ann. Med. Surg. 2022, 83, 104718. [Google Scholar] [CrossRef]
- Miller, K.M.; Liu, Y.C.; Weinstein, J.E.; Cohen, M.S.; Chi, D.H.; Anne, S. Outcomes in Pediatric Cholesteatoma. Otolaryngol. Head Neck Surg. 2025, 172, 299–306. [Google Scholar] [CrossRef]
- Lin, L.-J.; Chen, B.-C.; Chen, C.-Y.; Chen, P.-R.; Chou, Y.-F. Ossicular Reconstruction with Various Degrees of Malleus Preservation in Patients With Chronic Otitis Media. Ear Nose Throat J. 2024. ahead of print. [Google Scholar] [CrossRef]
- Kotzias, S.A.; Seerig, M.M.; Mello, M.F.P.C.; Chueiri, L.; Jacques, J.; Silva, M.B.C.D.; Zatt, D.B. Ossicular chain reconstruction in chronic otitis media: Hearing results and analysis of prognostic factors. Braz. J. Otorhinolaryngol. 2020, 86, 49–55. [Google Scholar] [CrossRef]
- Stankovic, M.D. Audiologic results of surgery for cholesteatoma: Short- and long-term follow-up of influential factors. Otol. Neurotol. 2008, 29, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Shiao, A.S.; Liao, W.H.; Ho, C.Y.; Lien, C.F. How long is long enough to follow up children after cholesteatoma surgery? A 29-year study. Laryngoscope 2012, 122, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.C.; Wang, C.Y.; Tsai, M.H.; Lin, C.D. Long-term follow-up of applying autologous bone grafts for reconstructing tympanomastoid defects in functional cholesteatoma surgery. PeerJ 2021, 9, e12522. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.H.; Kuo, C.L.; Huang, T.C. The anatomic applicability of transcanal endoscopic ear surgery in children. Int. J. Pediatr. Otorhinolaryngol. 2018, 105, 118–122. [Google Scholar] [CrossRef]




| Manual Dissection Clean Area | Manual Dissection Total Area | ||
|---|---|---|---|
| Sample size | 350 | Sample size | 350 |
| Lowest value | 159,840,000 | Lowest value | 5,288,240,000 |
| Highest value | 840,050,000 | Highest value | 9,926,240,000 |
| Arithmetic mean | 383,111,829 | Arithmetic mean | 8,143,257,686 |
| 95% CI for the Arithmetic mean | 366,715,635 to 399,508,022 | 95% CI for the Arithmetic mean | 8,014,627,013 to 8,271,888,359 |
| Standard deviation | 155,962,530 | Standard deviation | 1,223,550,150 |
| Shapiro–Wilk test | W = 0.9642 reject normality (p < 0.0001) | Shapiro–Wilk test | W = 0.9622 reject normality (p < 0.0001) |
| CADISS Clean Area | CADISS Total Area | ||
|---|---|---|---|
| Sample size | 320 | Sample size | 320 |
| Lowest value | 740,350,000 | Lowest value | 5,121,330,000 |
| Highest value | 2,217,450,000 | Highest value | 9,723,100,000 |
| Arithmetic mean | 1,554,416,875 | Arithmetic mean | 7,683,080,531 |
| 95% CI for the Arithmetic mean | 1,508,981,353 to 1,599,852,397 | 95% CI for the Arithmetic mean | 7,543,183,101 to 7,822,977,962 |
| Standard deviation | 413,115,583 | Standard deviation | 1,271,996,139 |
| Shapiro–Wilk test | W = 0.9678 | Shapiro–Wilk test | W = 0.9691 |
| Manual Dissection Clean Area/Total Area | CADISS Clean Area/Total Area | ||
|---|---|---|---|
| Sample size | 350 | Sample size | 320 |
| Lowest value | 0.01610 | Lowest value | 0.07650 |
| Highest value | 0.1277 | Highest value | 0.4284 |
| Arithmetic mean | 0.04782 | Arithmetic mean | 0.2095 |
| 95% CI for the Arithmetic mean | 0.04567 to 0.04997 | 95% CI for the Arithmetic mean | 0.2017 to 0.2173 |
| Standard deviation | 0.02044 | Standard deviation | 0.07092 |
| Shapiro–Wilk test | W = 0.9563 reject normality (p < 0.0001) | Shapiro–Wilk test | W = 0.9788 reject normality (p = 0.0001) |
| Manual Dissection Clean Area/Total Area | CADISS Clean Area/Total Area | |
|---|---|---|
| Sample size | 350 | 320 |
| Lowest value | 0.01610 | 0.07650 |
| Highest value | 0.1277 | 0.4284 |
| Median | 0.04635 | 0.2004 |
| 95% CI for the median | 0.04297 to 0.04898 | 0.1927 to 0.2107 |
| Interquartile range | 0.03300 to 0.05960 | 0.1587 to 0.2551 |
| Hodges–Lehmann median difference | 0.1546 | |
| 95% Confidence interval | 0.1464 to 0.1627 | |
| Mann–Whitney test (independent samples) | ||
| Average rank of the first group | 1,763,229 | |
| Average rank of the second group | 5,096,000 | |
| Mann–Whitney U | 28,800 | |
| Test statistic Z | −22,262 | |
| Two-tailed probability | p < 0.0001 | |
| Patients | Positive MRI 1 Month After Surgery | |
|---|---|---|
| Manual dissection | 35 | 4 (11.4%) |
| CADISS | 32 | 1 (3.1%) |
| Total | 67 | 5 (7.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Relucenti, M.; Romeo Plastina, U.; Fino, P.; Filippi, C.; Barbara, M.; Covelli, E. Improved Cholesteatoma Removal with CADISS: A Quantitative Ultrastructural Comparison Using VP-SEM. J. Clin. Med. 2025, 14, 4192. https://doi.org/10.3390/jcm14124192
Relucenti M, Romeo Plastina U, Fino P, Filippi C, Barbara M, Covelli E. Improved Cholesteatoma Removal with CADISS: A Quantitative Ultrastructural Comparison Using VP-SEM. Journal of Clinical Medicine. 2025; 14(12):4192. https://doi.org/10.3390/jcm14124192
Chicago/Turabian StyleRelucenti, Michela, Ubaldo Romeo Plastina, Pasquale Fino, Chiara Filippi, Maurizio Barbara, and Edoardo Covelli. 2025. "Improved Cholesteatoma Removal with CADISS: A Quantitative Ultrastructural Comparison Using VP-SEM" Journal of Clinical Medicine 14, no. 12: 4192. https://doi.org/10.3390/jcm14124192
APA StyleRelucenti, M., Romeo Plastina, U., Fino, P., Filippi, C., Barbara, M., & Covelli, E. (2025). Improved Cholesteatoma Removal with CADISS: A Quantitative Ultrastructural Comparison Using VP-SEM. Journal of Clinical Medicine, 14(12), 4192. https://doi.org/10.3390/jcm14124192







