Stereotactically Guided Microsurgical Approach for Deep-Seated Eloquently Located Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Study Design
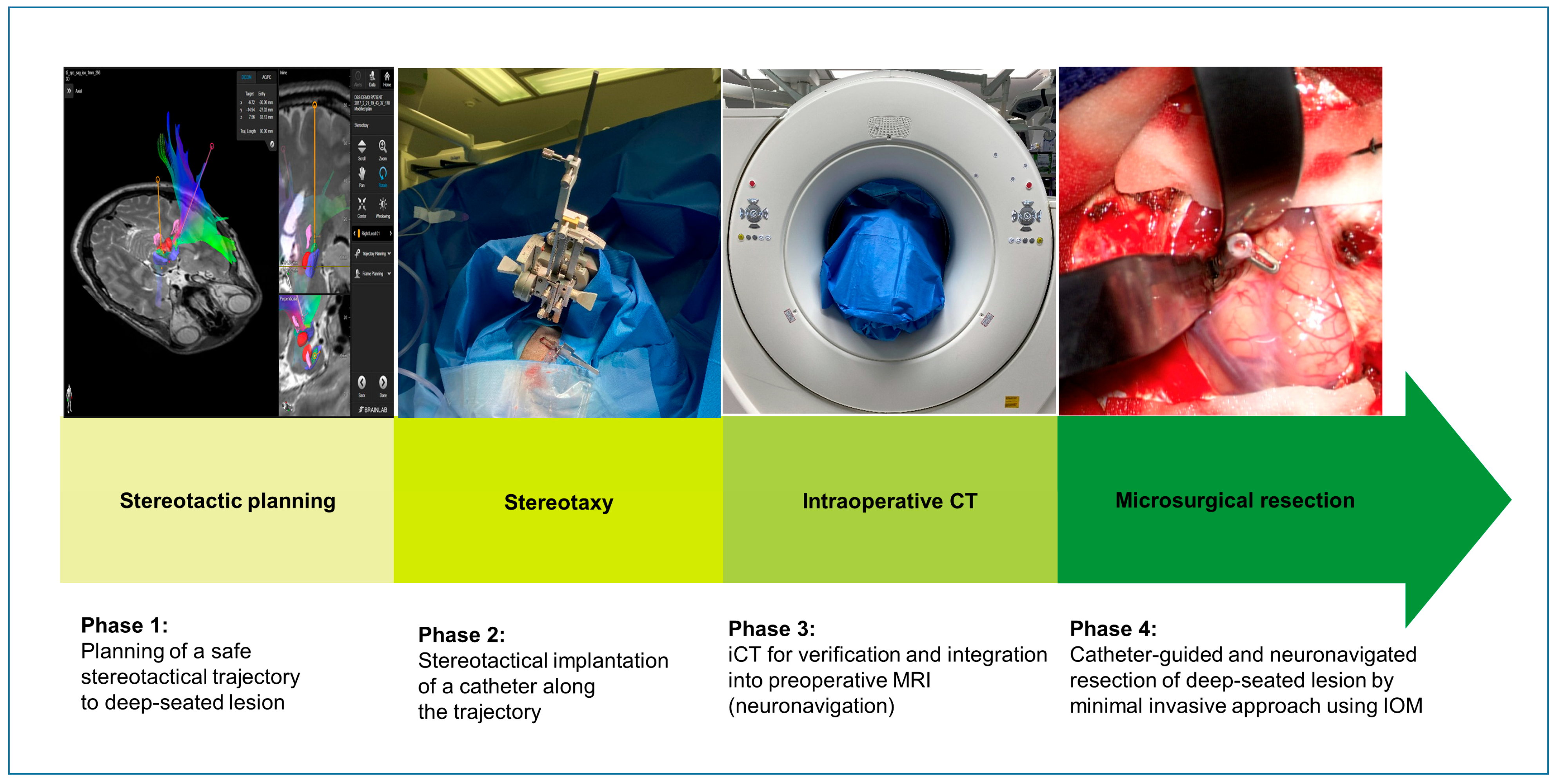
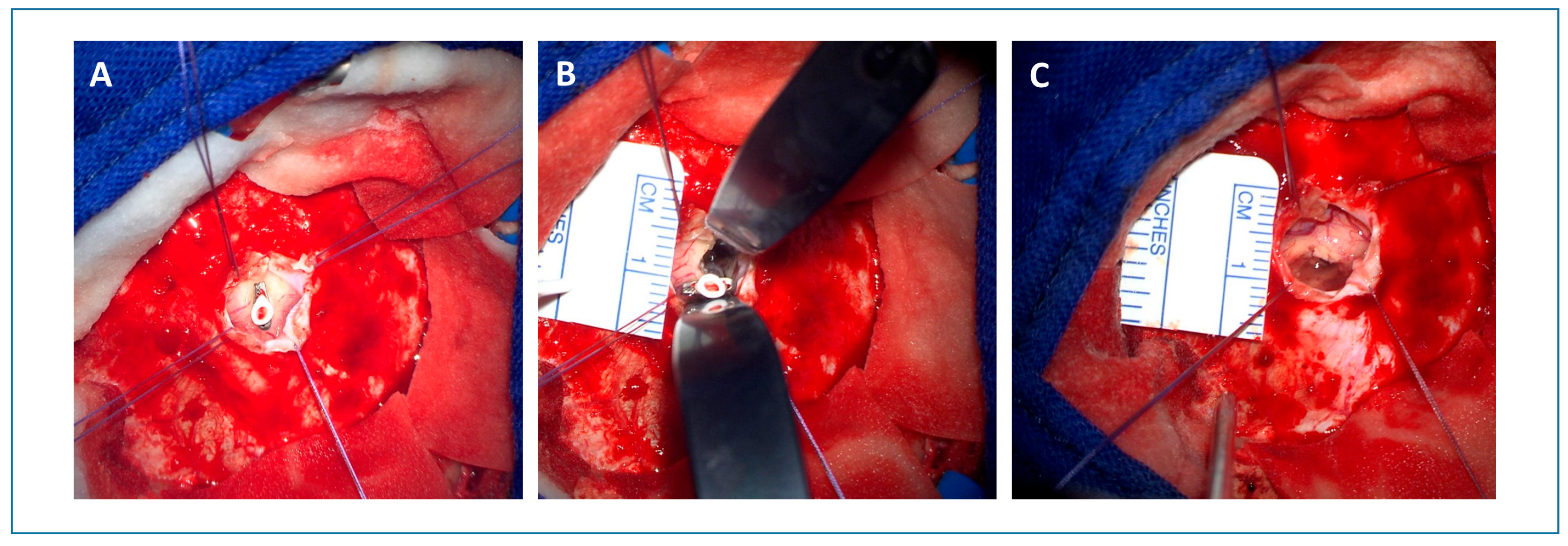
2.2. Surgical Technique
3. Results
3.1. Patient Characteristics
3.2. Surgical Results Using the Stereotactically Guided Neuronavigated Microsurgical Approach
3.3. Neurological Outcome
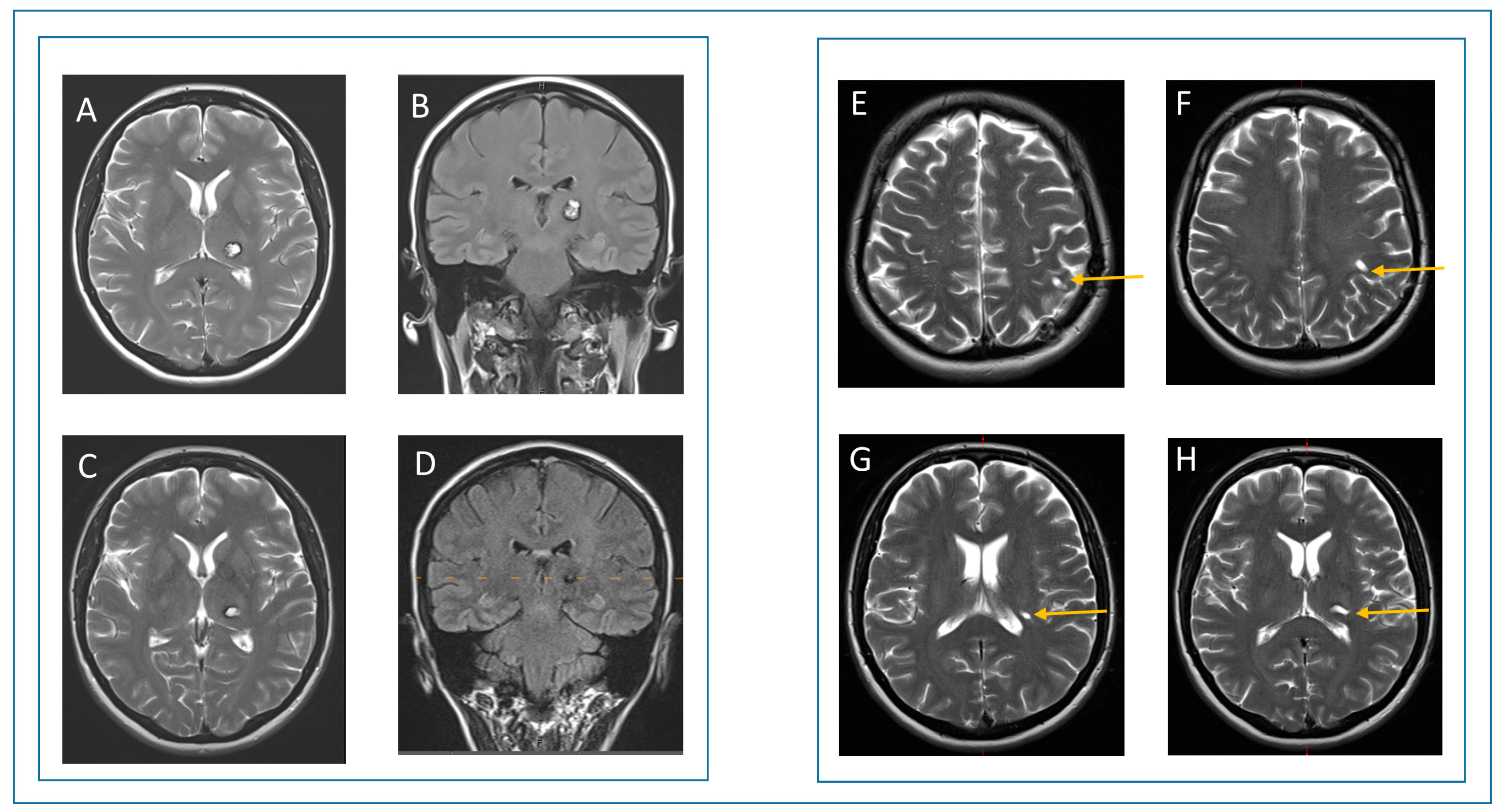
3.4. Case Report
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| iCT | Intraoperative computer tomography |
| DTI | Diffusion-tensor-imaging |
| mRS | modified Rankin score |
| GOS | Glasgow Outcome Scale |
| CSF | Cerebrospinal fluid |
| iUS | Intraoperative ultrasound |
| iMRI | Intraoperative magnetic resonance imaging |
| GTR | gross total resection |
| STR | subtotal resection |
| NTR | near total resection |
| DVT | deep vein thrombosis |
References
- Gassie, K.; Wijesekera, O.; Chaichana, K.L. Minimally invasive tubular retractor-assisted biopsy and resection of subcortical intra-axial gliomas and other neoplasms. J. Neurosurg. Sci. 2018, 62, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Chaichana, K.L. Minimally Invasive Resection of Deep-seated High-grade Gliomas Using Tubular Retractors and Exoscopic Visualization. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2018, 79, 330–336. [Google Scholar] [CrossRef]
- Jackson, C.; Gallia, G.L.; Chaichana, K.L. Minimally Invasive Biopsies of Deep-Seated Brain Lesions Using Tubular Retractors Under Exoscopic Visualization. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2017, 78, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Vivas-Buitrago, T.; Jackson, C.; Ehresman, J.; Olivi, A.; Bettegowda, C.; Quinones-Hinojosa, A. The Radiographic Effects of Surgical Approach and Use of Retractors on the Brain After Anterior Cranial Fossa Meningioma Resection. World Neurosurg. 2018, 112, e505–e513. [Google Scholar] [CrossRef]
- Rosenorn, J.; Diemer, N.H. Reduction of regional cerebral blood flow during brain retraction pressure in the rat. J. Neurosurg. 1982, 56, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Dujovny, M.; Perlin, A.R.; Perez-Arjona, E.; Park, H.K.; Diaz, F.G. Brain retraction injury. Neurol. Res. 2003, 25, 831–838. [Google Scholar] [CrossRef]
- Newman, W.C.; Engh, J.A. Stereotactic-Guided Dilatable Endoscopic Port Surgery for Deep-Seated Brain Tumors: Technical Report with Comparative Case Series Analysis. World Neurosurg. 2019, 125, e812–e819. [Google Scholar] [CrossRef]
- Bander, E.D.; Jones, S.H.; Kovanlikaya, I.; Schwartz, T.H. Utility of tubular retractors to minimize surgical brain injury in the removal of deep intraparenchymal lesions: A quantitative analysis of FLAIR hyperintensity and apparent diffusion coefficient maps. J. Neurosurg. 2016, 124, 1053–1060. [Google Scholar] [CrossRef]
- Cohen-Gadol, A.A. Minitubular transcortical microsurgical approach for gross total resection of third ventricular colloid cysts: Technique and assessment. World Neurosurg. 2013, 79, 207.e7–207.e10. [Google Scholar] [CrossRef]
- Marenco-Hillembrand, L.; Alvarado-Estrada, K.; Chaichana, K.L. Contemporary Surgical Management of Deep-Seated Metastatic Brain Tumors Using Minimally Invasive Approaches. Front. Oncol. 2018, 8, 558. [Google Scholar] [CrossRef]
- Shapiro, S.Z.; Sabacinski, K.A.; Mansour, S.A.; Echeverry, N.B.; Shah, S.S.; Stein, A.A.; Snelling, B.M. Use of Vycor Tubular Retractors in the Management of Deep Brain Lesions: A Review of Current Studies. World Neurosurg. 2020, 133, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Flores, B.C.; Whittemore, A.R.; Samson, D.S.; Barnett, S.L. The utility of preoperative diffusion tensor imaging in the surgical management of brainstem cavernous malformations. J. Neurosurg. 2015, 122, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Holodny, A.I.; Ollenschleger, M.D.; Liu, W.C.; Schulder, M.; Kalnin, A.J. Identification of the corticospinal tracts achieved using blood-oxygen-level-dependent and diffusion functional MR imaging in patients with brain tumors. AJNR Am. J. Neuroradiol. 2001, 22, 83–88. [Google Scholar] [PubMed]
- Holodny, A.I.; Schwartz, T.H.; Ollenschleger, M.; Liu, W.C.; Schulder, M. Tumor involvement of the corticospinal tract: Diffusion magnetic resonance tractography with intraoperative correlation. J. Neurosurg. 2001, 95, 1082. [Google Scholar] [CrossRef] [PubMed]
- Bastos, D.C.A.; Juvekar, P.; Tie, Y.; Jowkar, N.; Pieper, S.; Wells, W.M.; Bi, W.L.; Golby, A.; Frisken, S.; Kapur, T. Challenges and Opportunities of Intraoperative 3D Ultrasound With Neuronavigation in Relation to Intraoperative MRI. Front. Oncol. 2021, 11, 656519. [Google Scholar] [CrossRef]
- Katzendobler, S.; Do, A.; Weller, J.; Dorostkar, M.M.; Albert, N.L.; Forbrig, R.; Niyazi, M.; Egensperger, R.; Thon, N.; Tonn, J.C.; et al. Diagnostic Yield and Complication Rate of Stereotactic Biopsies in Precision Medicine of Gliomas. Front. Neurol. 2022, 13, 822362. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ahmadian, A.; Azar, A.D.; Sheykh, A.D.; Amiri, F.; Alirezaie, J. Estimation of intraoperative brain shift by combination of stereovision and doppler ultrasound: Phantom and animal model study. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 1753–1764. [Google Scholar] [CrossRef]
- Ardeshiri, A.; Radina, C.; Edlauer, M.; Ardeshiri, A.; Riepertinger, A.; Nerlich, A.; Tonn, J.C.; Winkler, P.A. Evaluation of new radiolucent polymer headholder pins for use in intraoperative computed tomography. J. Neurosurg. 2009, 111, 1168–1174. [Google Scholar] [CrossRef]
- Schichor, C.; Rachinger, W.; Morhard, D.; Zausinger, S.; Heigl, T.J.; Reiser, M.; Tonn, J.C. Intraoperative computed tomography angiography with computed tomography perfusion imaging in vascular neurosurgery: Feasibility of a new concept. J. Neurosurg. 2010, 112, 722–728. [Google Scholar] [CrossRef]
- Schichor, C.; Terpolilli, N.; Thorsteinsdottir, J.; Tonn, J.C. Intraoperative Computed Tomography in Cranial Neurosurgery. Neurosurg. Clin. N. Am. 2017, 28, 595–602. [Google Scholar] [CrossRef]
- Schnell, O.; Morhard, D.; Holtmannspotter, M.; Reiser, M.; Tonn, J.C.; Schichor, C. Near-infrared indocyanine green videoangiography (ICGVA) and intraoperative computed tomography (iCT): Are they complementary or competitive imaging techniques in aneurysm surgery? Acta Neurochir. 2012, 154, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Szelenyi, A.; Langer, D.; Kothbauer, K.; De Camargo, A.B.; Flamm, E.S.; Deletis, V. Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: Intraoperative changes and postoperative outcome. J. Neurosurg. 2006, 105, 675–681. [Google Scholar] [CrossRef]
- Spena, G.; Garbossa, D.; Panciani, P.P.; Griva, F.; Fontanella, M.M. Purely subcortical tumors in eloquent areas: Awake surgery and cortical and subcortical electrical stimulation (CSES) ensure safe and effective surgery. Clin. Neurol. Neurosurg. 2013, 115, 1595–1601. [Google Scholar] [CrossRef]
- Zanello, M.; Meyer, B.; Still, M.; Goodden, J.R.; Colle, H.; Schichor, C.; Bello, L.; Wager, M.; Smits, A.; Rydenhag, B.; et al. Surgical resection of cavernous angioma located within eloquent brain areas: International survey of the practical management among 19 specialized centers. Seizure 2019, 69, 31–40. [Google Scholar] [CrossRef]
- Beggio, G.; Raneri, F.; Rustemi, O.; Scerrati, A.; Zambon, G.; Piacentino, M. Techniques for pneumocephalus and brain shift reduction in DBS surgery: A review of the literature. Neurosurg. Rev. 2020, 43, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Brimley, C.; Shimony, N. Accuracy and Utility of Frameless Stereotactic Placement of Stereoelectroencephalography Electrodes. World Neurosurg. 2023, 180, e226–e232. [Google Scholar] [CrossRef]
- Nimsky, C.; Ganslandt, O.; Cerny, S.; Hastreiter, P.; Greiner, G.; Fahlbusch, R. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery 2000, 47, 1070–1079; discussion 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Reinges, M.H.; Nguyen, H.H.; Krings, T.; Hutter, B.O.; Rohde, V.; Gilsbach, J.M. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: Limits of conventional neuronavigation. Acta Neurochir. 2004, 146, 369–377; discussion 377. [Google Scholar] [CrossRef]
- Trantakis, C.; Tittgemeyer, M.; Schneider, J.P.; Lindner, D.; Winkler, D.; Strauss, G.; Meixensberger, J. Investigation of time-dependency of intracranial brain shift and its relation to the extent of tumor removal using intra-operative MRI. Neurol. Res. 2003, 25, 9–12. [Google Scholar] [CrossRef]
- Hassenbusch, S.J.; Anderson, J.S.; Pillay, P.K. Brain tumor resection aided with markers placed using stereotaxis guided by magnetic resonance imaging and computed tomography. Neurosurgery 1991, 28, 801–805; discussion 805–806. [Google Scholar] [CrossRef]
- Dixon, L.; Lim, A.; Grech-Sollars, M.; Nandi, D.; Camp, S. Intraoperative ultrasound in brain tumor surgery: A review and implementation guide. Neurosurg. Rev. 2022, 45, 2503–2515. [Google Scholar] [CrossRef]
- Mazzucchi, E.; La Rocca, G.; Hiepe, P.; Pignotti, F.; Galieri, G.; Policicchio, D.; Boccaletti, R.; Rinaldi, P.; Gaudino, S.; Ius, T.; et al. Intraoperative Integration of Multimodal Imaging to Improve Neuronavigation: A Technical Note. World Neurosurg. 2022, 164, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Sass, B.; Zivkovic, D.; Pojskic, M.; Nimsky, C.; Bopp, M.H.A. Navigated Intraoperative 3D Ultrasound in Glioblastoma Surgery: Analysis of Imaging Features and Impact on Extent of Resection. Front. Neurosci. 2022, 16, 883584. [Google Scholar] [CrossRef] [PubMed]
- Sommer, B.; Kasper, B.S.; Coras, R.; Blumcke, I.; Hamer, H.M.; Buchfelder, M.; Roessler, K. Surgical management of epilepsy due to cerebral cavernomas using neuronavigation and intraoperative MR imaging. Neurol. Res. 2013, 35, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Forbrig, R.; Geyer, L.L.; Stahl, R.; Thorsteinsdottir, J.; Schichor, C.; Kreth, F.W.; Patzig, M.; Herzberg, M.; Liebig, T.; Dorn, F.; et al. Radiation dose and image quality in intraoperative CT (iCT) angiography of the brain with stereotactic head frames. Eur. Radiol. 2019, 29, 2859–2867. [Google Scholar] [CrossRef]
- Gassie, K.; Alvarado-Estrada, K.; Bechtle, P.; Chaichana, K.L. Surgical Management of Deep-Seated Metastatic Brain Tumors Using Minimally Invasive Approaches. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2019, 80, 198–204. [Google Scholar] [CrossRef]
- Takeuchi, K.; Nagata, Y.; Tanahashi, K.; Araki, Y.; Mizuno, A.; Sasaki, H.; Harada, H.; Ito, K.; Saito, R. Efficacy and safety of the endoscopic “wet-field” technique for removal of supratentorial cavernous malformations. Acta Neurochir. 2022, 164, 2587–2594. [Google Scholar] [CrossRef]
- Liu, S.; Wu, S.; Xie, T.; Yeh, Y.Y.; Li, C.; Liu, T.; Sun, C.; Yang, L.; Li, Z.; Yu, Y.; et al. Neuronavigation-Guided Transcortical-Transventricular Endoport-Assisted Endoscopic Resection for Thalamic Lesions: Preliminary Experience. World Neurosurg. 2022, 166, 19–27. [Google Scholar] [CrossRef]
- Achey, R.; Kashkoush, A.; Potter, T.; Davison, M.; Moore, N.Z.; Kshettry, V.R.; Bain, M. Surgical Resection of Deep-Seated Arteriovenous Malformations Through Stereotactically Guided Tubular Retractor Systems: A Case Series. Oper Neurosurg. 2023, 24, 499–506. [Google Scholar] [CrossRef]
- Hey, G.; Guyot, M.; Carter, A.; Lucke-Wold, B. Augmented Reality in Neurosurgery: A New Paradigm for Training. Medicina 2023, 59, 1721. [Google Scholar] [CrossRef]
- Satoh, M.; Nakajima, T.; Watanabe, E.; Kawai, K. Augmented Reality in Stereotactic Neurosurgery: Current Status and Issues. Neurol. Med. Chir. 2023, 63, 137–140. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| - single lesion | - multiple lesions |
| - intraparenchymatous/intraventricular lesion | - no anatomical association to nerval structures |
| - well circumscribed in T1-weighted imaging with contrast enhancement | - infiltrative growth |
| - without significant perilesional edema (on T2-weighted imaging) | - perilesional edema (on T2-weighted imaging) |
| - 10–30 mm in diameter | - >30 mm in diameter |
| - ≥4 cm from the cortical surface AND/OR location in eloquent regions, e.g., basal ganglia, thalamus, mesencephalon or language-associated cortical areas | |
| - suspicion of a benign lesion | - suspicion of a highly aggressive tumor |
| Patient No. | Age | Gender | Side | Location | Preoperative Neurological Deficit | Preoperative mRS | Preoperative GOS | Postoperative Neurological Deficit | Postoperative mRS | Postoperative GOS | Neurological Deficit at Last FU | mRS, Last FU | GOS Last FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | F | L | white matter of the central region | focal seizures (facial numbness and spasms) | 1 | 4 | none | 0 | 5 | none | 0 | 5 |
| 2 | 18 | F | L | dorsal capsula interna, lentiform nucleus | focal seizures, ataxia, spasticity | 1 | 4 | none | 0 | 5 | none | 0 | 5 |
| 3 | 16 | F | R | thalamus, cerebral crus, dorsal internal capsule | left hemiparesis (proximal 3/5, distal 1/5), hemihypesthesia | 3 | 3 | left hemiparesis (proximal 4/5, distal 2/5), hemihypesthesia improved | 2 | 4 | left hemiparesis (distal 3/5), hemihypesthesia improved | 2 | 4 |
| 4 | 66 | M | R | frontal operculum | hemihypesthesia | 1 | 4 | none | 0 | 5 | none | 0 | 5 |
| 5 | 59 | F | R | basal ganglia | no deficit (incidental finding) | 0 | 5 | none | 0 | 5 | none | 0 | 5 |
| 6 | 28 | F | R | insula, external capsule, putamen | hemihypesthesia | 1 | 4 | none | 0 | 5 | one seizure after cessation of anticonvulsants | 1 | 5 |
| 7 | 37 | F | L | eloquent superior temporal gyrus | focal seizures (expressive aphasia) | 1 | 4 | none | 0 | 5 | none | 0 | 5 |
| 8 | 27 | F | L | eloquent superior temporal gyrus | focal seizures (expressive aphasia) | 1 | 4 | none | 0 | 5 | none | 0 | 5 |
| 9 | 36 | F | R | anterior part of the insula | focal seizures (hypesthesia right arm, dysarthria) | 1 | 4 | none | 0 | 5 | none | 0 | 5 |
| 10 | 19 | M | R | trigonum | no deficit (incidental finding) | 0 | 5 | none | 0 | 5 | none | 0 | 5 |
| 11 | 52 | F | L | thalamus | hypesthesia right arm | 1 | 4 | transient right hemiparesis (2/5), hypesthesia right arm | 3 | 3 | none | 0 | 5 |
| 12 | 46 | F | R | splenium | focal seizures | 1 | 5 | none | 0 | 0 | none | 0 | 5 |
| Patient No. | Lesion Size [mm] | Lesion Depth [mm] | Histology | Craniotomy Size [mm] | Trajectory Length [mm] | Mean Trajectory Diameter [mm] | Std Mean Trajectory Diameter [mm] | Duration of Planning and Stereotaxy [min] | Duration of Surgery [min] | Extent of Resection (in MRI) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | 43 | Cavernoma | 43 | 61.4 | 5.9 | 1.6 | 00:23 | 03:43 | GTR |
| 2 | 22 | 46 | Cavernoma | 45 | 62.7 | 4.9 | 1.0 | 00:34 | 04:00 | GTR |
| 3 | 31 | 62 | pilocytic astrocytoma | 34 | 64.5 | 6.8 | 2.5 | 00:23 | 05:42 | GTR |
| 4 | 19 | 41 | Cavernoma | 36 | 48.6 | 6.5 | 1.3 | 00:25 | 02:44 | GTR |
| 5 | 27 | 52 | Cavernoma | 33 | 66.6 | 4.7 | 1.5 | 00:15 | 03:14 | GTR |
| 6 | 23 | 36 | Cavernoma | 27 | 43.2 | 5.5 | 0.4 | 00:22 | 03:48 | GTR |
| 7 | 11 | 13 | Cavernoma | 32 | 45.7 | 7.0 | 0.6 | 00:23 | 02:16 | GTR |
| 8 | 22 | 14 | Cavernoma | 31 | 44.0 | 9.4 | 3.1 | 00:22 | 03:00 | GTR |
| 9 | 20 | 24 | Cavernoma | 36 | 68.8 | 4.2 | 0.6 | 00:19 | 02:53 | GTR |
| 10 | 18 | 36 | Meningioma | 30 | 66.8 | 6.1 | 0.4 | 00:46 | 03:25 | GTR |
| 11 | 10 | 46 | Cavernoma | 41 | 72.1 | 5.2 | 0.8 | 00:25 | 02:55 | GTR |
| 12 | 19 | 57 | Cavernoma | 19 | 63 | 5.1 | 0.3 | 00:32 | 01:57 | GTR (after second surgery) |
| Study (Year) | Approach | No. of Patients | Diameter [mm] | Median Size of Lesion [mm] | Median Craniotomy Size [mm] | Operative Time | Median Blood Loss [mL] | Complication Rate | Neurological Outcome | Extent of Resection |
|---|---|---|---|---|---|---|---|---|---|---|
| Liu et al. [38] (2022) | Neuronavigation-Guided Transcortical-Transventricular Endoport-Assisted Endoscopic Resection for Thalamic Lesions: Preliminary Experience | 8 | NA | 31 | NA | NA | NA | n = 2 (electrolyte disturbance) n = 1 (subdural hematoma) n = 1 (severe pneumonia) n = 1 (DVT) | Improved (n = 3) Stable (n = 3) Worsened (n = 2) | GTR (n = 4) NTR (n = 3) STR (n = 1) |
| Achey et al. [39] (2023) | Surgical Resection of Deep-Seated Arteriovenous Malformations Through Stereotactically Guided Tubular Retractor Systems: A Case Series. | 5 | NA | 8.2 | NA | NA | NA | NA | NA | GTR (n = 5) |
| Takeuchi et al. [37] (2022) | Efficacy and safety of the endoscopic “wet-field” technique for removal of supratentorial cavernous malformations | 13 | 6 (n = 8) 10 (n = 4) 17 (n = 1) | 22.9 | 55 (range: 40–70) | NA | NA | NA | Improved (n = 12) Stable (n = 1) | GTR (n = 12) STR (n = 1) |
| Our study | Stereotactically guided microsurgical resection of deep-seated or eloquently located lesions using intraoperative computed tomography | 12 | 6 ± 1.2 | 19.5 | 35 (range: 27–45) | 23 min/3 h 7 min | 100 (range: 30–300) | NA | Improved (n = 1) No deficit (n = 11) | GTR (n = 12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorsteinsdottir, J.; Siller, S.; Harapan, B.N.; Forbrig, R.; Tonn, J.-C.; Greve, T.; Quach, S.; Schichor, C. Stereotactically Guided Microsurgical Approach for Deep-Seated Eloquently Located Lesions. J. Clin. Med. 2025, 14, 4175. https://doi.org/10.3390/jcm14124175
Thorsteinsdottir J, Siller S, Harapan BN, Forbrig R, Tonn J-C, Greve T, Quach S, Schichor C. Stereotactically Guided Microsurgical Approach for Deep-Seated Eloquently Located Lesions. Journal of Clinical Medicine. 2025; 14(12):4175. https://doi.org/10.3390/jcm14124175
Chicago/Turabian StyleThorsteinsdottir, Jun, Sebastian Siller, Biyan Nathanael Harapan, Robert Forbrig, Jörg-Christian Tonn, Tobias Greve, Stefanie Quach, and Christian Schichor. 2025. "Stereotactically Guided Microsurgical Approach for Deep-Seated Eloquently Located Lesions" Journal of Clinical Medicine 14, no. 12: 4175. https://doi.org/10.3390/jcm14124175
APA StyleThorsteinsdottir, J., Siller, S., Harapan, B. N., Forbrig, R., Tonn, J.-C., Greve, T., Quach, S., & Schichor, C. (2025). Stereotactically Guided Microsurgical Approach for Deep-Seated Eloquently Located Lesions. Journal of Clinical Medicine, 14(12), 4175. https://doi.org/10.3390/jcm14124175







