Catheter-Based Therapies in Acute Pulmonary Embolism—Mortality and Safety Outcomes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection and Eligibility Criteria
2.2. Data Extraction and Quality Assessment
2.3. Study Outcomes and Statistical Analysis
3. Results
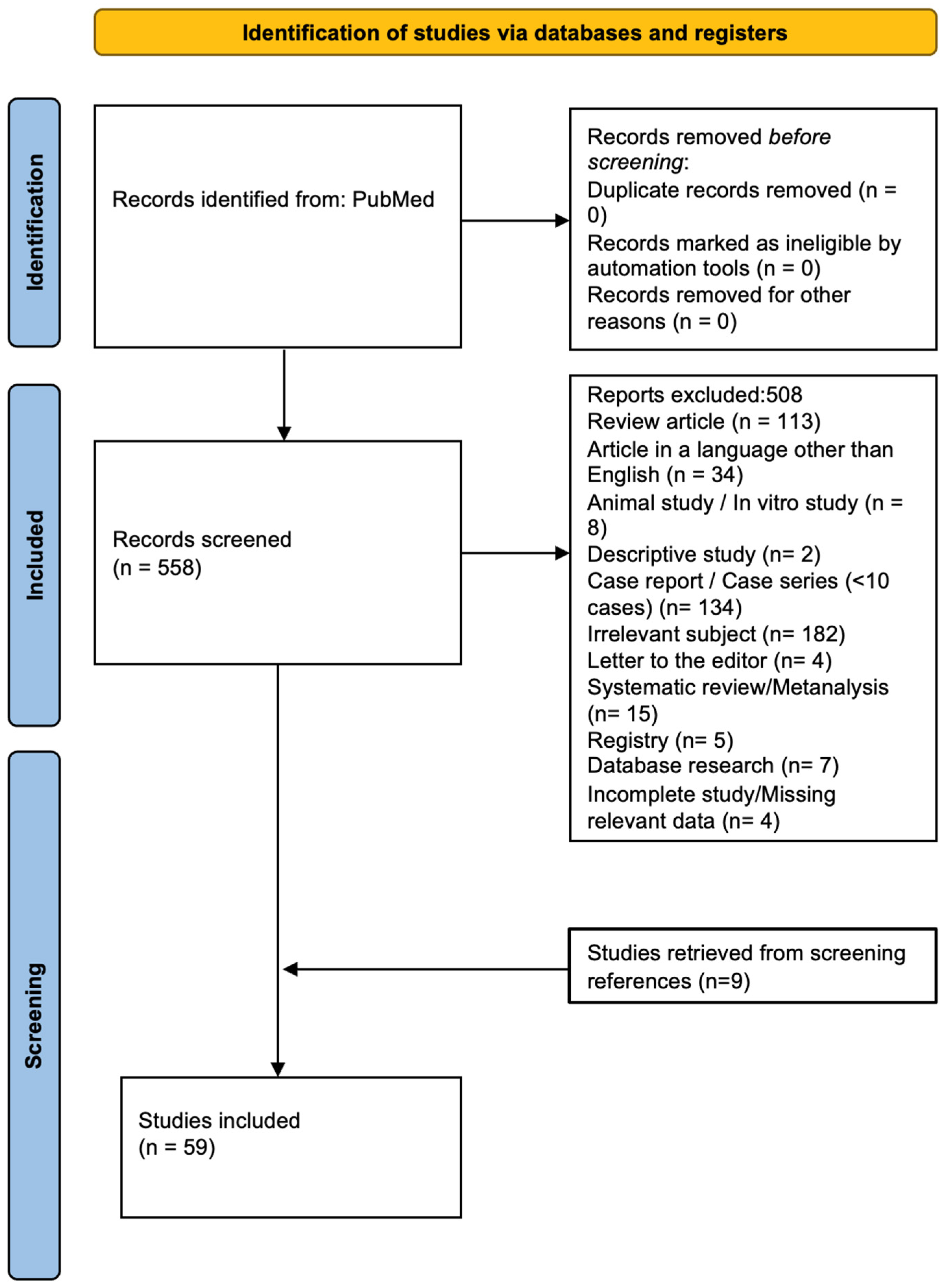
3.1. Search Results
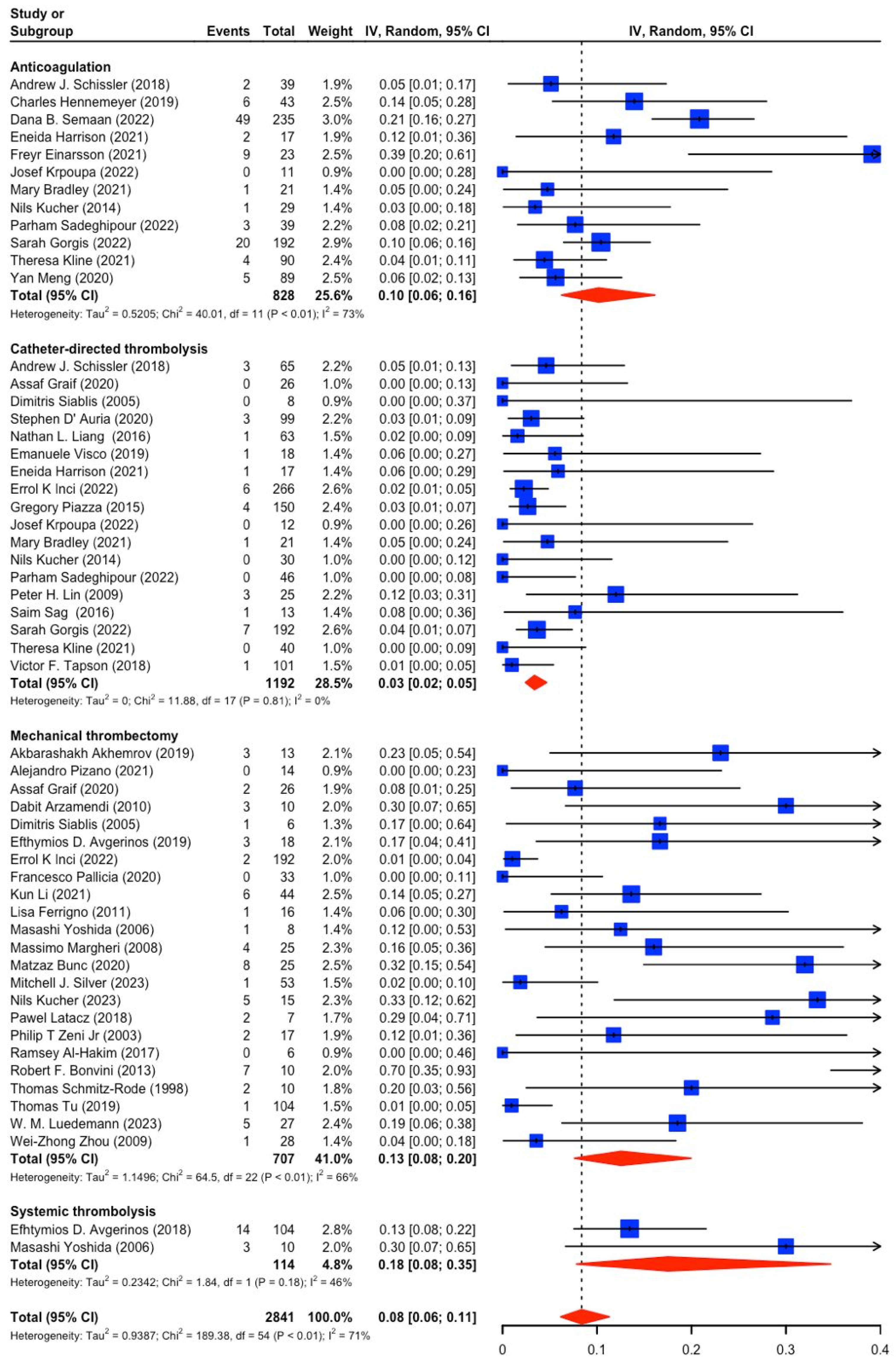
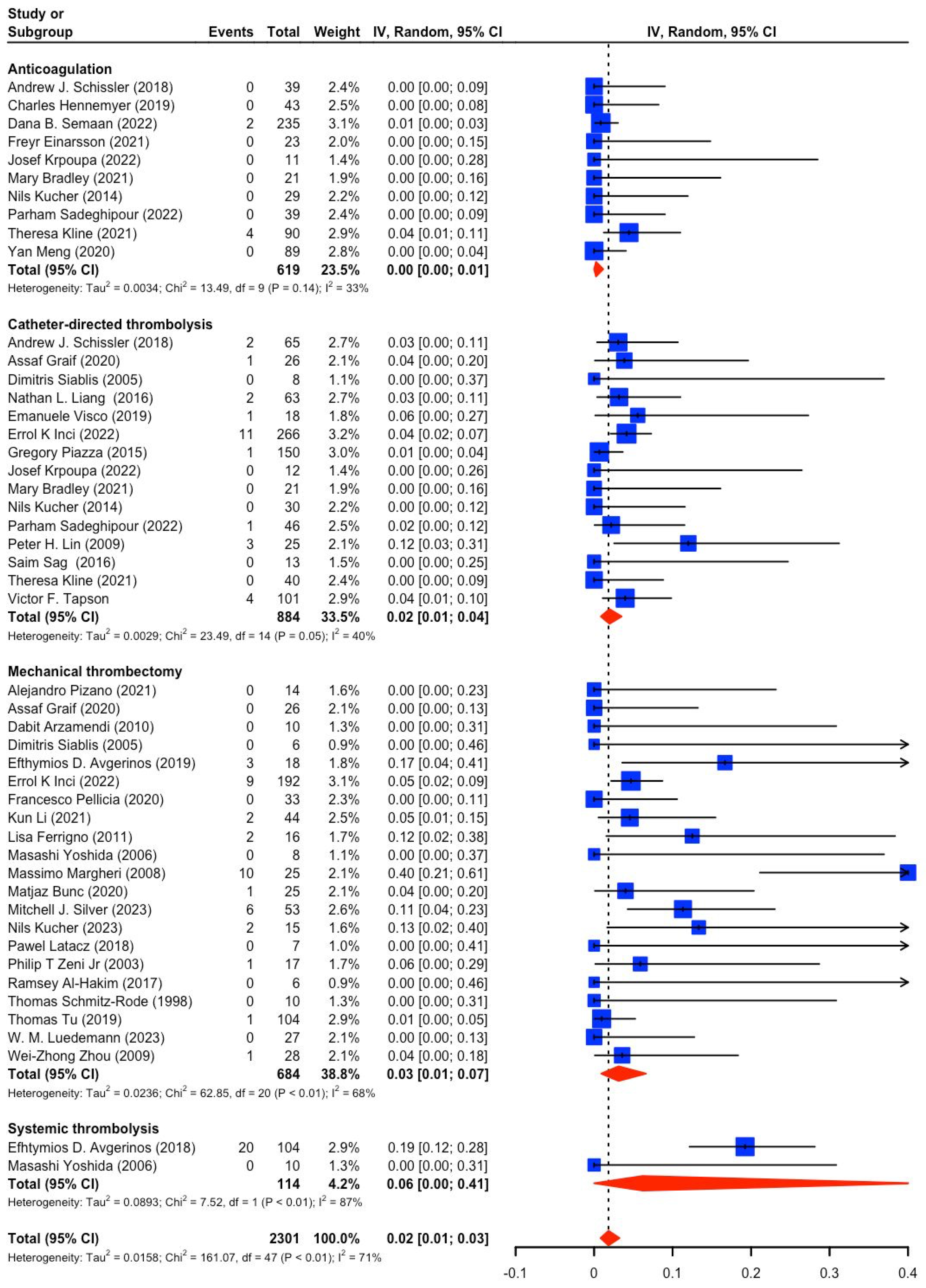
3.2. Study and Patient Characteristics (PE Severity, Mortality, Bleeding)
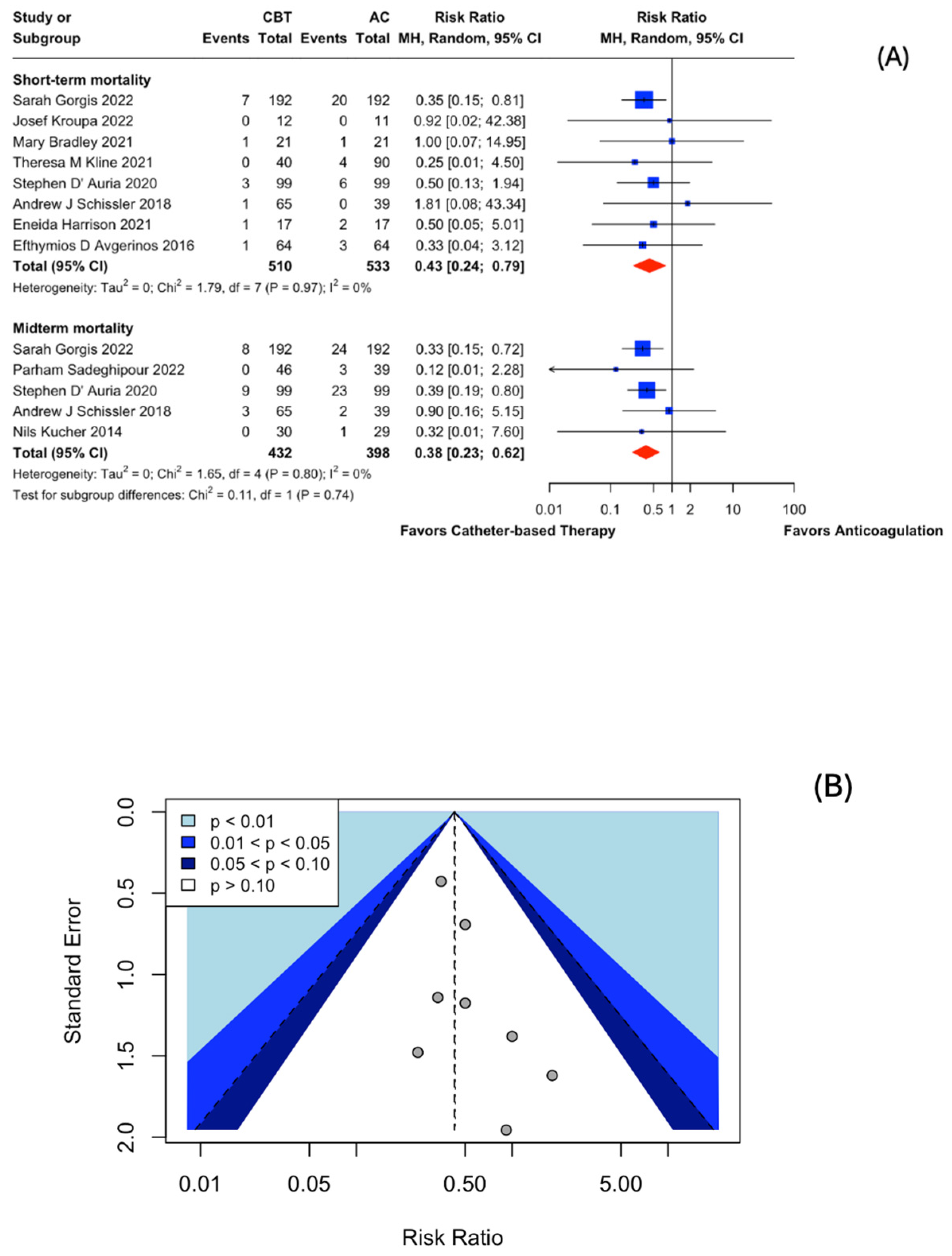
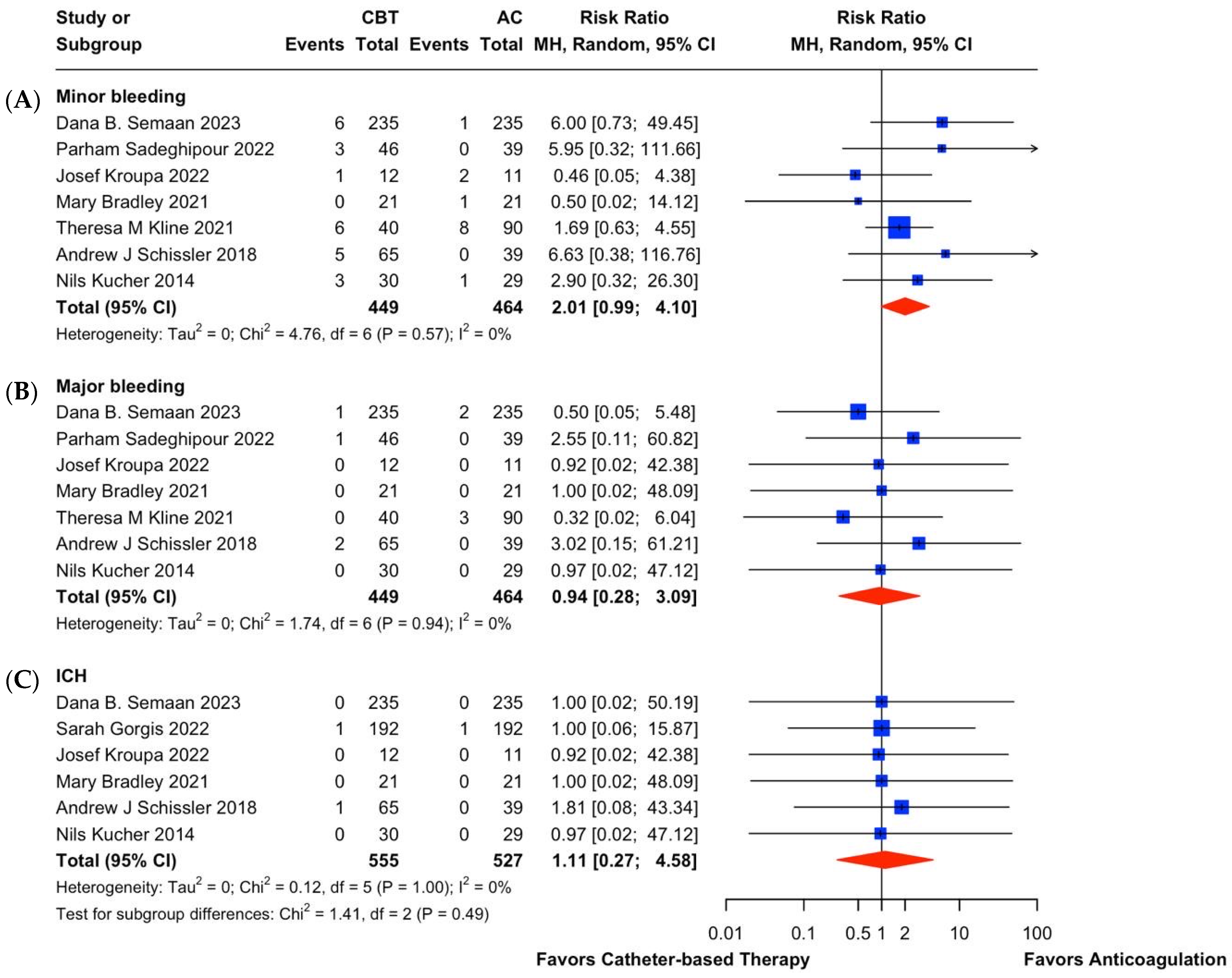
3.3. Catheter-Based Therapies (CBT) vs. Anticoagulation (AC) in Intermediate-Risk PE
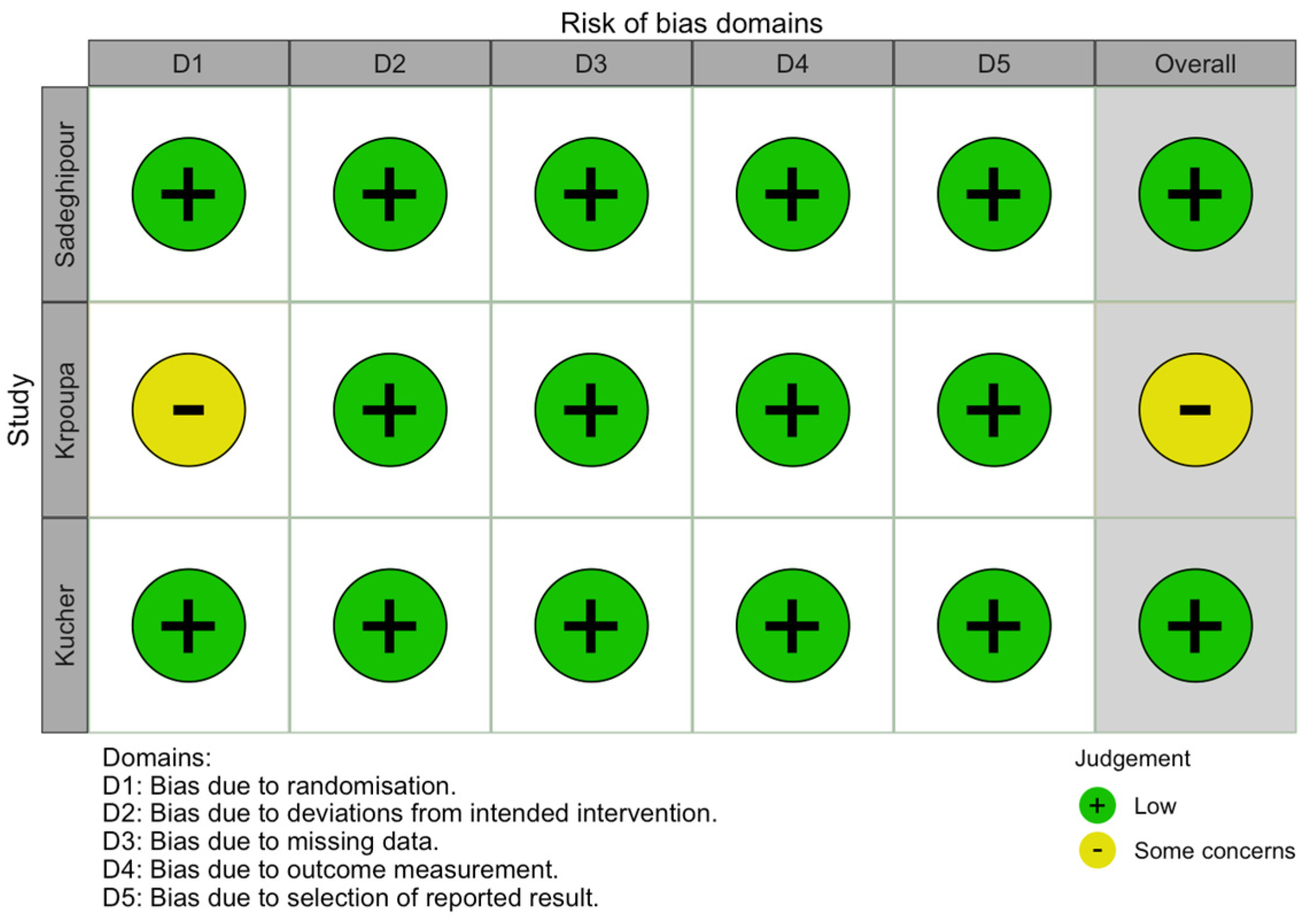
3.4. Quality (Risk of Bias) Assessment
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S. V 2014 ESC Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism. Eur. Heart J. 2014, 35, 3145–3146. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism Developed in Collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Sista, A.K.; Faintuch, S.; Dariushnia, S.R.; Baerlocher, M.O.; Lookstein, R.A.; Haskal, Z.J.; Nikolic, B.; Gemmete, J.J. Society of Interventional Radiology Position Statement on Catheter-Directed Therapy for Acute Pulmonary Embolism. J. Vasc. Interv. Radiol. 2018, 29, 293–297. [Google Scholar] [CrossRef]
- Rivera-Lebron, B.N.; Rali, P.M.; Tapson, V.F. The PERT Concept. Chest 2021, 159, 347–355. [Google Scholar] [CrossRef]
- Stevens, S.M.; Woller, S.C.; Kreuziger, L.B.; Bounameaux, H.; Doerschug, K.; Geersing, G.-J.; Huisman, M.V.; Kearon, C.; King, C.S.; Knighton, A.J.; et al. Antithrombotic Therapy for VTE Disease. Chest 2021, 160, e545–e608. [Google Scholar] [CrossRef]
- Giri, J.; Sista, A.K.; Weinberg, I.; Kearon, C.; Kumbhani, D.J.; Desai, N.D.; Piazza, G.; Gladwin, M.T.; Chatterjee, S.; Kobayashi, T.; et al. Interventional Therapies for Acute Pulmonary Embolism: Current Status and Principles for the Development of Novel Evidence: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e774–e801. [Google Scholar] [CrossRef]
- Frat, J.-P.; Ciurzyński, M. Intermediate-Risk Acute Pulmonary Embolism. Chest 2024, 165, 484–485. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wright, C.; Pietropaoli, A.P.; Elbadawi, A.; Delehanty, J.; Barrus, B.; Gosev, I.; Trawick, D.; Patel, D.; Cameron, S.J. Right Ventricular Dysfunction Is Superior and Sufficient for Risk Stratification by a Pulmonary Embolism Response Team. J. Thromb. Thrombolysis 2020, 49, 34–41. [Google Scholar] [CrossRef]
- Schoepf, U.J.; Kucher, N.; Kipfmueller, F.; Quiroz, R.; Costello, P.; Goldhaber, S.Z. Right Ventricular Enlargement on Chest Computed Tomography: A Predictor of Early Death in Acute Pulmonary Embolism. Circulation 2004, 110, 3276–3280. [Google Scholar] [CrossRef]
- Kucher, N.; Boekstegers, P.; Müller, O.J.; Kupatt, C.; Beyer-Westendorf, J.; Heitzer, T.; Tebbe, U.; Horstkotte, J.; Müller, R.; Blessing, E.; et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circulation 2014, 129, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Lauder, L.; Pérez Navarro, P.; Götzinger, F.; Ewen, S.; Al Ghorani, H.; Haring, B.; Lepper, P.M.; Kulenthiran, S.; Böhm, M.; Link, A.; et al. Mechanical Thrombectomy in Intermediate- and High-Risk Acute Pulmonary Embolism: Hemodynamic Outcomes at Three Months. Respir. Res. 2023, 24, 257. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Inci, E.K.; Khandhar, S.; Toma, C.; Licitra, G.; Brown, M.J.; Herzig, M.; Matthai, W.; Palevsky, H.; Schwartz, A.; Wight, J.A.; et al. Mechanical Thrombectomy versus Catheter Directed Thrombolysis in Patients with Pulmonary Embolism: A Multicenter Experience. Catheter. Cardiovasc. Interv. 2023, 101, 140–146. [Google Scholar] [CrossRef]
- Avgerinos, E.D.; Abou Ali, A.; Toma, C.; Wu, B.; Saadeddin, Z.; McDaniel, B.; Al-Khoury, G.; Chaer, R.A. Catheter-Directed Thrombolysis versus Suction Thrombectomy in the Management of Acute Pulmonary Embolism. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 623–628. [Google Scholar] [CrossRef]
- Bunc, M.; Steblovnik, K.; Zorman, S.; Popovic, P. Percutaneous Mechanical Thrombectomy in Patients with High-Risk Pulmonary Embolism and Contraindications for Thrombolytic Therapy. Radiol. Oncol. 2020, 54, 62–67. [Google Scholar] [CrossRef]
- Pelliccia, F.; De Luca, A.; Pasceri, V.; Tanzilli, G.; Speciale, G.; Gaudio, C. Safety and Outcome of Rheolytic Thrombectomy for the Treatment of Acute Massive Pulmonary Embolism. J. Invasive Cardiol. 2020, 32, 412–416. [Google Scholar] [CrossRef]
- Kucher, N.; Ouda, A.; Voci, D.; Barco, S.; Micieli, E.; Münger, M.; Pleming, W.; Grigorean, A.; Sromicki, J.; Schmiady, M.O.; et al. Percutaneous Large-Bore Aspiration Embolectomy with Veno-Arterial Extracorporal Membrane Oxygenation Support or Standby in Patients with High-Risk Pulmonary Embolism and Contraindications to Thrombolysis: A Preliminary Single Centre Experience. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 232–236. [Google Scholar] [CrossRef]
- Graif, A.; Patel, K.D.; Wimmer, N.J.; Kimbiris, G.; Grilli, C.J.; Upparapalli, D.; Kaneria, A.R.; Leung, D.A. Large-Bore Aspiration Thrombectomy versus Catheter-Directed Thrombolysis for Acute Pulmonary Embolism: A Propensity Score-Matched Comparison. J. Vasc. Interv. Radiol. 2020, 31, 2052–2059. [Google Scholar] [CrossRef]
- Pizano, A.; Ray, H.M.; Cambiaghi, T.; Saqib, N.U.; Afifi, R.; Khan, S.; Martin, G.; Harlin, S.A. Initial Experience and Early Outcomes of the Management of Acute Pulmonary Embolism Using the FlowTriever Mechanical Thrombectomy Device. J. Cardiovasc. Surg. 2022, 63, 222–228. [Google Scholar] [CrossRef]
- Bradley, M.; Bull, T.; Hountras, P.; MacLaren, R. Pragmatic Use of Catheter-Directed Thrombolysis in Venous Thromboembolism and a Comparative Evaluation With Traditional Therapies in Submassive Pulmonary Embolism. J. Pharm. Pract. 2022, 35, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cui, M.; Zhang, K.; Liang, K.; Liu, H.; Zhai, S. Treatment of Acute Pulmonary Embolism Using Rheolytic Thrombectomy. EuroIntervention 2021, 17, e158–e166. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, J.; Ma, Q.; Qin, H.; Zhang, B.; Pang, H.; Yin, Q.; Tian, H. Pulmonary Interventional Therapy for Acute Massive and Submassive Pulmonary Embolism in Cases Where Thrombolysis Is Contraindicated. Ann. Vasc. Surg. 2020, 64, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Latacz, P.; Simka, M.; Brzegowy, P.; Serednicki, W.; Konduracka, E.; Mrowiecki, W.; Słowik, A.; Łasocha, B.; Mrowiecki, T.; Popiela, T. Treatment of High- and Intermediate-Risk Pulmonary Embolism Using the AngioJet Percutaneous Mechanical Thrombectomy System in Patients with Contraindications for Thrombolytic Treatment—A Pilot Study. Wideochir Inne Tech. Maloinwazyjne 2018, 13, 233–242. [Google Scholar] [CrossRef]
- Liang, N.L.; Avgerinos, E.D.; Marone, L.K.; Singh, M.J.; Makaroun, M.S.; Chaer, R.A. Comparative Outcomes of Ultrasound-Assisted Thrombolysis and Standard Catheter-Directed Thrombolysis in the Treatment of Acute Pulmonary Embolism. Vasc. Endovasc. Surg. 2016, 50, 405–410. [Google Scholar] [CrossRef]
- Liu, B.; Liu, M.; Yan, L.; Yan, J.; Wu, J.; Jiao, X.; Guo, M. Percutaneous Mechanical Thrombectomy Combined with Catheter-Directed Thrombolysis in the Treatment of Acute Pulmonary Embolism and Lower Extremity Deep Venous Thrombosis: A Novel One-Stop Endovascular Strategy. J. Int. Med. Res. 2018, 46, 836–851. [Google Scholar] [CrossRef]
- Luedemann, W.M.; Zickler, D.; Kruse, J.; Koerner, R.; Lenk, J.; Erxleben, C.; Torsello, G.F.; Fehrenbach, U.; Jonczyk, M.; Guenther, R.W.; et al. Percutaneous Large-Bore Pulmonary Thrombectomy with the FlowTriever Device: Initial Experience in Intermediate-High and High-Risk Patients. Cardiovasc. Interv. Radiol. 2023, 46, 35–42. [Google Scholar] [CrossRef]
- Liu, S.; Shi, H.-B.; Gu, J.-P.; Yang, Z.-Q.; Chen, L.; Lou, W.-S.; He, X.; Zhou, W.-Z.; Zhou, C.-G.; Zhao, L.-B.; et al. Massive Pulmonary Embolism: Treatment with the Rotarex Thrombectomy System. Cardiovasc. Interv. Radiol. 2011, 34, 106–113. [Google Scholar] [CrossRef]
- Siablis, D.; Karnabatidis, D.; Katsanos, K.; Kagadis, G.C.; Zabakis, P.; Hahalis, G. AngioJet Rheolytic Thrombectomy versus Local Intrapulmonary Thrombolysis in Massive Pulmonary Embolism: A Retrospective Data Analysis. J. Endovasc. Ther. 2005, 12, 206–214. [Google Scholar] [CrossRef]
- Margheri, M.; Vittori, G.; Vecchio, S.; Chechi, T.; Falchetti, E.; Spaziani, G.; Giuliani, G.; Rovelli, S.; Consoli, L.; Biondi Zoccai, G.G.L. Early and Long-Term Clinical Results of AngioJet Rheolytic Thrombectomy in Patients with Acute Pulmonary Embolism. Am. J. Cardiol. 2008, 101, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.J.; Gibson, C.M.; Giri, J.; Khandhar, S.; Jaber, W.; Toma, C.; Mina, B.; Bowers, T.; Greenspon, L.; Kado, H.; et al. Outcomes in High-Risk Pulmonary Embolism Patients Undergoing FlowTriever Mechanical Thrombectomy or Other Contemporary Therapies: Results From the FLAME Study. Circ. Cardiovasc. Interv. 2023, 16, e013406. [Google Scholar] [CrossRef] [PubMed]
- Bonvini, R.F.; Roffi, M.; Bounameaux, H.; Noble, S.; Müller, H.; Keller, P.-F.; Jolliet, P.; Sarasin, F.P.; Rutschmann, O.T.; Bendjelid, K.; et al. AngioJet Rheolytic Thrombectomy in Patients Presenting with High-Risk Pulmonary Embolism and Cardiogenic Shock: A Feasibility Pilot Study. EuroIntervention 2013, 8, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Chen, G.; Zhao, B.; Kong, J.; Gu, J.; He, X. Rescue Catheter-Based Therapies for the Treatment of Acute Massive Pulmonary Embolism after Unsuccessful Systemic Thrombolysis. J. Thromb. Thrombolysis 2021, 51, 805–813. [Google Scholar] [CrossRef]
- Müller-Hülsbeck, S.; Brossmann, J.; Jahnke, T.; Grimm, J.; Reuter, M.; Bewig, B.; Heller, M. Mechanical Thrombectomy of Major and Massive Pulmonary Embolism with Use of the Amplatz Thrombectomy Device. Investig. Radiol. 2001, 36, 317–322. [Google Scholar] [CrossRef]
- Ribas, J.; Valcárcel, J.; Alba, E.; Ruíz, Y.; Cuartero, D.; Iriarte, A.; Mora-Luján, J.M.; Huguet, M.; Cerdà, P.; Martínez-Yélamos, S.; et al. Catheter-Directed Therapies in Patients with Pulmonary Embolism: Predictive Factors of In-Hospital Mortality and Long-Term Follow-Up. J. Clin. Med. 2021, 10, 4716. [Google Scholar] [CrossRef]
- Visco, E.; Adamo, M.; Locantore, E.; Fiorina, C.; Chizzola, G.; Branca, L.; Abbenante, A.; Castiello, A.; Metra, M.; Curello, S.; et al. EkoSonic Endovascular System for Patients with Acute Pulmonary Embolism and Contraindication to Systemic Fibrinolysis. J. Cardiovasc. Med. 2019, 20, 131–136. [Google Scholar] [CrossRef]
- Hennemeyer, C.; Khan, A.; McGregor, H.; Moffett, C.; Woodhead, G. Outcomes of Catheter-Directed Therapy Plus Anticoagulation Versus Anticoagulation Alone for Submassive and Massive Pulmonary Embolism. Am. J. Med. 2019, 132, 240–246. [Google Scholar] [CrossRef]
- Tu, T.; Toma, C.; Tapson, V.F.; Adams, C.; Jaber, W.A.; Silver, M.; Khandhar, S.; Amin, R.; Weinberg, M.; Engelhardt, T.; et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study. JACC Cardiovasc. Interv. 2019, 12, 859–869. [Google Scholar] [CrossRef]
- Ruzsa, Z.; Vámosi, Z.; Berta, B.; Nemes, B.; Tóth, K.; Kovács, N.; Zima, E.; Becker, D.; Merkely, B. Catheter Directed Thrombolytic Therapy and Aspiration Thrombectomy in Intermediate Pulmonary Embolism with Long Term Results. Cardiol. J. 2020, 27, 368–375. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Kawamura, A. Percutaneous Rheolytic Thrombectomy for Large Pulmonary Embolism: A Promising Treatment Option. Catheter. Cardiovasc. Interv. 2007, 70, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Arzamendi, D.; Bilodeau, L.; Ibrahim, R.; Noble, S.; Gallo, R.; Lavoie-L’allier, P.; Gosselin, G.; Deguise, P.; Ly, H.; Tanguay, J.-F.; et al. Role of Rheolytic Thrombectomy in Massive Pulmonary Embolism with Contraindication to Systemic Thrombolytic Therapy. EuroIntervention 2010, 5, 716–721. [Google Scholar] [CrossRef]
- Semaan, D.B.; Phillips, A.R.; Reitz, K.; Sridharan, N.; Mulukutla, S.; Avgerinos, E.; Eslami, M.H.; Chaer, R. Improved Long-Term Outcomes with Catheter-Directed Therapies over Medical Management in Patients with Submassive Pulmonary Embolism-a Retrospective Matched Cohort Study. J. Vasc. Surg. Venous Lymphat. Disord. 2023, 11, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Murai, K.; Tokita, Y.; Kato, K.; Iwasaki, Y.-K.; Sato, N.; Tajima, H.; Mizuno, K.; Tanaka, K. Thrombolysis with a Novel Modified Tissue-Type Plasminogen Activator, Monteplase, Combined with Catheter-Based Treatment for Major Pulmonary Embolism. Circ. J. 2009, 73, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Tajima, H.; Murata, S.; Kumita, S.-I.; Yamamoto, T.; Tanaka, K. Catheter Fragmentation of Acute Massive Pulmonary Thromboembolism: Distal Embolisation and Pulmonary Arterial Pressure Elevation. Br. J. Radiol. 2008, 81, 848–854. [Google Scholar] [CrossRef]
- Andresen, M.; González, A.; Mercado, M.; Díaz, O.; Meneses, L.; Fava, M.; Córdova, S.; Castro, R. Natriuretic Peptide Type-B Can Be a Marker of Reperfusion in Patients with Pulmonary Thromboembolism Subjected to Invasive Treatment. Int. J. Cardiovasc. Imaging 2012, 28, 659–666. [Google Scholar] [CrossRef]
- Meng, X.; Fu, M.; Wang, J.; Xu, H. Effects of Recombinant Human Brain Natriuretic Peptide in Patients with Acute Pulmonary Embolism Complicated with Right Ventricular Dysfunction Who Underwent Catheter-Directed Therapy. Int. Heart J. 2022, 63, 8–14. [Google Scholar] [CrossRef]
- Schmitz-Rode, T.; Janssens, U.; Schild, H.H.; Basche, S.; Hanrath, P.; Günther, R.W. Fragmentation of Massive Pulmonary Embolism Using a Pigtail Rotation Catheter. Chest 1998, 114, 1427–1436. [Google Scholar] [CrossRef][Green Version]
- Akhmerov, A.; Reich, H.; Mirocha, J.; Ramzy, D. Effect of Percutaneous Suction Thromboembolectomy on Improved Right Ventricular Function. Tex. Heart Inst. J. 2019, 46, 115–119. [Google Scholar] [CrossRef]
- Sag, S.; Nas, O.F.; Kaderli, A.A.; Ozdemir, B.; Baran, İ.; Erdoğan, C.; Gullulu, S.; Hakyemez, B.; Aydinlar, A. Catheter-Directed Ultrasound-Accelerated Thrombolysis May Be Life-Saving in Patients with Massive Pulmonary Embolism after Failed Systemic Thrombolysis. J. Thromb. Thrombolysis 2016, 42, 322–328. [Google Scholar] [CrossRef]
- Zeni, P.T.; Blank, B.G.; Peeler, D.W. Use of Rheolytic Thrombectomy in Treatment of Acute Massive Pulmonary Embolism. J. Vasc. Interv. Radiol. 2003, 14, 1511–1515. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Einarsson, F.; Sandström, C.; Svennerholm, K.; Oras, J.; Rylander, C. Outcomes of Catheter-Directed Interventions in High-Risk Pulmonary Embolism-a Retrospective Analysis. Acta Anaesthesiol. Scand. 2021, 65, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, L.; Bloch, R.; Threlkeld, J.; Demlow, T.; Kansal, R.; Karmy-Jones, R. Management of Pulmonary Embolism with Rheolytic Thrombectomy. Can. Respir. J. 2011, 18, e52–e58. [Google Scholar] [CrossRef]
- Villalba, L.; Nguyen, T.; Feitosa, R.L.; Gunanayagam, P.; Anning, N.; Dwight, K. Single-Session Catheter-Directed Lysis Using Adjunctive Power-Pulse Spray with AngioJet for the Treatment of Acute Massive and Submassive Pulmonary Embolism. J. Vasc. Surg. 2019, 70, 1920–1926. [Google Scholar] [CrossRef]
- Lin, P.H.; Annambhotla, S.; Bechara, C.F.; Athamneh, H.; Weakley, S.M.; Kobayashi, K.; Kougias, P. Comparison of Percutaneous Ultrasound-Accelerated Thrombolysis versus Catheter-Directed Thrombolysis in Patients with Acute Massive Pulmonary Embolism. Vascular 2009, 17 (Suppl. 3), S137–S147. [Google Scholar] [CrossRef]
- Liang, N.L.; Chaer, R.A.; Marone, L.K.; Singh, M.J.; Makaroun, M.S.; Avgerinos, E.D. Midterm Outcomes of Catheter-Directed Interventions for the Treatment of Acute Pulmonary Embolism. Vascular 2017, 25, 130–136. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, H.; Yang, Z.; Liu, S.; Zhou, C.; Zhao, L.; Xia, J.; Feng, Y.; Li, L. Value of Percutanous Catheter Fragmentation in the Management of Massive Pulmonary Embolism. Chin. Med. J. (Engl.) 2009, 122, 1723–1727. [Google Scholar]
- Yoshida, M.; Inoue, I.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Kurisu, S.; Kusano, K.F.; Ohe, T. Novel Percutaneous Catheter Thrombectomy in Acute Massive Pulmonary Embolism: Rotational Bidirectional Thrombectomy (ROBOT). Catheter. Cardiovasc. Interv. 2006, 68, 112–117. [Google Scholar] [CrossRef]
- Hubbard, J.; Saad, W.E.A.; Sabri, S.S.; Turba, U.C.; Angle, J.F.; Park, A.W.; Matsumoto, A.H. Rheolytic Thrombectomy with or without Adjunctive Indwelling Pharmacolysis in Patients Presenting with Acute Pulmonary Embolism Presenting with Right Heart Strain and/or Pulseless Electrical Activity. Thrombosis 2011, 2011, 246410. [Google Scholar] [CrossRef][Green Version]
- Piazza, G.; Hohlfelder, B.; Jaff, M.R.; Ouriel, K.; Engelhardt, T.C.; Sterling, K.M.; Jones, N.J.; Gurley, J.C.; Bhatheja, R.; Kennedy, R.J.; et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc. Interv. 2015, 8, 1382–1392. [Google Scholar] [CrossRef]
- Tapson, V.F.; Sterling, K.; Jones, N.; Elder, M.; Tripathy, U.; Brower, J.; Maholic, R.L.; Ross, C.B.; Natarajan, K.; Fong, P.; et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc. Interv. 2018, 11, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Gorgis, S.; Mawri, S.; Dabbagh, M.F.; Aurora, L.; Ali, M.; Mitchell, G.; Jacobsen, G.; Hegab, S.; Schwartz, S.; Kelly, B.; et al. Ultrasound-Assisted Catheter-Directed Thrombolysis versus Anticoagulation Alone for Management of Submassive Pulmonary Embolism. J. Cardiol. 2022, 80, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Sadeghipour, P.; Jenab, Y.; Moosavi, J.; Hosseini, K.; Mohebbi, B.; Hosseinsabet, A.; Chatterjee, S.; Pouraliakbar, H.; Shirani, S.; Shishehbor, M.H.; et al. Catheter-Directed Thrombolysis vs. Anticoagulation in Patients With Acute Intermediate-High-Risk Pulmonary Embolism: The CANARY Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 1189–1197. [Google Scholar] [CrossRef]
- Kroupa, J.; Buk, M.; Weichet, J.; Malikova, H.; Bartova, L.; Linkova, H.; Ionita, O.; Kozel, M.; Motovska, Z.; Kocka, V. A Pilot Randomised Trial of Catheter-Directed Thrombolysis or Standard Anticoagulation for Patients with Intermediate-High Risk Acute Pulmonary Embolism. EuroIntervention 2022, 18, e639–e646. [Google Scholar] [CrossRef]
- Kline, T.M.; Rodino, A.M.; Dorszynski, A.; Murray, B.; Cicci, J.; Iyer, P. Ultrasound-Assisted Catheter-Directed Thrombolysis versus Systemic Anticoagulation Alone for Submassive Pulmonary Embolism. J. Thromb. Thrombolysis 2021, 52, 130–137. [Google Scholar] [CrossRef]
- D’Auria, S.; Sezer, A.; Thoma, F.; Sharbaugh, M.; McKibben, J.; Maholic, R.; Avgerinos, E.D.; Rivera-Lebron, B.N.; Toma, C. Outcomes of Catheter-Directed Thrombolysis vs. Standard Medical Therapy in Patients with Acute Submassive Pulmonary Embolism. Pulm. Circ. 2020, 10, 2045894019898368. [Google Scholar] [CrossRef]
- Schissler, A.J.; Gylnn, R.J.; Sobieszczyk, P.S.; Waxman, A.B. Ultrasound-Assisted Catheter-Directed Thrombolysis Compared with Anticoagulation Alone for Treatment of Intermediate-Risk Pulmonary Embolism. Pulm. Circ. 2018, 8, 2045894018800265. [Google Scholar] [CrossRef]
- Harrison, E.; Kim, J.S.; Lakhter, V.; Lio, K.U.; Alashram, R.; Zhao, H.; Gupta, R.; Patel, M.; Harrison, J.; Panaro, J.; et al. Safety and Efficacy of Catheter Directed Thrombolysis (CDT) in Elderly with Pulmonary Embolism (PE). BMJ Open Respir. Res. 2021, 8, e000894. [Google Scholar] [CrossRef]
- Avgerinos, E.D.; Liang, N.L.; El-Shazly, O.M.; Toma, C.; Singh, M.J.; Makaroun, M.S.; Chaer, R.A. Improved Early Right Ventricular Function Recovery but Increased Complications with Catheter-Directed Interventions Compared with Anticoagulation Alone for Submassive Pulmonary Embolism. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 268–275. [Google Scholar] [CrossRef]
- Al-Hakim, R.; Bhatt, A.; Benenati, J.F. Continuous Aspiration Mechanical Thrombectomy for the Management of Submassive Pulmonary Embolism: A Single-Center Experience. J. Vasc. Interv. Radiol. 2017, 28, 1348–1352. [Google Scholar] [CrossRef]
- De Gregorio, M.A.; Guirola, J.A.; Kuo, W.T.; Serrano, C.; Urbano, J.; Figueredo, A.L.; Sierre, S.; Quezada, C.A.; Barbero, E.; Jiménez, D. Catheter-Directed Aspiration Thrombectomy and Low-Dose Thrombolysis for Patients with Acute Unstable Pulmonary Embolism: Prospective Outcomes from a PE Registry. Int. J. Cardiol. 2019, 287, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, E.D.; Abou Ali, A.N.; Liang, N.L.; Rivera-Lebron, B.; Toma, C.; Maholic, R.; Makaroun, M.S.; Chaer, R.A. Catheter-Directed Interventions Compared with Systemic Thrombolysis Achieve Improved Ventricular Function Recovery at a Potentially Lower Complication Rate for Acute Pulmonary Embolism. J. Vasc. Surg. Venous Lymphat. Disord. 2018, 6, 425–432. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, K.M.; Sasahara, A.A. The Hemodynamic Response to Pulmonary Embolism in Patients without Prior Cardiopulmonary Disease. Am. J. Cardiol. 1971, 28, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Lankhaar, J.-W.; Westerhof, N.; Faes, T.J.C.; Marques, K.M.J.; Marcus, J.T.; Postmus, P.E.; Vonk-Noordegraaf, A. Quantification of Right Ventricular Afterload in Patients with and without Pulmonary Hypertension. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1731–H1737. [Google Scholar] [CrossRef]
- Mauritz, G.-J.; Marcus, J.T.; Westerhof, N.; Postmus, P.E.; Vonk-Noordegraaf, A. Prolonged Right Ventricular Post-Systolic Isovolumic Period in Pulmonary Arterial Hypertension Is Not a Reflection of Diastolic Dysfunction. Heart 2011, 97, 473–478. [Google Scholar] [CrossRef]
- Begieneman, M.P.V.; van de Goot, F.R.W.; van der Bilt, I.A.C.; Vonk Noordegraaf, A.; Spreeuwenberg, M.D.; Paulus, W.J.; van Hinsbergh, V.W.M.; Visser, F.C.; Niessen, H.W.M. Pulmonary Embolism Causes Endomyocarditis in the Human Heart. Heart 2008, 94, 450–456. [Google Scholar] [CrossRef][Green Version]
- Toma, C.; Jaber, W.A.; Weinberg, M.D.; Bunte, M.C.; Khandhar, S.; Stegman, B.; Gondi, S.; Chambers, J.; Amin, R.; Leung, D.A.; et al. Acute Outcomes for the Full US Cohort of the FLASH Mechanical Thrombectomy Registry in Pulmonary Embolism. EuroIntervention 2023, 18, 1201–1212. [Google Scholar] [CrossRef]
- Ranade, M.; Foster, M.T.; Brady, P.S.; Sokol, S.I.; Butty, S.; Klein, A.; Maholic, R.; Safar, A.; Patel, T.; Zlotnick, D.; et al. Novel Mechanical Aspiration Thrombectomy in Patients With Acute Pulmonary Embolism: Results From the Prospective APEX-AV Trial. J. Soc. Cardiovasc. Angiogr. Interv. 2025, 4, 102463. [Google Scholar] [CrossRef]
- Planer, D.; Yanko, S.; Matok, I.; Paltiel, O.; Zmiro, R.; Rotshild, V.; Amir, O.; Elbaz-Greener, G.; Raccah, B.H. Catheter-Directed Thrombolysis Compared with Systemic Thrombolysis and Anticoagulation in Patients with Intermediate- or High-Risk Pulmonary Embolism: Systematic Review and Network Meta-Analysis. CMAJ 2023, 195, E833–E843. [Google Scholar] [CrossRef]
- Furfaro, D.; Stephens, R.S.; Streiff, M.B.; Brower, R. Catheter-Directed Thrombolysis for Intermediate-Risk Pulmonary Embolism. Ann. Am. Thorac. Soc. 2018, 15, 134–144. [Google Scholar] [CrossRef]
- Giri, J.; Mahfoud, F.; Gebauer, B.; Andersen, A.; Friedman, O.; Gandhi, R.T.; Jaber, W.A.; Pereira, K.; West, F.M. PEERLESS II: A Randomized Controlled Trial of Large-Bore Thrombectomy Versus Anticoagulation in Intermediate-Risk Pulmonary Embolism. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101982. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Piazza, G.; Sharp, A.S.P.; Ní Ainle, F.; Jaff, M.R.; Chauhan, N.; Patel, B.; Barco, S.; Goldhaber, S.Z.; Kucher, N.; et al. Ultrasound-Facilitated, Catheter-Directed Thrombolysis vs. Anticoagulation Alone for Acute Intermediate-High-Risk Pulmonary Embolism: Rationale and Design of the HI-PEITHO Study. Am. Heart J. 2022, 251, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Jaber, W.A.; Gonsalves, C.F.; Stortecky, S.; Horr, S.; Pappas, O.; Gandhi, R.T.; Pereira, K.; Giri, J.; Khandhar, S.J.; Ammar, K.A.; et al. Large-Bore Mechanical Thrombectomy Versus Catheter-Directed Thrombolysis in the Management of Intermediate-Risk Pulmonary Embolism: Primary Results of the PEERLESS Randomized Controlled Trial. Circulation 2025, 151, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Budaj-Fidecka, A.; Kurzyna, M.; Fijałkowska, A.; Żyłkowska, J.; Wieteska, M.; Florczyk, M.; Szewczyk, G.; Torbicki, A.; Filipiak, K.J.; Opolski, G.; et al. In-Hospital Major Bleeding Predicts Mortality in Patients with Pulmonary Embolism: An Analysis of ZATPOL Registry Data. Int. J. Cardiol. 2013, 168, 3543–3549. [Google Scholar] [CrossRef]
- Pietrasik, A.; Gąsecka, A.; Szarpak, Ł.; Pruc, M.; Kopiec, T.; Darocha, S.; Banaszkiewicz, M.; Niewada, M.; Grabowski, M.; Kurzyna, M. Catheter-Based Therapies Decrease Mortality in Patients With Intermediate and High-Risk Pulmonary Embolism: Evidence From Meta-Analysis of 65,589 Patients. Front. Cardiovasc. Med. 2022, 9, 861307. [Google Scholar] [CrossRef]
- Meyer, G.; Vicaut, E.; Danays, T.; Agnelli, G.; Becattini, C.; Beyer-Westendorf, J.; Bluhmki, E.; Bouvaist, H.; Brenner, B.; Couturaud, F.; et al. Fibrinolysis for Patients with Intermediate-Risk Pulmonary Embolism. N. Engl. J. Med. 2014, 370, 1402–1411. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chakraborty, A.; Weinberg, I.; Kadakia, M.; Wilensky, R.L.; Sardar, P.; Kumbhani, D.J.; Mukherjee, D.; Jaff, M.R.; Giri, J. Thrombolysis for Pulmonary Embolism and Risk of All-Cause Mortality, Major Bleeding, and Intracranial Hemorrhage: A Meta-Analysis. JAMA 2014, 311, 2414–2421. [Google Scholar] [CrossRef]
- Monteleone, P.; Ahern, R.; Banerjee, S.; Desai, K.R.; Kadian-Dodov, D.; Webber, E.; Omidvar, S.; Troy, P.; Parikh, S.A. Modern Treatment of Pulmonary Embolism (USCDT vs. MT): Results From a Real-World, Big Data Analysis (REAL-PE). J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101192. [Google Scholar] [CrossRef]
| First Author (Year) | Country | Total N | Age, Mean (SD) | Sex (M/F) | Arm 1 | Arm 2 | PE Severitiy |
|---|---|---|---|---|---|---|---|
| Nils Kucher (2014) | Multinational | 59 | 63 (14) | 28/31 | CDT | AC | Intermediate-risk |
| Errol K. Inci (2022) | USA | 458 | 57 (1.67) | 217/241 | MT | CDT | Intermediate-risk/High-risk |
| Efthymios D. Avgerinos (2019) | USA | 72 | 63.6 (15) | 31/41 | CDT | MT | Intermediate-risk/High-risk |
| Matjaz Bunc (2020) | Slovenia | 62.6 (12.7) | 16/9 | MT | NA | High-risk | |
| Francesco Pelliccia (2020) | Italy | 33 | 43 (13) | 20/13 | MT | NA | High-risk |
| Nils Kucher (2023) | Switzerland | 15 | 63 (12) | 14/1 | MT | NA | High-risk |
| Assaf Graif (2020) | USA | 52 | 59.7 (20.9) | 24/28 | MT | CDT | Intermediate-risk |
| Alejandro Pizano (2021) | USA | 14 | 60 (median) | 9/5 | MT | NA | Intermediate-risk |
| Mary Bradley (2021) | USA | 42 | 56.8 (24.7) | 25/17 | CDT | AC | Intermediate-risk |
| Kun Li (2021) | China | 23 | 58.6 (12.2) | 11/12 | MT | NA | Intermediate-risk |
| Kun Li (2021) | China | 21 | 58 (15) | 10/11 | MT | NA | High-risk |
| Yan Meng (2020) | China | 186 | 60.3 (12.6) | 90/96 | CBT | AC | Intermediate-risk/High-risk |
| Miguel Angel De Gregorio (2019) | Spain | 54 | 59.7 (16.8) | 23/31 | CBT | NA | High-risk |
| Pawel Latacz (2018) | Poland | 7 | 52.7 (16.6) | 4/3 | MT | NA | Intermediate-risk/High risk |
| Nathan L. Liang (2016) | USA | 63 | 59 (19.2) | 27/36 | CDT | NA | Intermediate-risk/High-risk |
| Bing Liu (2018) | China | 20 | 61.4 (13.5) | 7/13 | CBT | NA | NA |
| W. M. Luedemann (2023) | Germany | 27 | 56.1 (15.3) | 12/15 | MT | NA | Intermediate-risk/High-risk |
| Sheng Liu (2010) | China | 14 | 55.4 | 8/6 | CBT | NA | Intermediate-risk/High-risk |
| Dimitris Siablis (2005) | Greece | 14 | 63.6 (15.6) | 7/7 | CDT | MT | NA |
| Massimo Margheri (2008) | Italy | 17 | 64.2 (13.9) | 11/6 | MT | NA | Intermediate-risk |
| Massimo Margheri (2008) | Italy | 8 | 67.4 (11.9) | 5/3 | MT | NA | High-risk |
| Mitchell J. Silver (2023) | USA | 114 | 63 (15) | 53/61 | MT | ST/AC | High-risk |
| Robert F. Bonvini (2013) | Switzerland | 10 | 73 (9) | 5/5 | MT | NA | High-risk |
| Maofeng Gong (2021) | China | 48 | 59.9 (12.3) | 29/19 | CBT | NA | High-risk |
| S. Muller-Hulsbeck (2001) | Germany | 9 | 55 | 4/5 | CBT | NA | Intermediate-risk |
| Jesus Ribas (2021) | Spain | 20 | 57.3 (12.4) | 10/10 | CBT | NA | Intermediate-risk |
| Jesus Ribas (2021) | Spain | 43 | 61.6 (14.5) | 22/21 | CBT | NA | High-risk |
| Ramsey Al-Hakim (2017) | USA | 6 | 62.7 (19) | 3/3 | MT | NA | Intermediate-risk |
| Emanuele Visco (2019) | Italy | 18 | 74 (12.7) | 5/13 | CDT | NA | Intermediate-risk/High-risk |
| Charles Hennemeyer (2019) | USA | 79 | 59.5 (18.5) | 37/42 | CBT | AC | Intermediate-risk/High-risk |
| Thomas Tu (2019) | USA | 104 | 55.6 (13.7) | 56/48 | MT | NA | Intermediate-risk |
| Zoltan Ruzsa (2020) | Hungary | 80 | 59 (16.8) | 42/38 | CBT | NA | Intermediate-risk |
| Manish Chauhan (2007) | USA | 6 | 57.8 (12.6) | 2/4 | CBT | NA | High-risk |
| Manish Chauhan (2007) | USA | 8 | 67.6 (7.8) | 5/3 | CBT | NA | Intermediate-risk |
| Dabit Arzamendi (2010) | Canada | 10 | 43.7 (18.8) | 3/7 | MT | NA | High-risk |
| Dana B. Semaan (2022) | USA | 470 | 59.4 | 244/226 | CBT | AC | Intermediate-risk |
| Takeshi Yamamoto (2008) | Japan | 50 | 62 (15) | 19/31 | CBT | NA | High-risk |
| Kazuhiro Nakazawa (2008) | Japan | 25 | 60 (15) | 8/17 | CBT | NA | High-risk |
| Max Andresen (2012) | Chile | 14 | 67 (19) | 5/9 | CBT | NA | Intermediate-risk |
| Xiangdong Meng (2022) | China | 159 | 61.4 (10.5) | 71/88 | CBT | NA | Intermediate-risk/High-risk |
| Thomas Schmitz-Rode (1998) | Germany | 10 | 53.8 (9.5) | 6/4 | MT | NA | High-risk |
| Akbarashakh Akhmerov (2019) | USA | 13 | 56 (15) | 10/3 | MT | NA | NA |
| Saim Sag (2016) | Turkey | 13 | 51.6 (18.2) | 6/7 | CDT | NA | High-risk |
| Philip T Zeni Jr (2003) | USA | 17 | 51.7 (16.6) | 9/8 | MT | NA | High-risk |
| Freyr Einarsson (2021) | Sweden | 45 | 70,68 (median for each group) | 20/25 | CBT | AC | High-risk |
| Efhtymios D. Avgerinos (2018) | USA | 90 | 58.8 (15.8) | 152/165 | CBT | ST | High-risk |
| Efhtymios D. Avgerinos (2018) | USA | 227 | 58.8 (15.8) | 152/165 | CBT | ST | Intermediate-risk |
| Lisa Ferrigno (2011) | USA | 5 | 54 (16.4) | 3/2 | MT | NA | High-risk |
| Lisa Ferrigno (2011) | USA | 11 | 54.2 (13.5) | 4/7 | MT | NA | Intermediate-risk |
| Laurencia Villalba (2019) | Australia | 32 | 65.8 | 17/15 | CBT | NA | Intermediate-risk/High-risk |
| Peter H. Lin (2009) | USA | 25 | 60.7 (29.6) | 12/13 | CDT | NA | Intermediate-risk/High-risk |
| Nathan L. Liang (2017) | USA | 69 | 59.2 (15.4) | 30/39 | CBT | NA | Intermediate-risk/High-risk |
| Wei-Zhong Zhou (2009) | China | 28 | 63.5 (11.5) | 20/8 | MT | NA | Intermediate-risk/High-risk |
| Masashi Yoshida (2006) | Japan | 18 | 64.3 (25.5) | 7/11 | MT | ST | NA |
| J. Hubbard (2011) | USA | 11 | 60.2 | 9/2 | CBT | NA | Intermediate-risk/High-risk |
| Gregory Piazza (2015) | USA | 150 | 59 (16.1) | 73/77 | CDT | NA | Intermediate-risk/High-risk |
| Victor F. Tapson (2018) | Multinational | 101 | 60 (median) | 53/48 | CDT | NA | Intermediate-risk |
| Sarah Gorgis (2022) | Italy | 384 | 59.3 (15.1) | 188/196 | CDT | AC | Intermediate-risk |
| Parham Sadeghipour (2022) | Iran | 94 | 57.6 (2.4) | 61/24 | CDT | AC | Intermediate-risk |
| Josef Kroupa (2022) | Czech Republic | 23 | 61.9 (25.6) | 13/10 | CDT | AC | Intermediate-risk |
| Theresa Kline (2021) | USA | 130 | 63 (median) | 57/73 | CDT | AC | Intermediate-risk |
| Stephen D’ Auria (2020) | USA | 198 | NA | 103/95 | CDT | AC | Intermediate-risk |
| Andrew J. Schissler (2018) | USA | 104 | 55.5 (16.7) | 46/58 | CDT | AC | Intermediate-risk |
| Eneida Harrison (2021) | USA | 34 | 75.6 (11) | NA | CDT | AC | Intermediate-risk |
| Efthymios D. Avgerinos (2016) | USA | 128 | 59.3 (16.7) | 63/65 | CBT | AC | Intermediate-risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoumpourlis, P.; Mangeshkar, S.; Chi, K.-Y.; Varrias, D.; Spanos, M.; Fahimuddin, M.; Langston, M.D.; Khan, U.A.; Grushko, M.J.; Singh, P.; et al. Catheter-Based Therapies in Acute Pulmonary Embolism—Mortality and Safety Outcomes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4167. https://doi.org/10.3390/jcm14124167
Zoumpourlis P, Mangeshkar S, Chi K-Y, Varrias D, Spanos M, Fahimuddin M, Langston MD, Khan UA, Grushko MJ, Singh P, et al. Catheter-Based Therapies in Acute Pulmonary Embolism—Mortality and Safety Outcomes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(12):4167. https://doi.org/10.3390/jcm14124167
Chicago/Turabian StyleZoumpourlis, Panagiotis, Shaunak Mangeshkar, Kuan-Yu Chi, Dimitrios Varrias, Michail Spanos, Muhammad Fahimuddin, Matthew D. Langston, Usman A. Khan, Michael J. Grushko, Prabhjot Singh, and et al. 2025. "Catheter-Based Therapies in Acute Pulmonary Embolism—Mortality and Safety Outcomes: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 12: 4167. https://doi.org/10.3390/jcm14124167
APA StyleZoumpourlis, P., Mangeshkar, S., Chi, K.-Y., Varrias, D., Spanos, M., Fahimuddin, M., Langston, M. D., Khan, U. A., Grushko, M. J., Singh, P., & Sokol, S. I. (2025). Catheter-Based Therapies in Acute Pulmonary Embolism—Mortality and Safety Outcomes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(12), 4167. https://doi.org/10.3390/jcm14124167