Effects of Neuromuscular Priming with Spinal Cord Transcutaneous Stimulation on Lower Limb Motor Performance in Humans: A Randomized Crossover Sham-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Participants
2.2. Experimental Protocol
2.3. Data Acquisition and Experimental Procedures
2.3.1. Anthropometric Characteristics
2.3.2. Data Acquisition System
2.3.3. Surface EMG Recordings
2.3.4. Isometric Knee Extension: Torque Steadiness and MVC
2.3.5. Maximal Explosive Power
2.3.6. Spinal Cord Transcutaneous Stimulation
2.3.7. Recruitment Curves by Spinal Cord Transcutaneous Stimulation
2.3.8. Neuromuscular Priming with scTS or Sham Stimulation
2.4. Data Analysis and Computation of Experimental Variables
2.4.1. Recruitment Curves
2.4.2. Torque Steadiness
2.4.3. MVC
2.4.4. Maximal Explosive Power
2.4.5. Statistical Analysis
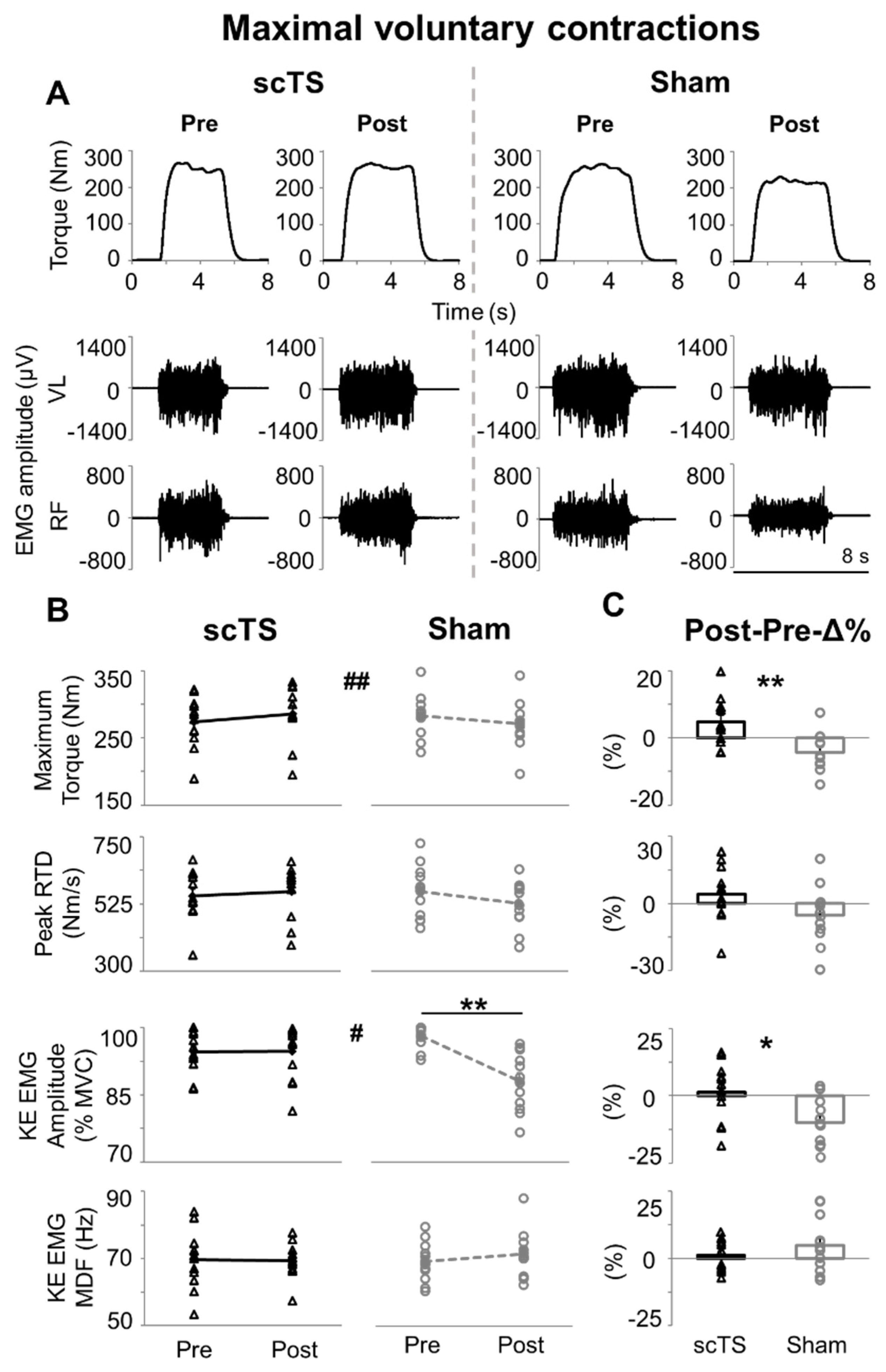
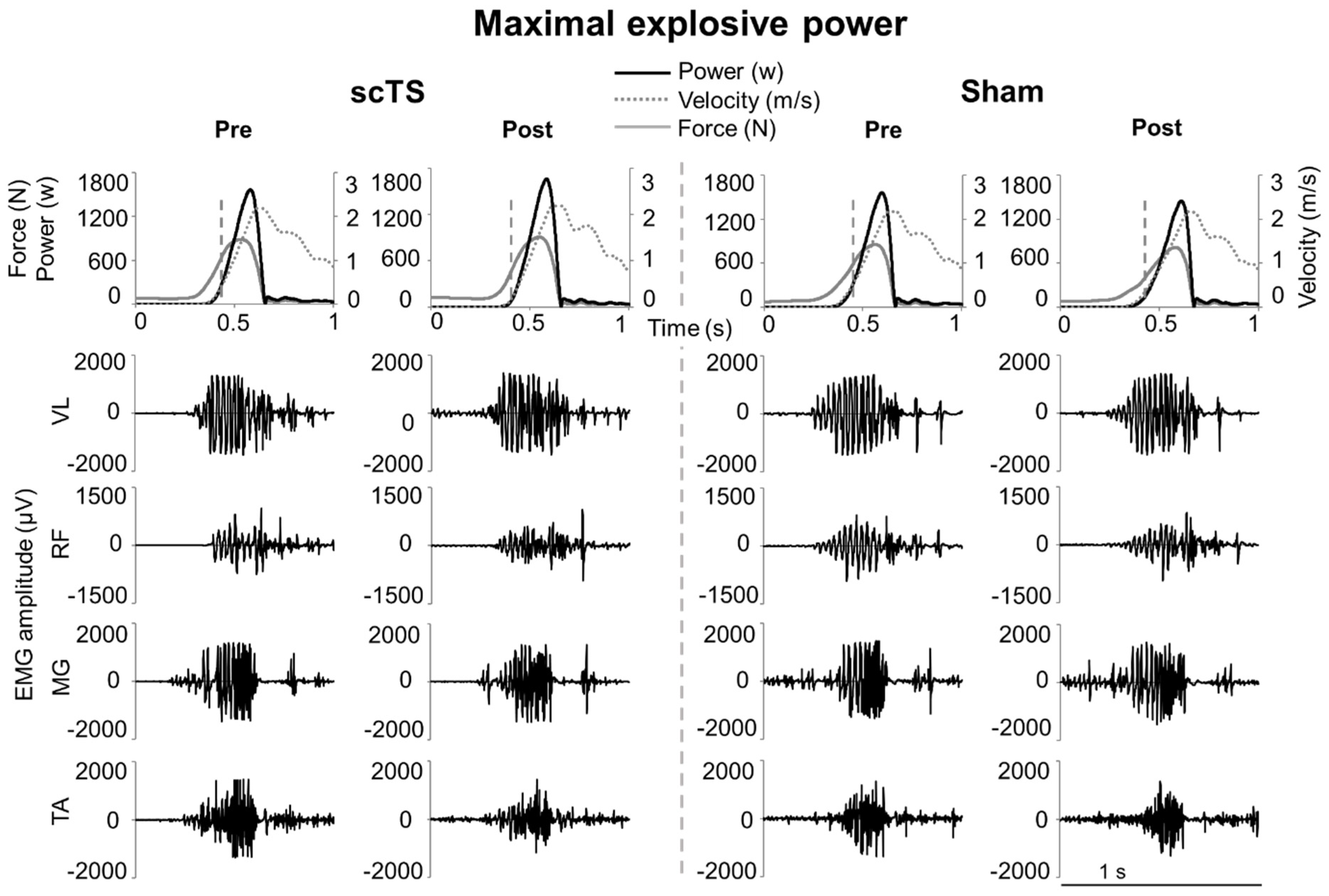
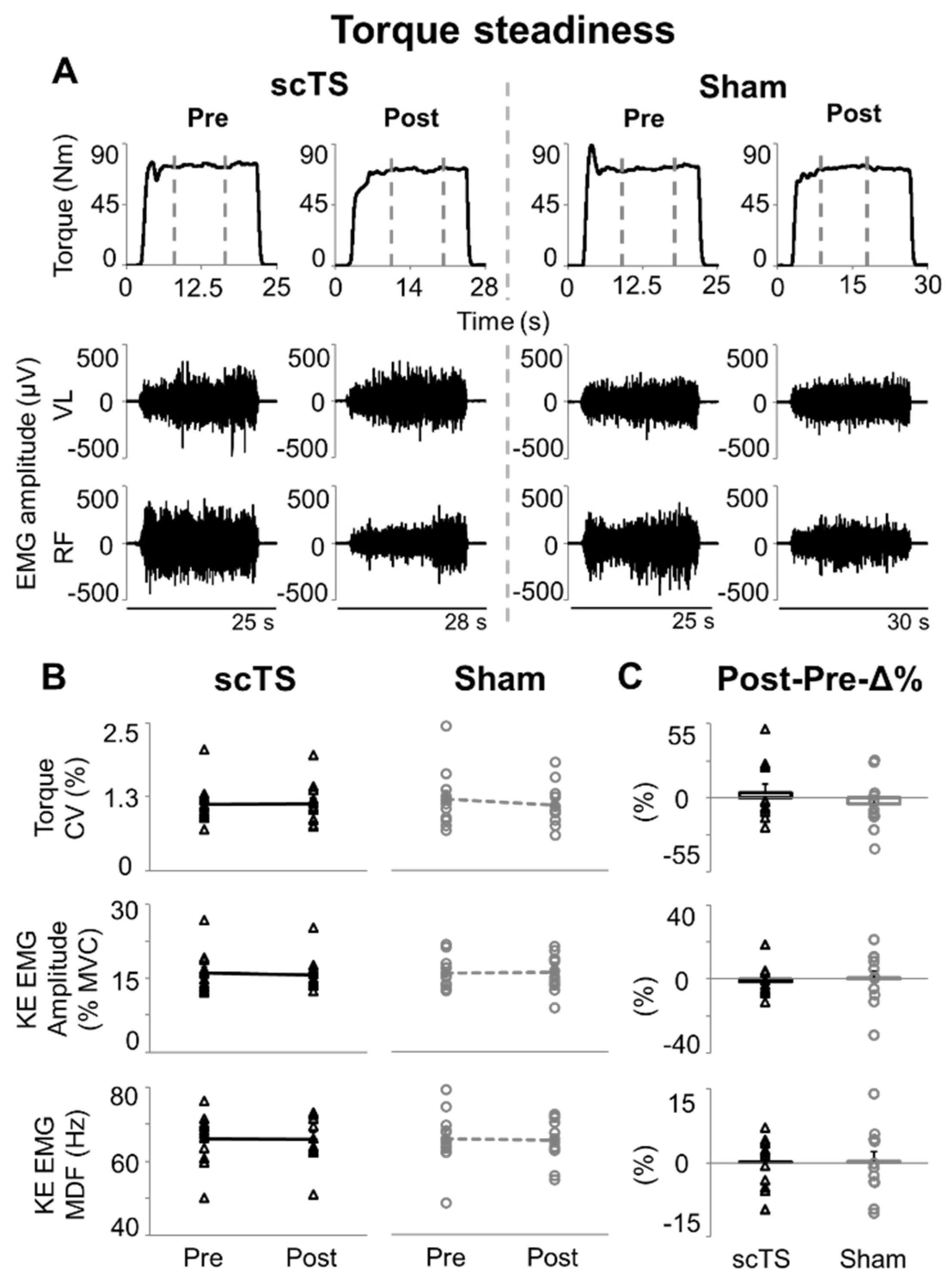
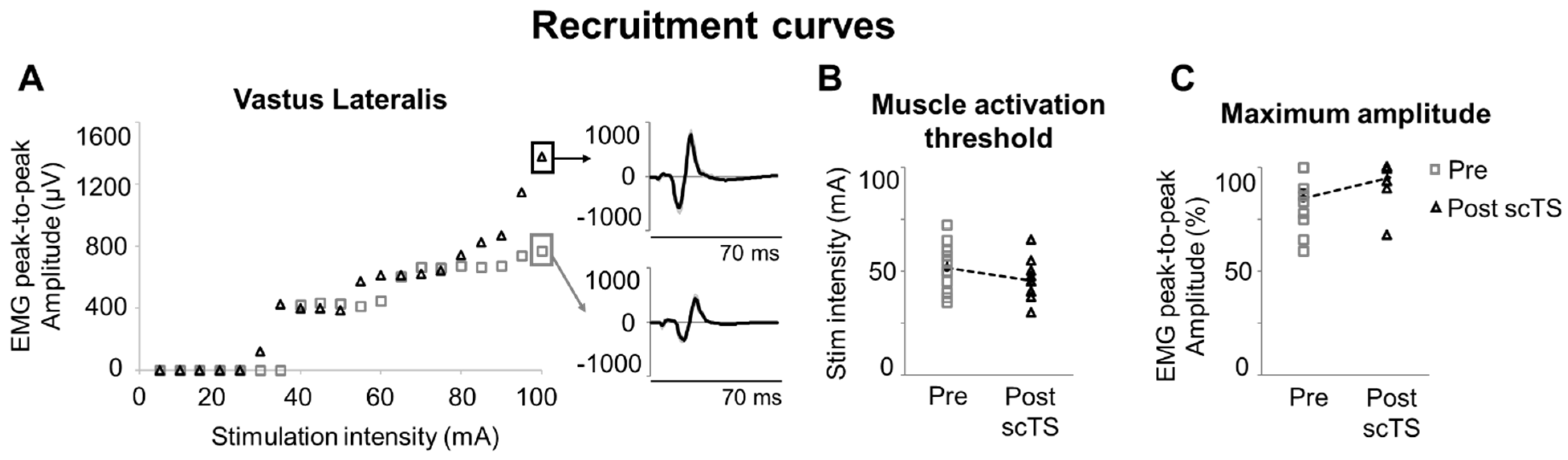
3. Results
4. Discussion
4.1. Maximal Neuromuscular Performance of Lower Limbs
4.2. Low-Level Torque Steadiness
4.3. scTS for Neuromuscular Priming
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarabia, J.M.; Moya-Ramón, M.; Hernández-Davó, J.L.; Fernandez-Fernandez, J.; Sabido, R. The Effects of Training with Loads That Maximise Power Output and Individualised Repetitions vs. Traditional Power Training. PLoS ONE 2017, 12, e0186601. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, T.J.; Nimphius, S.; Bellon, C.R.; Stone, M.H. The Importance of Muscular Strength: Training Considerations. Sports Med. 2018, 48, 765–785. [Google Scholar] [CrossRef]
- Sayer, A.A.; Kirkwood, T.B.L. Grip Strength and Mortality: A Biomarker of Ageing? Lancet 2015, 386, 226–227. [Google Scholar] [CrossRef]
- Li, R.; Xia, J.; Zhang, X.I.; Gathirua-Mwangi, W.G.; Guo, J.; Li, Y.; McKenzie, S.; Song, Y. Associations of Muscle Mass and Strength with All-Cause Mortality among US Older Adults. Med. Sci. Sports Exerc. 2018, 50, 458–467. [Google Scholar] [CrossRef]
- McKinnon, N.B.; Connelly, D.M.; Rice, C.L.; Hunter, S.W.; Doherty, T.J. Neuromuscular Contributions to the Age-Related Reduction in Muscle Power: Mechanisms and Potential Role of High Velocity Power Training. Ageing Res. Rev. 2017, 35, 147–154. [Google Scholar] [CrossRef]
- Cormie, P.; McGuigan, M.R.; Newton, R.U. Developing Maximal Neuromuscular Power: Part 1—Biological Basis of Maximal Power Production. Sports Med. 2011, 41, 17–38. [Google Scholar] [CrossRef]
- Narici, M.V.; de Boer, M.D. Disuse of the Musculo-Skeletal System in Space and on Earth. Eur. J. Appl. Physiol. 2011, 111, 403–420. [Google Scholar] [CrossRef]
- Farina, D.; Negro, F.; Dideriksen, J.L. The Effective Neural Drive to Muscles Is the Common Synaptic Input to Motor Neurons. J. Physiol. 2014, 592, 3427–3441. [Google Scholar] [CrossRef]
- Taylor, J.L.; Todd, G.; Gandevia, S.C. Evidence for a Supraspinal Contribution to Human Muscle Fatigue. Clin. Exp. Pharmacol. Physiol. 2006, 33, 400–405. [Google Scholar] [CrossRef]
- Zellers, J.A.; Parker, S.; Marmon, A.; Grävare Silbernagel, K. Muscle Activation during Maximum Voluntary Contraction and M-Wave Related in Healthy but Not in Injured Conditions: Implications When Normalizing Electromyography. Clin. Biomech. 2019, 69, 104–108. [Google Scholar] [CrossRef]
- Chmielewski, T.L.; Stackhouse, S.; Axe, M.J.; Snyder-Mackler, L. A Prospective Analysis of Incidence and Severity of Quadriceps Inhibition in a Consecutive Sample of 100 Patients with Complete Acute Anterior Cruciate Ligament Rupture. J. Orthop. Res. 2004, 22, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; Nitsche, M.A.; Kibele, A. Noninvasive Brain Stimulation and Neural Entrainment Enhance Athletic Performance—A Review. J. Cogn. Enhanc. 2017, 1, 73–79. [Google Scholar] [CrossRef]
- Maudrich, T.; Ragert, P.; Perrey, S.; Kenville, R. Single-Session Anodal Transcranial Direct Current Stimulation to Enhance Sport-Specific Performance in Athletes: A Systematic Review and Meta-Analysis. Brain Stimul. 2022, 15, 1517–1529. [Google Scholar] [CrossRef]
- Tanaka, S.; Hanakawa, T.; Honda, M.; Watanabe, K. Enhancement of Pinch Force in the Lower Leg by Anodal Transcranial Direct Current Stimulation. Exp. Brain Res. 2009, 196, 459–465. [Google Scholar] [CrossRef]
- Vargas, V.Z.; Baptista, A.F.; Pereira, G.O.C.; Pochini, A.C.; Ejnisman, B.; Santos, M.B.; João, S.M.A.; Hazime, F.A. Modulation of Isometric Quadriceps Strength in Soccer Players with Transcranial Direct Current Stimulation: A Crossover Study. J. Strength Cond. Res. 2018, 32, 1336–1341. [Google Scholar] [CrossRef]
- Cates, A.; Lin, R.; Mayberry, A.; Clark, R.; Chao, D.; Taylor, T.; Stray-Gundersen, J.; Wingeier, B. Repeated Sessions of Transcranial Direct Current Stimulation (tDCS) with Vertical Jump Training Improves Vertical Jump Performance in Elite Athletes. Brain Stimul. 2019, 12, 560. [Google Scholar] [CrossRef]
- Holgado, D.; Zandonai, T.; Ciria, L.F.; Zabala, M.; Hopker, J.; Sanabria, D. Transcranial Direct Current Stimulation (tDCS) over the Left Prefrontal Cortex Does Not Affect Time-Trial Self-Paced Cycling Performance: Evidence from Oscillatory Brain Activity and Power Output. PLoS ONE 2019, 14, e0210873. [Google Scholar] [CrossRef]
- Mesquita, P.H.C.; Lage, G.M.; Franchini, E.; Romano-Silva, M.A.; Albuquerque, M.R. Bi-Hemispheric Anodal Transcranial Direct Current Stimulation Worsens Taekwondo-Related Performance. Hum. Mov. Sci. 2019, 66, 578–586. [Google Scholar] [CrossRef]
- Maeda, K.; Yamaguchi, T.; Tatemoto, T.; Kondo, K.; Otaka, Y.; Tanaka, S. Transcranial Direct Current Stimulation Does Not Affect Lower Extremity Muscle Strength Training in Healthy Individuals: A Triple-Blind, Sham-Controlled Study. Front. Neurosci. 2017, 11, 179. [Google Scholar] [CrossRef]
- Fortes, L.d.S.; Mazini-Filho, M.; Lima-Júnior, D.; Machado, D.G.S.; Albuquerque, M.R.; Fonseca, F.d.S.; Ferreira, M.E.C. Transcranial Stimulation Improves Volume and Perceived Exertion but Does Not Change Power. Int. J. Sports Med. 2021, 42, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.d.S.; Unal, G.; Andrade, S.M.; Moreira, A.; Altimari, L.R.; Brunoni, A.R.; Perrey, S.; Mauger, A.R.; Bikson, M.; Okano, A.H. Effect of Transcranial Direct Current Stimulation on Exercise Performance: A Systematic Review and Meta-Analysis. Brain Stimul. 2019, 12, 593–605. [Google Scholar] [CrossRef]
- Dissanayaka, T.; Zoghi, M.; Farrell, M.; Egan, G.F.; Jaberzadeh, S. Does Transcranial Electrical Stimulation Enhance Corticospinal Excitability of the Motor Cortex in Healthy Individuals? A Systematic Review and Meta-Analysis. Eur. J. Neurosci. 2017, 46, 1968–1990. [Google Scholar] [CrossRef]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]
- Rejc, E.; Angeli, C.A. Spinal Cord Epidural Stimulation for Lower Limb Motor Function Recovery in Individuals with Motor Complete Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2019, 30, 337–354. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; Lu, D.C.; Modaber, M.; Zdunowski, S.; Gad, P.; Sayenko, D.G.; Morikawa, E.; Haakana, P.; Ferguson, A.R.; Roy, R.R.; et al. Noninvasive Reactivation of Motor Descending Control after Paralysis. J. Neurotrauma 2015, 32, 1968–1980. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Rath, M.; Ferguson, A.R.; Burdick, J.W.; Havton, L.A.; Edgerton, V.R.; Gerasimenko, Y.P. Self-Assisted Standing Enabled by Non-Invasive Spinal Stimulation after Spinal Cord Injury. J. Neurotrauma 2019, 36, 1435–1450. [Google Scholar] [CrossRef]
- Berry, H.R.; Tate, R.J.; Conway, B.A. Transcutaneous Spinal Direct Current Stimulation Induces Lasting Fatigue Resistance and Enhances Explosive Vertical Jump Performance. PLoS ONE 2017, 12, e0173846. [Google Scholar] [CrossRef]
- Awosika, O.O.; Sandrini, M.; Volochayev, R.; Thompson, R.M.; Fishman, N.; Wu, T.; Floeter, M.K.; Hallett, M.; Cohen, L.G. Transcutaneous Spinal Direct Current Stimulation Improves Locomotor Learning in Healthy Humans. Brain Stimul. 2019, 12, 628–634. [Google Scholar] [CrossRef]
- Shamantseva, N.; Timofeeva, O.; Gvozdeva, A.; Andreeva, I.; Moshonkina, T. Posture of Healthy Subjects Modulated by Transcutaneous Spinal Cord Stimulation. Life 2023, 13, 1909. [Google Scholar] [CrossRef]
- Harrison, P.W.; James, L.P.; McGuigan, M.R.; Jenkins, D.G.; Kelly, V.G. Resistance Priming to Enhance Neuromuscular Performance in Sport: Evidence, Potential Mechanisms and Directions for Future Research. Sports Med. 2019, 49, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Zarkou, A.; Stackhouse, S.; Binder-Macleod, S.A.; Lee, S.C.K. Comparison of Techniques to Determine Human Skeletal Muscle Voluntary Activation. J. Electromyogr. Kinesiol. 2017, 36, 8–15. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.M.; Waddington, G.; Scarvell, J.M.; Ball, N.B.; Creer, R.; Woods, K.; Smith, D. The Effect of Limb Dominance on Lower Limb Functional Performance—A Systematic Review. J. Sports Sci. 2016, 34, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Rejc, E.; Zaccaron, S.; D’Alleva, M.; Botter, A.; Lazzer, S. Effects of Bilateral or Unilateral Plyometric Training of Lower Limbs on the Bilateral Deficit During Explosive Efforts. J. Musculoskelet. Neuronal Interact. 2024, 24, 200–208. [Google Scholar]
- Sayenko, D.G.; Atkinson, D.A.; Dy, C.J.; Gurley, K.M.; Smith, V.L.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Spinal Segment-Specific Transcutaneous Stimulation Differentially Shapes Activation Pattern among Motor Pools in Humans. J. Appl. Physiol. 2015, 118, 1364–1374. [Google Scholar] [CrossRef]
- Hawkins, K.A.; DeMark, L.A.; Vistamehr, A.; Snyder, H.J.; Conroy, C.; Wauneka, C.; Tonuzi, G.; Fuller, D.D.; Clark, D.J.; Fox, E.J. Feasibility of Transcutaneous Spinal Direct Current Stimulation Combined with Locomotor Training after Spinal Cord Injury. Spinal Cord 2022, 60, 971–977. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Neuromodulation of Evoked Muscle Potentials Induced by Epidural Spinal-Cord Stimulation in Paralyzed Individuals. J. Neurophysiol. 2014, 111, 1088–1099. [Google Scholar] [CrossRef]
- Rejc, E.; di Prampero, P.E.; Lazzer, S.; Grassi, B.; Simunic, B.; Pisot, R.; Antonutto, G.; Narici, M. A 35-Day Bed Rest Does Not Alter the Bilateral Deficit of the Lower Limbs during Explosive Efforts. Eur. J. Appl. Physiol. 2015, 115, 1323–1330. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Kang, H. Sample Size Determination and Power Analysis Using the G*Power Software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Walker, S.; Davis, L.; Avela, J.; Häkkinen, K. Neuromuscular Fatigue during Dynamic Maximal Strength and Hypertrophic Resistance Loadings. J. Electromyogr. Kinesiol. 2012, 22, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.; Kamen, G.; Rowland, D.M. Adaptations in Maximal Motor Unit Discharge Rate to Strength Training in Young and Older Adults. Muscle Nerve 2001, 24, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Elgueta-Cancino, E.; Evans, E.; Martinez-Valdes, E.; Falla, D. The Effect of Resistance Training on Motor Unit Firing Properties: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 817631. [Google Scholar] [CrossRef] [PubMed]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef]
- Kotikangas, J.; Walker, S.; Toivonen, S.; Peltonen, H.; Häkkinen, K. Acute Neuromuscular and Hormonal Responses to Power, Strength, and Hypertrophic Protocols and Training Background. Front. Sports Act. Living 2022, 4, 919228. [Google Scholar] [CrossRef]
- Millet, G.Y.; Lepers, R. Alterations of Neuromuscular Function After Prolonged Running, Cycling and Skiing Exercises. Sports Med. 2004, 34, 105–116. [Google Scholar] [CrossRef]
- Duchateau, J.; Enoka, R.M. Neural Control of Lengthening Contractions. J. Exp. Biol. 2016, 219, 197–204. [Google Scholar] [CrossRef]
- Herman, K.; Barton, C.; Malliaras, P.; Morrissey, D. The Effectiveness of Neuromuscular Warm-up Strategies, That Require No Additional Equipment, for Preventing Lower Limb Injuries during Sports Participation: A Systematic Review. BMC Med. 2012, 10, 75. [Google Scholar] [CrossRef]
- McGowan, C.J.; Pyne, D.B.; Thompson, K.G.; Rattray, B. Warm-Up Strategies for Sport and Exercise: Mechanisms and Applications. Sports Med. 2015, 45, 1523–1546. [Google Scholar] [CrossRef]
- Fischer, J.; Paternoster, F.K. Post-Activation-Performance Enhancement: Possible Contributing Factors. J. Sports Sci. Med. 2024, 23, 34–45. [Google Scholar] [CrossRef]
- Mayr, W.; Krenn, M.; Dimitrijevic, M.R. Epidural and Transcutaneous Spinal Electrical Stimulation for Restoration of Movement after Incomplete and Complete Spinal Cord Injury. Curr. Opin. Neurol. 2016, 29, 721. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, J.; Angeli, C.A.; Harkema, S.J.; Farina, D.; Rejc, E. Recruitment Order of Motor Neurons Promoted by Epidural Stimulation in Individuals with Spinal Cord Injury. J. Appl. Physiol. 2021, 131, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Barss, T.S.; Parhizi, B.; Porter, J.; Mushahwar, V.K. Neural Substrates of Transcutaneous Spinal Cord Stimulation: Neuromodulation across Multiple Segments of the Spinal Cord. J. Clin. Med. 2022, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering Spinal Cord Excitability Enables Voluntary Movements after Chronic Complete Paralysis in Humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef]
- Rejc, E.; Smith, A.C.; Weber, K.A.; Ugiliweneza, B.; Bert, R.J.; Negahdar, M.; Boakye, M.; Harkema, S.J.; Angeli, C.A. Spinal Cord Imaging Markers and Recovery of Volitional Leg Movement with Spinal Cord Epidural Stimulation in Individuals with Clinically Motor Complete Spinal Cord Injury. Front. Syst. Neurosci. 2020, 14, 559313. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Enoka, R.M. The Extraction of Neural Strategies from the Surface EMG. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef]
- D’Emanuele, S.; Maffiuletti, N.A.; Tarperi, C.; Rainoldi, A.; Schena, F.; Boccia, G. Rate of Force Development as an Indicator of Neuromuscular Fatigue: A Scoping Review. Front. Hum. Neurosci. 2021, 15, 701916. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Negro, F.; Holobar, A.; Casolo, A.; Folland, J.P.; Felici, F.; Farina, D. You Are as Fast as Your Motor Neurons: Speed of Recruitment and Maximal Discharge of Motor Neurons Determine the Maximal Rate of Force Development in Humans. J. Physiol. 2019, 597, 2445–2456. [Google Scholar] [CrossRef]
- Enoka, R.M.; Farina, D. Force Steadiness: From Motor Units to Voluntary Actions. Physiology 2021, 36, 114–130. [Google Scholar] [CrossRef]
- Søgaard, K.; Gandevia, S.C.; Todd, G.; Petersen, N.T.; Taylor, J.L. The Effect of Sustained Low-Intensity Contractions on Supraspinal Fatigue in Human Elbow Flexor Muscles. J. Physiol. 2006, 573, 511–523. [Google Scholar] [CrossRef]
- Muddle, T.W.D.; Colquhoun, R.J.; Magrini, M.A.; Luera, M.J.; DeFreitas, J.M.; Jenkins, N.D.M. Effects of Fatiguing, Submaximal High- versus Low-Torque Isometric Exercise on Motor Unit Recruitment and Firing Behavior. Physiol. Rep. 2018, 6, e13675. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Common Neural Structures Activated by Epidural and Transcutaneous Lumbar Spinal Cord Stimulation: Elicitation of Posterior Root-Muscle Reflexes. PLoS ONE 2018, 13, e0192013. [Google Scholar] [CrossRef] [PubMed]
- Moraud, E.M.; Capogrosso, M.; Formento, E.; Wenger, N.; DiGiovanna, J.; Courtine, G.; Micera, S. Mechanisms Underlying the Neuromodulation of Spinal Circuits for Correcting Gait and Balance Deficits after Spinal Cord Injury. Neuron 2016, 89, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Musienko, P.E.; Zelenin, P.V.; Orlovsky, G.N.; Deliagina, T.G. Facilitation of Postural Limb Reflexes with Epidural Stimulation in Spinal Rabbits. J. Neurophysiol. 2010, 103, 1080–1092. [Google Scholar] [CrossRef]
- Skiadopoulos, A.; Famodimu, G.O.; Solomon, S.K.; Agarwal, P.; Harel, N.Y.; Knikou, M. Priming Locomotor Training with Transspinal Stimulation in People with Spinal Cord Injury: Study Protocol of a Randomized Clinical Trial. Trials 2023, 24, 145. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Gad, P. Spinal Automaticity of Movement Control and Its Role in Recovering Function after Spinal Injury. Expert Rev. Neurother. 2022, 22, 655–667. [Google Scholar] [CrossRef]


| Subject | Age (Yrs) | Body Mass (Kg) | Stature (cm) | BMI (Kg/m2) | Sport Practiced | Training Activities (Days/Week) |
|---|---|---|---|---|---|---|
| 1 | 23 | 74 | 169 | 25.9 | Mixed martial arts | 3 |
| 2 | 23 | 71 | 176 | 22.9 | Resistance training | 2 |
| 3 | 21 | 66 | 170 | 22.8 | Resistance training | 3 |
| 4 | 28 | 70 | 181 | 21.4 | Fencing | 2 |
| 5 | 23 | 96 | 184 | 28.4 | Resistance training | 2 |
| 6 | 22 | 73 | 175 | 23.8 | Futsal | 5 |
| 7 | 19 | 94 | 195 | 24.7 | Basketball | 2 |
| 8 | 22 | 73 | 174 | 24.1 | Soccer | 4 |
| 9 | 21 | 73 | 177 | 23.3 | Soccer | 4 |
| 10 | 24 | 91 | 182 | 27.5 | Basketball | 3 |
| 11 | 23 | 74 | 172 | 25.0 | Soccer | 5 |
| 12 | 23 | 75 | 181 | 22.9 | Resistance training | 4 |
| Mean | 22.7 | 77.5 | 178 | 24.4 | - | 3.3 |
| SD | 2.1 | 10.1 | 7.2 | 2.0 | - | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccaron, S.; Mari, L.; D’Alleva, M.; Stafuzza, J.; Parpinel, M.; Lazzer, S.; Rejc, E. Effects of Neuromuscular Priming with Spinal Cord Transcutaneous Stimulation on Lower Limb Motor Performance in Humans: A Randomized Crossover Sham-Controlled Trial. J. Clin. Med. 2025, 14, 4143. https://doi.org/10.3390/jcm14124143
Zaccaron S, Mari L, D’Alleva M, Stafuzza J, Parpinel M, Lazzer S, Rejc E. Effects of Neuromuscular Priming with Spinal Cord Transcutaneous Stimulation on Lower Limb Motor Performance in Humans: A Randomized Crossover Sham-Controlled Trial. Journal of Clinical Medicine. 2025; 14(12):4143. https://doi.org/10.3390/jcm14124143
Chicago/Turabian StyleZaccaron, Simone, Lara Mari, Mattia D’Alleva, Jacopo Stafuzza, Maria Parpinel, Stefano Lazzer, and Enrico Rejc. 2025. "Effects of Neuromuscular Priming with Spinal Cord Transcutaneous Stimulation on Lower Limb Motor Performance in Humans: A Randomized Crossover Sham-Controlled Trial" Journal of Clinical Medicine 14, no. 12: 4143. https://doi.org/10.3390/jcm14124143
APA StyleZaccaron, S., Mari, L., D’Alleva, M., Stafuzza, J., Parpinel, M., Lazzer, S., & Rejc, E. (2025). Effects of Neuromuscular Priming with Spinal Cord Transcutaneous Stimulation on Lower Limb Motor Performance in Humans: A Randomized Crossover Sham-Controlled Trial. Journal of Clinical Medicine, 14(12), 4143. https://doi.org/10.3390/jcm14124143






