Necrotizing Pneumonia as a Complication of Community-Acquired Pneumonia in Adults at a Tertiary Institution
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Variables
2.4. Quantitative Variables and Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAL | Bronchoalveolar lavage |
| CAP | Community-acquired pneumonia |
| CPPE | Complicated parapneumonic effusion |
| CT | Computed tomography |
| ICU | Intensive care unit |
| MRSA | Methicillin-resistant Staphylococcus Aureus |
| NP | Necrotizing pneumonia |
| VATS | Video-assisted thoracoscopic surgery |
References
- Chatha, N.; Fortin, D.; Bosma, K.J. Management of Necrotizing Pneumonia and Pulmonary Gangrene: A Case Series and Review of the Literature. Can. Respir. J. 2014, 21, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Gillet, Y.; Vanhems, P.; Lina, G.; Bes, M.; Vandenesch, F.; Floret, D.; Etienne, J. Factors Predicting Mortality in Necrotizing Community-Acquired Pneumonia Caused by Staphylococcus aureus Containing Panton-Valentine Leukocidin. Clin. Infect. Dis. 2007, 45, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Krutikov, M.; Rahman, A.; Tiberi, S. Necrotizing pneumonia (aetiology, clinical features and management). Curr. Opin. Pulm. Med. 2019, 25, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Larose, J.C.; Wang, H.T.; Rakovich, G. Survival with optimal medical management in a cohort of severe necrotizing bacterial lung infections. J. Thorac. Dis. 2023, 15, 3860–3869. [Google Scholar] [CrossRef]
- Kapania, E.M.; Cavallazzi, R. Necrotizing Pneumonia: A Practical Guide for the Clinician. Pathogens 2024, 13, 984. [Google Scholar] [CrossRef]
- Masters, I.B.; Isles, A.F.; Grimwood, K. Necrotizing pneumonia: An emerging problem in children? Pneumonia 2017, 9, 11. [Google Scholar] [CrossRef]
- Sawicki, G.S.; Lu, F.L.; Valim, C.; Cleveland, R.H.; Colin, A.A. Necrotising pneumonia is an increasingly detected complication of pneumonia in children. Eur. Respir. J. 2008, 31, 1285–1291. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Tsai, Y.T.; Ku, Y.H. Surgical Treatment of 26 Patients with Necrotizing Pneumonia. Eur. Surg. Res. 2011, 47, 13–18. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Torres, A.; Nagavci, B.; Aliberti, S.; Antonelli, M.; Bassetti, M.; Bos, L.; Chalmers, J.D.; Derde, L.; de Waele, J.; et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Eur. Respir. J. 2023, 61, 2200735. [Google Scholar] [CrossRef]
- Bover-Bauza, C.; Osona, B.; Gil, J.A.; Peña-Zarza, J.A.; Figuerola, J. Long-term outcomes of necrotizing pneumonia. An. Pediatría (Engl. Ed.) 2021, 95, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Reimel, B.A.; Krishnadasen, B.; Cuschieri, J.; Klein, M.B.; Gross, J.; Karmy-Jones, R. Surgical Management of Acute Necrotizing Lung Infections. Can. Respir. J. 2006, 13, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Wang, C.; Zhang, Y.; Zhou, Y. Necrotizing Pneumonia in Children: Early Recognition and Management. JCM 2023, 12, 2256. [Google Scholar] [CrossRef]
- Krenke, K.; Sanocki, M.; Urbankowska, E.; Kraj, G.; Krawiec, M.; Urbankowski, T.; Peradzyńska, J.; Kulus, M. Necrotizing Pneumonia and Its Complications in Children. In Pulmonary Infection; Advances in Experimental Medicine and, Biology; Pokorski, M., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; Volume 857, pp. 9–17. [Google Scholar] [CrossRef]
- Wong, K.S.; Chiu, C.H.; Yeow, K.M.; Huang, Y.C.; Liu, H.P.; Lin, T.Y. Necrotising pneumonitis in children. Eur. J. Pediatr. 2000, 159, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 1st ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 20 May 2024).
- Pande, A.; Nasir, S.; Rueda, A.M.; Matejowsky, R.; Ramos, J.; Doshi, S.; Kulkarni, P.; Musher, D.M. The Incidence of Necrotizing Changes in Adults With Pneumococcal Pneumonia. Clin. Infect. Dis. 2012, 54, 10–16. [Google Scholar] [CrossRef]
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar] [CrossRef]
- Rivero-Calle, I.; Cebey-López, M.; Pardo-Seco, J.; Yuste, J.; Redondo, E.; Vargas, A.D.; Mascarós, E.; Díaz-Maroto, J.L.; Linares-Rufo, M.; Jimeno, I.; et al. Lifestyle and comorbid conditions as risk factors for community-acquired pneumonia in outpatient adults (NEUMO-ES-RISK project). BMJ Open Resp. Res. 2019, 6, e000359. [Google Scholar] [CrossRef]
- Kreienbuehl, L.; Charbonney, E.; Eggimann, P. Community-acquired necrotizing pneumonia due to methicillin-sensitive Staphylococcus aureus secreting Panton-Valentine leukocidin: A review of case reports. Ann. Intensive Care 2011, 1, 52. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Ku, Y.H. Necrotizing pneumonia: A rare complication of pneumonia requiring special consideration. Curr. Opin. Pulm. Med. 2012, 18, 246–252. [Google Scholar] [CrossRef]
- Gillet, Y.; Issartel, B.; Vanhems, P.; Fournet, J.-C.; Lina, G.; Bes, M.; Vandenesch, F.; Piémont, Y.; Brousse, N.; Floret, D.; et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 2002, 359, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Alifano, M.; Lorut, C.; Lefebvre, A.; Khattar, L.; Damotte, D.; Huchon, G.; Regnard, J.-F.; Rabbat, A. Necrotizing pneumonia in adults: Multidisciplinary management. Intensive Care Med. 2011, 37, 1888–1889. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Canelas, C.; Fontoura, I.; Rodrigues, B.; Lemos, J.; Torres, A.; Girão, F. Lung abscess and necrotizing pneumonia: A hospital’s experience. Eur. J. Intern. Med. 2013, 24, e61. [Google Scholar] [CrossRef]
- Schweigert, M.; Dubecz, A.; Beron, M.; Ofner, D.; Stein, H. Surgical Therapy for Necrotizing Pneumonia and Lung Gangrene. Thorac. Cardiovasc. Surg. 2012, 61, 636–641. [Google Scholar] [CrossRef]
- Karmy-Jones, R.; Valli??res, E.; Harrington, R. Surgical Management of Necrotizing Pneumonia. Clin. Pulm. Med. 2003, 10, 17–25. [Google Scholar] [CrossRef]
- Seo, H.; Cha, S.; Shin, K.; Lim, J.; Yoo, S.; Lee, J.; Lee, S.; Kim, C.; Park, J. Focal necrotizing pneumonia is a distinct entity from lung abscess. Respirology 2013, 18, 1095–1100. [Google Scholar] [CrossRef]
- Pascual, F.E.; Matthay, M.A.; Bacchetti, P.; Wachter, R.M. Assessment of Prognosis in Patients With Community-Acquired Pneumonia Who Require Mechanical Ventilation. Chest 2000, 117, 503–512. [Google Scholar] [CrossRef]
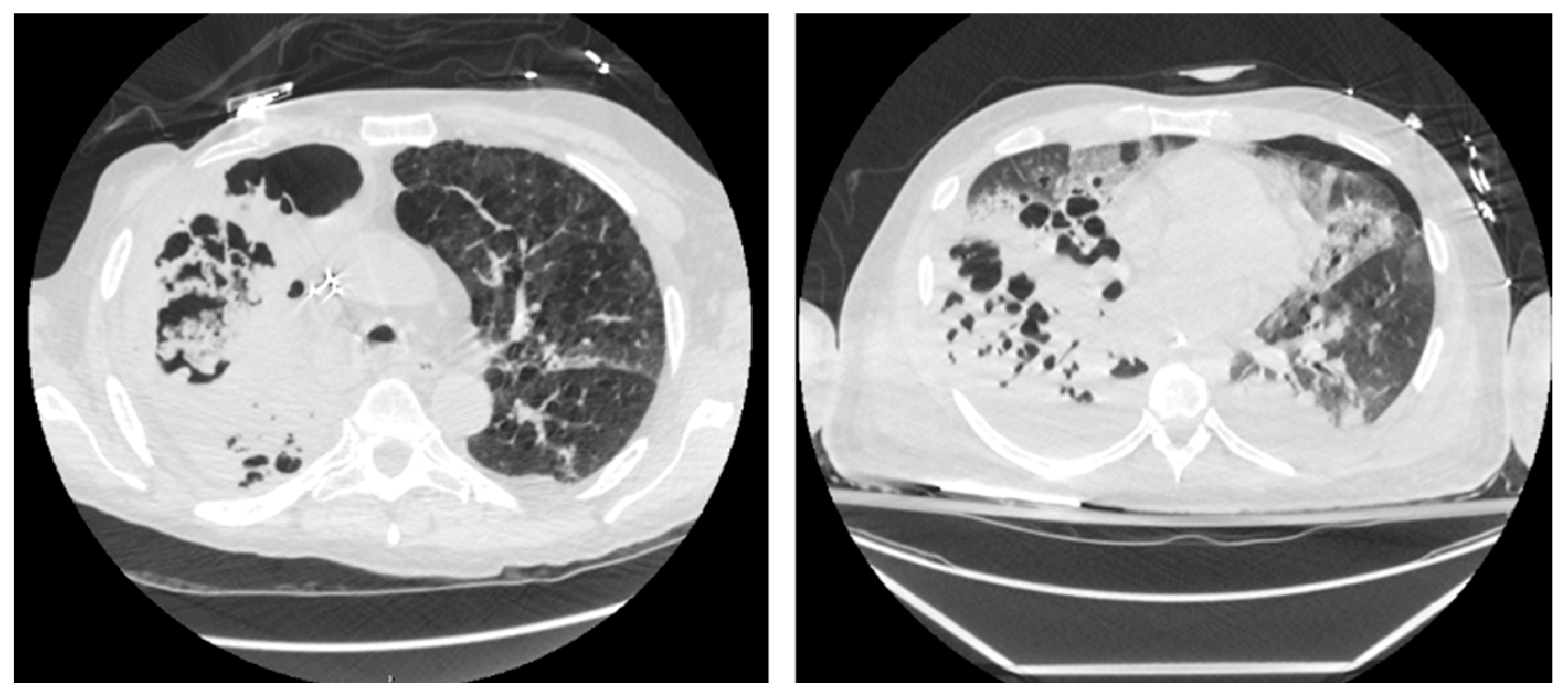
| N = 57 | |
|---|---|
| Age | |
| Mean | 55.0 (15.0) |
| Median | 57.0 [43.0, 66.0] |
| Male | 31 (54.4%) |
| Race | |
| White | 29 (50.9%) |
| Black or African American | 25 (43.9%) |
| Asian | 1 (1.8%) |
| Other | 2 (3.5%) |
| Co-Morbidities | |
| Smoker | 47 (82.5%) |
| Alcohol Use | 10 (17.5%) |
| Diabetes | 12 (21.1%) |
| Hypertension | 27 (47.4%) |
| Chronic kidney disease | 1 (1.8%) |
| Chronic obstructive pulmonary disease | 21 (36.8%) |
| HIV | 7 (12.3%) |
| History of malignancy | 9 (15.8%) |
| Need for mechanical ventilation | 20 (35.1%) |
| Septic shock needing vasopressors | 16 (28.1%) |
| N = 57 | |
|---|---|
| Onset of Symptoms | |
| <1 week | 35 (61.4%) |
| 1–2 week | 11 (19.3%) |
| >2 weeks | 11 (19.3%) |
| MAP on admission | |
| Mean | 86.6 (20.4) |
| Median | 85.0 [72.0, 98.0] |
| Extent of Pneumonia | |
| Single lobe | 19 (33.3%) |
| Multifocal ipsilateral | 17 (29.8%) |
| Multifocal bilateral | 21 (36.8%) |
| Pneumonia-associated complications | |
| Septic shock | 16 (28.1%) |
| Complicated parapneumonic effusion/empyema | 13 (22.8) |
| Bronchopleural fistula | 5 (8.8%) |
| Need for chest tube placement | 16 (28.1%) |
| Characteristics | N = 57 |
|---|---|
| Viral PCR positivity | |
| Influenza A | 1 (1.8%) |
| Influenza B | 1 (1.8%) |
| Rhinovirus | 2 (3.5%) |
| SARS-CoV-2 | 1 (1.8%) |
| Respiratory culture (sputum, tracheal aspirate, or bronchoalveolar lavage) growth | |
| Monomicrobial | 20 (35.1%) |
| Polymicrobial | 26 (61.4%) |
| None identified | 11 (19.3%) |
| Associated pathogens | |
| Methicillin-sensitive Staphylococcus Aureus (MSSA) | 4 |
| Methicillin-resistant Staphylococcus Aureus (MRSA) | 12 |
| Pseudomonas sp. | 5 |
| Escherichia coli | 3 |
| Streptococcus pneumoniae | 2 |
| Streptococcus pyogenes | 2 |
| Other Gram-negative rods | 15 |
| Mixed flora | 12 |
| Characteristics | N = 57 |
|---|---|
| Hospital length of stay (days) | |
| Mean | 26.6 (29.2) |
| Median | 16.0 [12.0, 29.0] |
| ICU admission from emergency department | 27 (47.4%) |
| Time spent on mechanical ventilation (days), mean | 8.54 (24.0) |
| Time spent on mechanical ventilation (days), median | 0 [0, 6] |
| Duration of antibiotics (days), median | 28 [21–42] |
| Surgery performed | 8 |
| Video-assisted thoracoscopic surgery (VATS) with decortication | 8 |
| Local debridement | 1 |
| Mortality | 14 (24.6%) |
| Yes (N = 14) | No (N = 43) | p-Value | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 57.7 (14.4) | 54.1 (15.3) | 0.458 * |
| Smoking history | |||
| Yes | 10 (71.4%) | 37 (86.0%) | 0.24 |
| No | 4 (28.6%) | 6 (14.0%) | |
| Presence of comorbidity | |||
| No | 2 (14.3%) | 5 (11.6%) | 1 |
| Yes | 12 (85.7%) | 38 (88.4%) | |
| Extent of pneumonia | |||
| Single lobe | 3 (21.4%) | 16 (37.2%) | 0.588 |
| Multifocal ipsilateral | 5 (35.7%) | 12 (27.9%) | |
| Multifocal bilateral | 6 (42.9%) | 15 (34.9%) | |
| Respiratory culture growth (sputum/tracheal aspirate or BAL) | |||
| No | 2 (14.3%) | 9 (20.9%) | 0.714 |
| Yes | 12 (85.7%) | 34 (79.1%) | |
| Monomicrobial vs. polymicrobial growth on respiratory culture | |||
| None | 2 (14.3%) | 9 (20.9%) | 0.664 |
| Monomicrobial | 4 (28.6%) | 16 (37.2%) | |
| Polymicrobial | 8 (57.1%) | 18 (41.9%) | |
| MRSA | |||
| No | 8 (57.1%) | 27 (62.8%) | 0.471 † |
| Yes | 4 (28.6%) | 8 (18.6%) | |
| Need for mechanical ventilation during hospital stay | |||
| Yes | 13 (92.9%) | 7 (16.3%) | <0.001 |
| No | 1 (7.1%) | 36 (83.7%) | |
| Septic shock | |||
| Yes | 3 (21.4%) | 38 (88.4%) | <0.001 |
| No | 11 (78.6%) | 5 (11.6%) | |
| Bronchopleural fistula | 12 (85.7%) | 40 (93.0%) | 0.587 |
| Yes | 2 (14.3%) | 3 (7.0%) | |
| No | |||
| Complicated parapneumonic effusion/empyema | |||
| Yes | 10 (71.4%) | 34 (79.1%) | 0.715 |
| No | 4 (28.6%) | 9 (20.9%) | |
| Inpatient surgery | |||
| Yes | 2 (14.3%) | 6 (14.0%) | 1 |
| No | 12 (85.7%) | 36 (83.7%) |
| Coefficient | exp(Coefficient) | 95% CI for Coefficient | 95% CI for exp(Coefficient) | p-Value | |
|---|---|---|---|---|---|
| Need for mechanical ventilation | 3.32 | 27.60 | (0.99, 6.51) | (2.69, 671.96) | 0.011 |
| Septic shock | 1.31 | 0.04 | (0.74, 3.43) | (0.48, 30.82) | 0.203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boppana, L.K.T.; Isern, S.; Romero, K.N.; Ferreira, J.; Garvan, G.; Ashby, T. Necrotizing Pneumonia as a Complication of Community-Acquired Pneumonia in Adults at a Tertiary Institution. J. Clin. Med. 2025, 14, 4086. https://doi.org/10.3390/jcm14124086
Boppana LKT, Isern S, Romero KN, Ferreira J, Garvan G, Ashby T. Necrotizing Pneumonia as a Complication of Community-Acquired Pneumonia in Adults at a Tertiary Institution. Journal of Clinical Medicine. 2025; 14(12):4086. https://doi.org/10.3390/jcm14124086
Chicago/Turabian StyleBoppana, Leela Krishna Teja, Samantha Isern, Kaitlyn N. Romero, Jason Ferreira, Gerard Garvan, and Tracy Ashby. 2025. "Necrotizing Pneumonia as a Complication of Community-Acquired Pneumonia in Adults at a Tertiary Institution" Journal of Clinical Medicine 14, no. 12: 4086. https://doi.org/10.3390/jcm14124086
APA StyleBoppana, L. K. T., Isern, S., Romero, K. N., Ferreira, J., Garvan, G., & Ashby, T. (2025). Necrotizing Pneumonia as a Complication of Community-Acquired Pneumonia in Adults at a Tertiary Institution. Journal of Clinical Medicine, 14(12), 4086. https://doi.org/10.3390/jcm14124086







