Lipopolysaccharide-Binding Protein (LBP) and Inflammatory Biomarkers in SARS-CoV-2 Hospitalized Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethical Approval
2.3. Clinical and Demographic Data
2.4. Blood Sampling and Laboratory Measurements
2.5. Criteria for Determining Disease Severity
2.6. Nutritional Assessment
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Demographic, Anthropometric and Clinical Characteristics
3.3. LBP and Its Relation to Inflammatory Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majumder, J.; Minko, T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.C.; Lau, S.K.P.; Woo, P.C.Y.; Kwok, Y.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007, 20, 660–694. [Google Scholar] [CrossRef]
- Girard, M.P.; Tam, J.S.; Assossou, O.M.; Kieny, M.P. The 2009 A (H1N1) influenza virus pandemic: A review. Vaccine 2010, 28, 4895–4902. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Hui, D.S.; Perlman, S. Middle East respiratory syndrome. Lancet 2015, 386, 995–1007. [Google Scholar] [CrossRef]
- Oleribe, O.O.; Salako, B.L.; Ka, M.M.; Akpalu, A.; McConnochie, M.; Foster, M.; Taylor-Robinson, S.D. Ebola virus disease epidemic in West Africa: Lessons learned and issues arising from West African countries. Clin. Med. 2015, 15, 54–57. [Google Scholar] [CrossRef]
- Agumadu, V.C.; Ramphul, K. Zika virus: A review of literature. Cureus 2018, 10, e3025. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Mirzazadeh, A.; Tavakolpour, S. Genetics and genomics of SARS-CoV-2: A review with special focus on genetic diversity and genome detection. Genomics 2020, 112, 4183–4191. [Google Scholar] [CrossRef]
- Alsharif, W.; Qurashi, A. Effectiveness of COVID-19 diagnosis and management tools: A review. Radiography 2021, 27, 682–687. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, A.; Kumar, V.; Poyoja, R.; Ghosh, A. Virology, pathogenesis, diagnosis and in-line treatment of COVID-19. Environ. Pollut. 2021, 274, 116468. [Google Scholar] [CrossRef]
- Angeletti, S.; Benvenuto, D.; Bianchi, M.; Giovanetti, M.; Pascarella, S.; Ciccozzi, M. COVID-2019: The role of the nsp2 and nsp3 in its pathogenesis. J. Med. Virol. 2020, 92, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Darif, D.; Hammi, I.; Kihel, A.; El Idrissi, I.; Saik, I.; Guessous, F. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021, 153, 104799. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine storm in COVID-19: The current evidence and treatment strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef]
- Maurya, R.; Sebastian, P.; Namdeo, M.; Devender, M.; Gertler, A. COVID-19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link Between High Morbidity and Mortality. Front. Immunol. 2021, 12, 649359. [Google Scholar] [CrossRef] [PubMed]
- Schröder, N.W.J.; Schumann, R.R. Non-LPS Targets and Actions of LPS Binding Protein (LBP). J. Endotoxin Res. 2005, 11, 237–242. [Google Scholar] [CrossRef]
- Utrata, A.; Schmidtner, N.; Mester, P.; Schmid, S.; Müller, M.; Pavel, V.; Buechler, C. Plasma Lipopolysaccharide-Binding Protein (LBP) Is Induced in Critically Ill Females with Gram-Negative Infections—Preliminary Study. Infect. Dis. Rep. 2025, 17, 10. [Google Scholar] [CrossRef]
- Godeau, D.; Petit, A.; Richard, I.; Roquelaure, Y.; Descatha, A. Return-to-Work, Disabilities and Occupational Health in the Age of COVID-19. Scand. J. Work. Environ. Health 2021, 47, 408–409. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Gyriki, D.; Nikolaidis, C.G.; Bezirtzoglou, E.; Voidarou, C.; Stavropoulou, E.; Tsigalou, C. The Gut Microbiota and Aging: Interactions, Implications, and Interventions. Microorganisms 2025, 11, 1452917. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, C.; Ba, Y.; Wang, Y.; Song, B. Nutritional Risk and Therapy for Severe and Critical COVID-19 Patients: A Multicenter Retrospective Observational Study. Nutr. Clin. Pract. 2020, 35, 1232–1240. [Google Scholar] [CrossRef]
- Sattar, N.; McInnes, I.B.; McMurray, J.J.V. Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms. Circulation 2020, 142, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, Y.; Huang, Y.-M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.-L. Obesity in Patients with COVID-19: A Systematic Review and Meta-Analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef]
- Meng, L.; Song, Z.; Liu, A.; Dahmen, U.; Yang, X.; Fang, H. Effects of Lipopolysaccharide-Binding Protein (LBP) Single Nucleotide Polymorphism (SNP) in Infections, Inflammatory Diseases, Metabolic Disorders and Cancers. Front. Immunol. 2021, 12, 699320. [Google Scholar] [CrossRef]
- Alabdullatif, W.; Almnaizel, A.; Alhijji, A.; Alshathri, A.; Albarrag, A.; Bindayel, I. Correlation of Plasma 25(OH)D3 and Vitamin D Binding Protein Levels with COVID-19 Severity and Outcome in Hospitalized Patients. Nutrients 2023, 15, 1818. [Google Scholar] [CrossRef]
- Centers for Disease Control Prevention (CDC) About Adult, B.M.I. 2022. Available online: https://www.cdc.gov/bmi/adult-calculator/bmi-categories.html (accessed on 1 February 2023).
- Porter Starr, K.N.; Bales, C.W. Excessive Body Weight in Older Adults. Clin. Geriatr. Med. 2015, 31, 311–326. [Google Scholar] [CrossRef]
- Acehan, S.; Gulen, M.; Isıkber, C.; Unlu, N.; Sumbul, H.E.; Gulumsek, E.; Satar, S. mNUTRIC Tool Is Capable to Predict Nutritional Needs and Mortality Early in Patients Suffering from Severe Pneumonia. Clin. Nutr. ESPEN 2021, 45, 184–191. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. Dynamic Lipopolysaccharide Transfer Cascade to TLR4/MD2 Complex via LBP and CD14. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Hoel, H.; Heggelund, L.; Reikvam, D.H.; Stiksrud, B.; Ueland, T.; Michelsen, A.E.; Otterdal, K.; Muller, K.E.; Lind, A.; Muller, F.; et al. Elevated Markers of Gut Leakage and Inflammasome Activation in COVID-19 Patients with Cardiac Involvement. J. Intern. Med. 2021, 289, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Cammisotto, V.; Cangemi, R.; Ferro, D.; Miele, M.C.; De Angelis, M.; Cancelli, F.; Pignatelli, P.; Venditti, M.; Pugliese, F.; et al. Low-Grade Endotoxemia and Thrombosis in COVID-19. Clin. Transl. Gastroenterol. 2021, 12, e00348. [Google Scholar] [CrossRef]
- Oliva, A.; Miele, M.C.; Di Timoteo, F.; De Angelis, M.; Mauro, V.; Aronica, R.; Al Ismail, D.; Ceccarelli, G.; Pinacchio, C.; D’ettorre, G.; et al. Persistent Systemic Microbial Translocation and Intestinal Damage During Coronavirus Disease-19. Front. Immunol. 2021, 12, 748361. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed]
- Worthmann, H.; Tryc, A.B.; Dirks, M.; Schuppner, R.; Brand, K.; Klawonn, F.; Lichtinghagen, R.; Weissenborn, K. Lipopolysaccharide Binding Protein, Interleukin-10, Interleukin-6 and C-Reactive Protein Blood Levels in Acute Ischemic Stroke Patients with Post-Stroke Infection. J. Neuroinflamm. 2015, 12, 13. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serino, M.; Luche, E.; Waget, A.; Pardo, G.; Salvador, J.; Ricart, W.; Frühbeck, G.; Burcelin, R.; et al. Circulating Lipopolysaccharide-Binding Protein (LBP) as a Marker of Obesity-Related Insulin Resistance. Int. J. Obes. 2012, 36, 1442–1449. [Google Scholar] [CrossRef]
- Dutheil, F.; Trousselard, M.; Perrier, C.; Lac, G.; Chamoux, A.; Duclos, M.; Naughton, G.; Mnatzaganian, G.; Schmidt, J. Urinary Interleukin-8 Is a Biomarker of Stress in Emergency Physicians, Especially with Advancing Age—The JOBSTRESS* Randomized Trial. PLoS ONE 2013, 8, e71668. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]

| Characteristic | Total (n = 167) | Non-Critical (n = 86) | Critical (n = 81) | p-Value |
|---|---|---|---|---|
| Age (years) | 59.54 ± 14.84 | 57.58 ± 13.90 | 62.62 ± 15.91 | 0.031 |
| Female | 84 (50.3%) | 46 (53.5%) | 38 (46.9%) | 0.396 |
| Male | 83 (49.7%) | 40 (46.5%) | 43 (53.1%) | |
| Weight, kg | 82.66 ± 19.58 | 82.11 ± 18.51 | 83.36 ± 20.48 | 0.678 |
| BMI, kg/m2 | 30.87 ± 7.17 | 30.46 ± 0.67 | 31.31 ± 8.08 | 0.447 |
| BMI Categories | ||||
| <25 kg/m2 | 34 (20.4%) | 17 (19.8%) | 17 (21%) | |
| 25–≤30 kg/m2 | 52 (31.1%) | 23 (26.7%) | 29 (35.8%) | 0.361 |
| >30 kg/m2 | 81 (48.5%) | 46 (53.5%) | 35 (43.2%) | |
| Inflammatory biomarkers | ||||
| LBP, ng/mL | 8.32 [5.19–10.1] | 8.7 [5.5–10.7] | 7.8 [5–9.7] | 0.140 |
| IL-6, pg/mL | 24.63 [4.3] | 23.93 [2.75] | 26.80 [7.57] | <0.001 |
| IL-8, pg/mL | 71.01 [153.59] | 32.47 [92.15] | 124.55 [245.89] | <0.001 |
| IL-10, pg/mL | 266.23 [456.53] | 206.76 [458.82] | 248.76 [479.08] | 0.674 |
| TNF-α, pg/mL | 131.17 [227.25] | 124.92 [171.26] | 151.88 [418.06] | 0.125 |
| IL-6/IL-10 | 0.1334 [0.23] | 0.12 [0.21] | 0.134 [0.24] | 0.353 |
| IL-10/TNF-α | 1.73 [2.96] | 1.85 [2.85] | 1.53 [3.05] | 0.315 |
| CRP, mg/L | 103.5 [110.6] | 88.95 [112.5] | 113.0 [120.13] | 0.057 |
| Variable | Correlation Coefficient | p Value |
|---|---|---|
| Age | 0.102 | 0.188 |
| Gender | - | 0.970 |
| BMI, kg/m2 | −0.063 | 0.420 |
| IL-6, pg/mL | 0.009 | 0.906 |
| IL-8, pg/mL | 0.077 | 0.320 |
| IL-10, pg/mL | 0.427 | <0.001 |
| TNF-α, pg/mL | 0.275 | <0.001 |
| IL-6/IL-10 | −0.397 | <0.001 |
| IL-10/TNF-α | 0.050 | 0.521 |
| CRP, mg/L | −0.084 | 0.299 |
| Variable | BMI < 25 (n = 34) | BMI 25–<30 (n = 52) | BMI > 30 (n = 81) | p-Value |
|---|---|---|---|---|
| LBP, ng/mL | 8.31 [5.01–11.1] | 8.61 [5.4–10.6] | 7.9 [5–9.7] | 0.613 |
| IL-6, pg/mL | 26.1 [23.8–30.1] | 23.9 [23.3–27.6] | 24.63 [23.2–26.8] | 0.154 |
| IL-8, pg/mL | 124.6 [63.04–316.6] | 63.2 [1.75–181.9] | 77.3 [17.1–155.34] | 0.049 |
| IL-10, pg/mL | 200.44 [96.9–517.5] | 316.7 [100.9–654.5] | 212.1 [93.68–543.3] | 0.384 |
| TNF-α, pg/mL | 127.3 [66–465.4] | 138.65 [73.8–257.8] | 130.6 [68.6–317.3] | 0.922 |
| IL-6/IL-10 | 0.15 [0.04–0.26] | 0.1 [0.04–0.3] | 0.12 [0.05–0.3] | 0.398 |
| IL-10/TNF-α | 1.08 [0.5–3.3] | 1.7 [1–4.4] | 1.78 [0.7–4.2] | 0.214 |
| CRP, mg/L | 115.5 [29.25–172.25] | 129 [83.02–188.5] | 85.25 [47.3–160.25] | 0.025 |
| Variable | Not at Risk of Malnutrition | At Risk of Malnutrition | p-Value |
|---|---|---|---|
| Critical cases | 8 (10.5%) | 73 (80.2%) | <0.001 |
| Non-critical cases | 68 (89.5%) | 18 (19.8%) | 0.154 |
| Age, years | 52.8 ± 10.2 | 66.0 ± 15.9 | <0.001 |
| Female | 37 (46.1%) | 47 (51.6%) | 0.73 |
| Male | 39 (51.3%) | 44 (48.4%) | 0.922 |
| BMI, kg/m2 | 31.2 ± 6.3 | 30.6 ± 7.84 | 0.641 |
| LBP, ng/mL | 8.2 [5.3–9.6] | 8.6 [5–10.7] | 0.565 |
| IL-6, pg/mL | 23.93 [2.75] | 26.10 [6.75] | <0.001 |
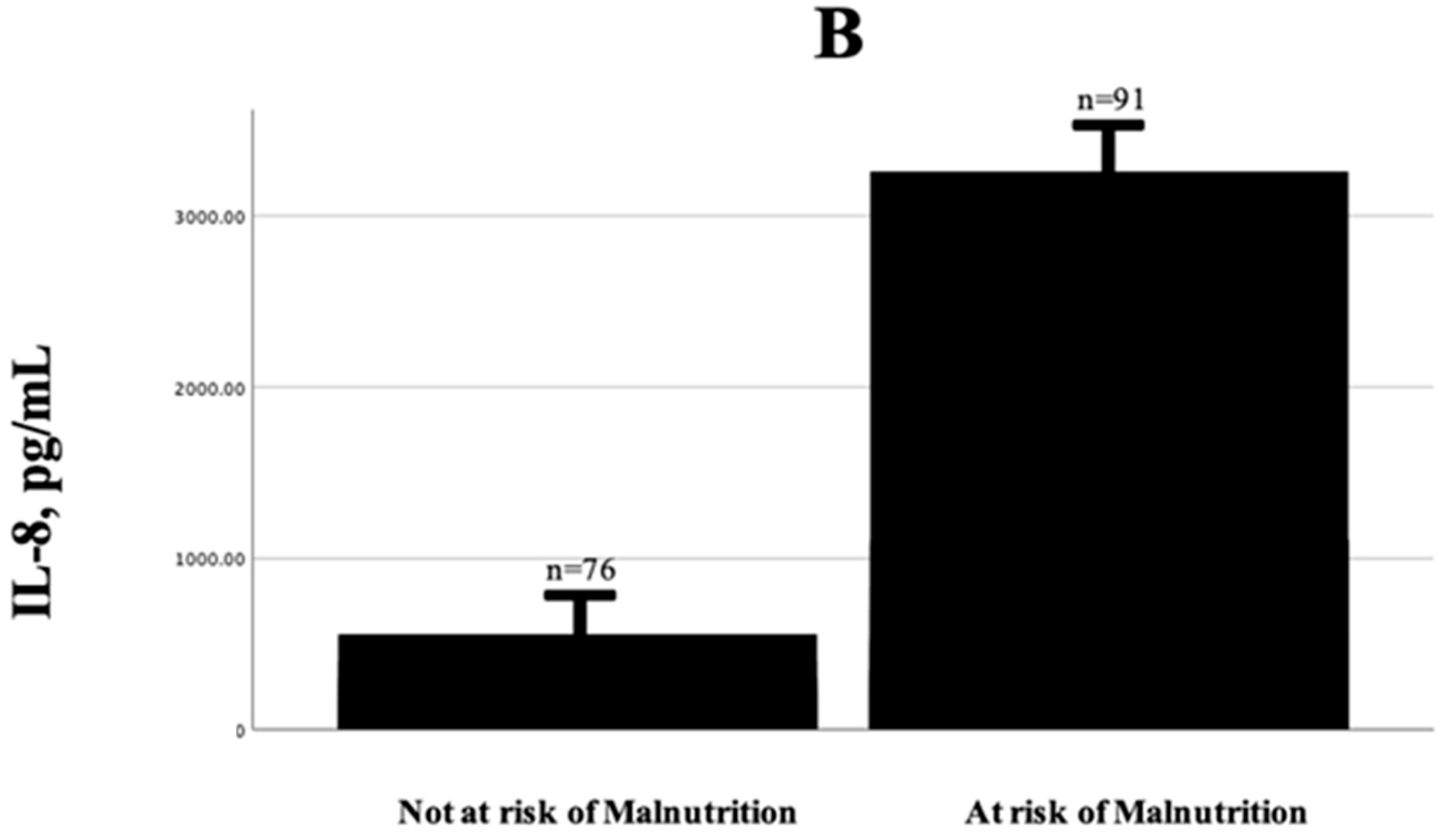
| IL-8, pg/mL | 32.5 [1.75–93.9] | 99.3 [63.04–285.9] | <0.001 |
| IL-10, pg/mL | 186.3 [87.5–547.1] | 266.9 [107.4–575.1] | 0.144 |
| TNF-α, pg/mL | 117.9 [72.1–235.1] | 148.2 [69.2–407.9] | 0.243 |
| IL-6/IL-10 | 0.14 [0.04–0.3] | 0.11 [0.04–0.27] | 0.836 |
| IL-10/TNF-α | 1.5 [0.75–3.6] | 1.9 [0.6–4.05] | 0.928 |
| CRP, mg/L | 86 [46.7–162] | 111.5 [61.37–179] | 0.169 |
| Variable | Survivors (n = 49) | Non-Survivors (n = 33) | p-Value |
|---|---|---|---|
| Age, years | 59.58 ± 14.71 | 67.03 ± 16.77 | 0.032 |
| Female | 20 (41.7%) | 18 (54.5%) | 0.254 |
| Male | 28 (58.3%) | 15 (45.5%) | |
| BMI, kg/m2 | 31.2 ± 6.3 | 30.6 ± 7.84 | 0.641 |
| <25 kg/m2 | 9 (18.4%) | 9 (27.7%) | 0.400 |
| 25–30 kg/m2 | 20 (40.8%) | 9 (27.7%) | |
| >30 kg/m2 | 20 (40.8%) | 15 (45.5%) | |
| LBP, ng/mL | 7 [4.7–9.5] | 8.46 [5.94–9.85] | 0.128 |
| IL-6, pg/mL | 25.3 [23.3–29.9] | 28.3 [23.9–36.2] | 0.035 |
| IL-8, pg/mL | 93.9 [32.5–278.2] | 155.3 [81.3–365.1] | 0.102 |
| IL-10, pg/mL | 248.75 [98.1–577.2] | 239.3 [102.5–556.0] | 0.974 |
| TNF-α, pg/mL | 110.8 [60.4–252.63] | 260.45 [83.3–659.8] | 0.102 |
| IL-6/IL-10 | 0.13 [0.04–0.3] | 0.1 [0.07–0.3] | 0.681 |
| IL-10/TNF-α | 1.9 [0.9–4.7] | 1.2 [0.4–2.5] | 0.061 |
| CRP, mg/L | 144.5 [69.4–188.5] | 98 [61.4–162.5] | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshathri, A.; Bindayel, I.; Alabdullatif, W.; Alhijji, A.; Albarrag, A. Lipopolysaccharide-Binding Protein (LBP) and Inflammatory Biomarkers in SARS-CoV-2 Hospitalized Patients. J. Clin. Med. 2025, 14, 4075. https://doi.org/10.3390/jcm14124075
Alshathri A, Bindayel I, Alabdullatif W, Alhijji A, Albarrag A. Lipopolysaccharide-Binding Protein (LBP) and Inflammatory Biomarkers in SARS-CoV-2 Hospitalized Patients. Journal of Clinical Medicine. 2025; 14(12):4075. https://doi.org/10.3390/jcm14124075
Chicago/Turabian StyleAlshathri, Aldanah, Iman Bindayel, Wajude Alabdullatif, Ali Alhijji, and Ahmed Albarrag. 2025. "Lipopolysaccharide-Binding Protein (LBP) and Inflammatory Biomarkers in SARS-CoV-2 Hospitalized Patients" Journal of Clinical Medicine 14, no. 12: 4075. https://doi.org/10.3390/jcm14124075
APA StyleAlshathri, A., Bindayel, I., Alabdullatif, W., Alhijji, A., & Albarrag, A. (2025). Lipopolysaccharide-Binding Protein (LBP) and Inflammatory Biomarkers in SARS-CoV-2 Hospitalized Patients. Journal of Clinical Medicine, 14(12), 4075. https://doi.org/10.3390/jcm14124075






