Differences in Exercise Performance in Fontan Patients with Extracardiac Conduit and Lateral Tunnel: A FORCE Fontan Registry Study
Abstract
1. Introduction
2. Methods
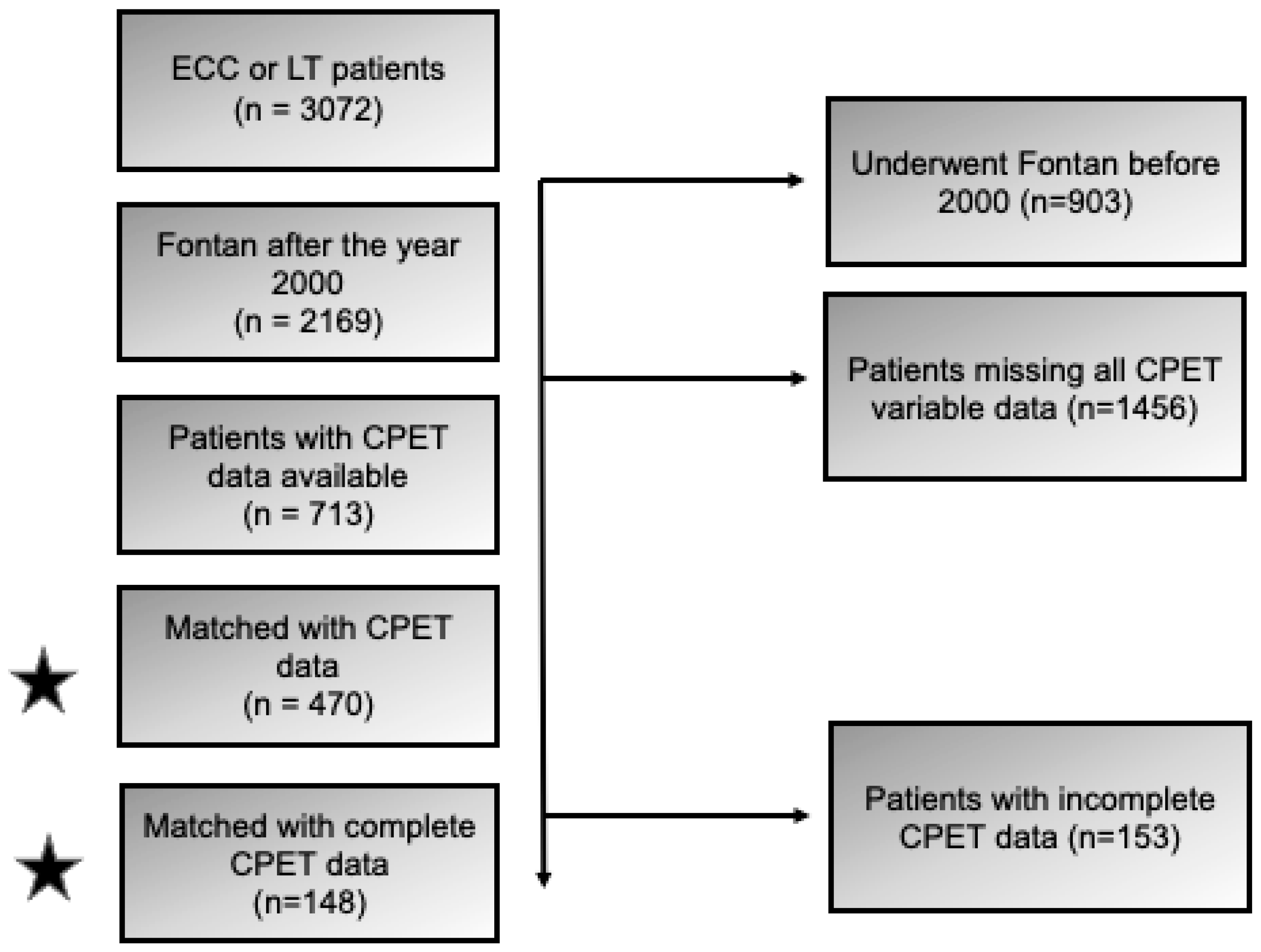
2.1. Study Design, Patient Population, Data Source, and Definitions
2.2. Cardiopulmonary Exercise Stress Testing Details
2.3. Statistical Analysis
3. Results
4. Comment
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fontan, F.; Baudet, E. Surgical repair of tricuspid atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, G.; Galíndez, E.; Bono, H.; De Palma, C.; Laura, J.P. An operation for the correction of tricuspid atresia. J. Thorac. Cardiovasc. Surg. 1973, 66, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Downing, T.E.; Allen, K.Y.; Glatz, A.C.; Rogers, L.S.; Ravishankar, C.; Rychik, J.; Faerber, J.A.; Fuller, S.; Montenegro, L.M.; Steven, J.M.; et al. Long-term survival after the Fontan operation: Twenty years of experience at a single center. J. Thorac. Cardiovasc. Surg. 2017, 154, 243–253.e2. [Google Scholar] [CrossRef]
- Iyengar, A.J.; Winlaw, D.S.; Grigg, L.E.; Celermajer, D.S.; D’udekem, Y. Redefining expectations of long-term survival after the Fontan procedure: Twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 2014, 130 (Suppl. 1), S32–S38. [Google Scholar] [CrossRef][Green Version]
- Mery, C.M.; De León, L.E.; Trujillo-Diaz, D.; Ocampo, E.C.; Dickerson, H.A.; Zhu, H.; Adachi, I.; Heinle, J.S.; Fraser, C.D.; Ermis, P.R. Contemporary Outcomes of the Fontan Operation: A Large Single-Institution Cohort. Ann. Thorac. Surg. 2019, 108, 1439–1446. [Google Scholar] [CrossRef]
- Jain, C.C.; Egbe, A.C.; Allison, T.G.; van de Bruaene, A.; Borlaug, B.A.; Connolly, H.M.; Burchill, L.J.; Miranda, W.R. Functional Capacity Assessment in Adults After Fontan Palliation: A Cardiopulmonary Exercise Test-Invasive Exercise Hemodynamics Correlation Study. Am. J. Cardiol. 2024, 232, 82–88. [Google Scholar] [CrossRef]
- Detterich, J.A. Exercise Performance: A Fontan Phenotype Predictive of Long-Term Survival. JACC Adv. 2024, 3, 101253. [Google Scholar] [CrossRef] [PubMed]
- Udholm, S.; Aldweib, N.; Hjortdal, V.E.; Veldtman, G.R. Prognostic power of cardiopulmonary exercise testing in Fontan patients: A systematic review. Open Heart 2018, 5, e000812. [Google Scholar] [CrossRef]
- Gorla, S.R.; Jhingoeri, N.K.; Chakraborty, A.; Raja, K.R.; Garg, A.; Sandhu, S.; Rosenkranz, E.R.; Swaminathan, S. Incidence and factors influencing the spontaneous closure of Fontan fenestration. Congenit. Heart Dis. 2018, 13, 776–781. [Google Scholar] [CrossRef]
- Poterucha, J.T.; Johnson, J.N.; Taggart, N.W.; Cabalka, A.K.; Hagler, D.J.; Driscoll, D.J.; Cetta, F. Embolization of veno-venous collaterals after the Fontan operation Is associated with decreased survival. Congenit. Heart Dis. 2015, 10, E230–E236. [Google Scholar] [CrossRef]
- La Gerche, A.; Gewillig, M. What Limits Cardiac Performance during Exercise in Normal Subjects and in Healthy Fontan Patients? Int. J. Pediatr. 2010, 2010, 791291. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.T., 2nd; Doshi, P.; Onukwube, J.; Fram, R.Y.; Robbins, J.M. Risk Factors for Increased Hospital Resource Utilization and In-Hospital Mortality in Adults with Single Ventricle Congenital Heart Disease. Am. J. Cardiol. 2016, 118, 453–462. [Google Scholar] [CrossRef]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.-Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult with Fontan Circulation: A Scientific Statement from the American Heart Association. Circulation 2019, 140, e234–e284. [Google Scholar] [CrossRef]
- Mayer, J.E., Jr.; Hill, K.; Jacobs, J.P.; Overman, D.M.; Kumar, S.R. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2020 Update on Outcomes and Research. Ann. Thorac. Surg. 2020, 110, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.A.; Arena, R.; Myers, J.; Peterman, J.E.; Bonikowske, A.R.; Harber, M.P.; Medina Inojosa, J.R.; Lavie, C.J.; Squires, R.W. Updated Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing: Data from the Fitness Registry and the Importance of Exercise National Database (FRIEND). Mayo Clin. Proc. 2022, 97, 285–293. [Google Scholar] [CrossRef]
- Cooper, K.H. A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. JAMA 1968, 203, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Dores, H.; Mendes, M.; Abreu, A.; Durazzo, A.; Rodrigues, C.; Vilela, E.; Cunha, G.; Gomes Pereira, J.; Bento, L.; Moreno, L.; et al. Cardiopulmonary exercise testing in clinical practice: Principles, applications, and basic interpretation. Rev. Port. Cardiol. 2024, 43, 525–536. [Google Scholar] [CrossRef]
- Marshall, K.H.; d’Udekem, Y.; Sholler, G.F.; Opotowsky, A.R.; Costa, D.S.J.; Sharpe, L.; Celermajer, D.S.; Winlaw, D.S.; Newburger, J.W.; Kasparian, N.A. Health-Related Quality of Life in Children, Adolescents, and Adults with a Fontan Circulation: A Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e014172. [Google Scholar] [CrossRef]
- Daley, M.; d’Udekem, Y. The optimal Fontan operation: Lateral tunnel or extracardiac conduit? J. Thorac. Cardiovasc. Surg. 2021, 162, 1825–1834. [Google Scholar] [CrossRef]
- Kumar, S.P.; Rubinstein, C.S.; Simsic, J.M.; Taylor, A.B.; Saul, J.P.; Bradley, S.M. Lateral tunnel versus extracardiac conduit Fontan procedure: A concurrent comparison. Ann. Thorac. Surg. 2003, 76, 1389–1396, discussion 1396–1397. [Google Scholar] [CrossRef]
- Bove, E.L.; de Leval, M.R.; Migliavacca, F.; Balossino, R.; Dubini, G. Toward optimal hemodynamics: Computer modeling of the Fontan circuit. Pediatr. Cardiol. 2007, 28, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Corsini, C.; Baker, C.; Kung, E.; Schievano, S.; Arbia, G.; Baretta, A.; Biglino, G.; Migliavacca, F.; Dubini, G.; Pennati, G.; et al. An integrated approach to patient-specific predictive modeling for single ventricle heart palliation. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 1572–1589. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.-P.; Giardini, A.; Dimopoulos, K.; Gargiulo, G.; Müller, J.; Derrick, G.; Giannakoulas, G.; Khambadkone, S.; Lammers, A.E.; Picchio, F.M.; et al. Predictors of morbidity and mortality in contemporary Fontan patients: Results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur. Heart J. 2010, 31, 3073–3083. [Google Scholar] [CrossRef]
- Giannakoulas, G.; Dimopoulos, K.; Yuksel, S.; Inuzuka, R.; Pijuan-Domenech, A.; Hussain, W.; Tay, E.L.; Gatzoulis, M.A.; Wong, T. Atrial tachyarrhythmias late after Fontan operation are related to increase in mortality and hospitalization. Int. J. Cardiol. 2012, 157, 221–226. [Google Scholar] [CrossRef]
- Saltin, B. Hemodynamic adaptations to exercise. Am. J. Cardiol. 1985, 55, 42D–47D. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.H.; Connor, C.E.; Gooding, L.; Rocchini, A.P. Relation of systemic venous return, pulmonary vascular resistance, and diastolic dysfunction to exercise capacity in patients with single ventricle receiving fontan palliation. Am. J. Cardiol. 2010, 105, 1169–1175. [Google Scholar] [CrossRef]
- Fernandes, S.M.; Alexander, M.E.; Graham, D.A.; Khairy, P.; Clair, M.; Rodriguez, E.; Pearson, D.D.; Landzberg, M.J.; Rhodes, J. Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery. Congenit. Heart Dis. 2011, 6, 294–303. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Nathan, A.S.; Rhodes, J.; Shafer, K.; Landzberg, M.J.; Opotowsky, A.R. Decline in peak oxygen consumption over time predicts death or transplantation in adults with a Fontan circulation. Am. Heart J. 2017, 189, 184–192. [Google Scholar] [CrossRef]
- Ohuchi, H.; Negishi, J.; Noritake, K.; Hayama, Y.; Sakaguchi, H.; Miyazaki, A.; Kagisaki, K.; Yamada, O. Prognostic value of exercise variables in 335 patients after the Fontan operation: A 23-year single-center experience of cardiopulmonary exercise testing. Congenit. Heart Dis. 2015, 10, 105–116. [Google Scholar] [CrossRef]
- Egbe, A.C.; Driscoll, D.J.; Khan, A.R.; Said, S.S.; Akintoye, E.; Berganza, F.M.; Connolly, H.M. Cardiopulmonary exercise test in adults with prior Fontan operation: The prognostic value of serial testing. Int. J. Cardiol. 2017, 235, 6–10. [Google Scholar] [CrossRef]
- Bossers, S.S.; Helbing, W.A.; Duppen, N.; Kuipers, I.M.; Schokking, M.; Hazekamp, M.G.; Bogers, A.J.; Harkel, A.D.T.; Takken, T. Exercise capacity in children after total cavopulmonary connection: Lateral tunnel versus extracardiac conduit technique. J. Thorac. Cardiovasc. Surg. 2014, 148, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Weixler, V.H.M.; Zurakowski, D.; Kheir, J.; Guariento, A.; Kaza, A.; Baird, C.W.; del Nido, P.J.; Emani, S.M. Fontan with lateral tunnel is associated with improved survival compared with extracardiac conduit. J. Thorac. Cardiovasc. Surg. 2020, 159, 1480–1491.e2. [Google Scholar] [CrossRef]
- Alsaied, T.; Li, R.; Christopher, A.B.; Fogel, M.A.; Slesnick, T.C.; Krishnamurthy, R.; Muthurangu, V.; Dorfman, A.L.; Lam, C.Z.; Weigand, J.D.; et al. High-Performing Fontan Patients: A Fontan Outcome Registry by Cardiac Magnetic Resonance Imaging Study. JACC Adv. 2024, 3, 101254. [Google Scholar] [CrossRef] [PubMed]
- Daley, M.; d’Udekem, Y. In patients undergoing Fontan completion, does a younger age at operation result in better long-term exercise capacity and prognosis? Interact. Cardiovasc. Thorac. Surg. 2019, 28, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.P.; Dimopoulos, K.; Okonko, D.; Li, W.; Babu-Narayan, S.V.; Broberg, C.S.; Johansson, B.; Bouzas, B.; Mullen, M.J.; Poole-Wilson, P.A.; et al. Exercise intolerance in adult congenital heart disease: Comparative severity, correlates, and prognostic implication. Circulation 2005, 112, 828–835. [Google Scholar] [CrossRef]
- Ko, H.; Song, J.; Chi, S.A.; Lee, S.-Y.; Kim, S.-J.; Lee, C.-H.; Park, C.S.; Choi, E.S.; An, H.S.; Kang, I.S.; et al. The long-term effects of the fenestration in patients with extracardiac Fontan circulation-a multicenter Korean cohort study based on national Fontan registry. Front. Cardiovasc. Med. 2024, 11, 1341882. [Google Scholar] [CrossRef]
- Greenleaf, C.E.; Lim, Z.N.; Li, W.; LaPar, D.J.; Salazar, J.D.; Corno, A.F. Impact on clinical outcomes from transcatheter closure of the Fontan fenestration: A systematic review and meta-analysis. Front. Pediatr. 2022, 10, 915045. [Google Scholar] [CrossRef]
| Equation | Reference | Population | Output |
|---|---|---|---|
| Wasserman | Wasserman, K.; et al. Principles of Exercise Testing and Interpretation, 5th ed., 2011. | Clinical populations (e.g., cardiopulmonary disease) | Predicted peak VO2 based on age, sex, weight, height |
| Cooper | Cooper, K.H. A means of assessing maximal oxygen intake. JAMA 1968, 203, 201–204. | General adult population (field-based estimation) | Predicted VO2 max from treadmill run time (Cooper test) |
| FRIEND | Kaminsky, L.A.; et al. Updated Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing: Data from the FRIEND Registry. Mayo Clin. Proc. 2022, 97, 285–293. | Healthy adults (normative CPET values) | Normative peak VO2 values using CPET in healthy adult populations |
| Overall (n = 713) | Lateral Tunnel (n = 241) | Extra Cardiac Conduit (n = 472) | p-Value | |
|---|---|---|---|---|
| Age at Fontan surgery, years | 3.2 (2.4, 4.1) | 2.4 (2.1, 3.1) | 3.5 (2.8, 4.5) | <0.0001 |
| Female | 292 (41.0) | 86 (35.7) | 206 (43.6) | 0.0409 |
| Suspected heterotaxy | 83 (11.7) | 20 (8.3) | 63 (13.4) | 0.0448 |
| Dominant ventricular morphology | 0.0611 | |||
| Balanced or mixed | 110 (15.7) | 34 (14.1) | 76 (16.5) | |
| Left | 271 (38.6) | 82 (34.0) | 189 (41.0) | |
| Right | 321 (45.7) | 125 (51.9) | 196 (42.5) | |
| Cardiac diagnosis | <0.0001 | |||
| Atrioventricular canal defect | 51 (7.2) | 8 (3.3) | 43 (9.1) | |
| Double inlet left or right ventricle | 81 (11.4) | 29 (12.0) | 52 (11.0) | |
| Double outlet right ventricle | 77 (10.8) | 22 (9.1) | 55 (11.7) | |
| Ebsteins | 9 (1.3) | 4 (1.7) | 5 (1.1) | |
| Hypoplastic left heart syndrome or small left-sided structures | 239 (33.5) | 107 (44.4) | 132 (28.0) | |
| Mitral atresia (including mitral atresia with DORV) | 18 (2.5) | 6 (2.5) | 12 (2.5) | |
| Pulmonary atresia with intact ventricular septum | 50 (7.0) | 9 (3.7) | 41 (8.7) | |
| Tricuspid atresia | 116 (16.3) | 41 (17.0) | 75 (15.9) | |
| Other hypoplastic right ventricle or small right-sided structures | 16 (2.2) | 6 (2.5) | 10 (2.1) | |
| Other | 56 (7.9) | 9 (3.7) | 47 (10.0) | |
| Year of Fontan surgery | 2007 (2004, 2010) | 2006 (2003, 2009) | 2008 (2005, 2011) | <0.0001 |
| Overall | LT | ECC | p-Value | |
|---|---|---|---|---|
| Age at time of exercise stress test visit (years) | 15.3 (12.7, 18.0) n = 713 | 16.2 (13.6, 19.0) n = 241 | 14.8 (12.3, 17.6) n = 472 | 0.0001 |
| Peak heart rate (beats/minute) | 172 (160, 181) n = 683 | 169 (155, 179) n = 237 | 173 (160, 184) n = 446 | 0.0003 |
| Percent of predicted peak heart rate (beats/minute) | 86 (80, 90) n = 647 | 85.5 (78, 90) n = 228 | 86 (80, 90) n = 419 | 0.1984 |
| Baseline/Pre-exercise heart rate (beats/minute) | 86 (75, 97) n = 669 | 86 (76, 96) n = 237 | 86 (75, 98) n = 432 | 0.8981 |
| Heart rate at VAT (beats/minute) | 125.0 (110.0, 143.5) n = 408 | 119.0 (103.0, 133.0) n = 163 | 132.0 (113.0, 148.0) n = 245 | <0.0001 |
| Peak O2 pulse (%) | 8.3 (6.7, 11.0) n = 455 | 8.9 (6.6, 11.1) n = 175 | 8.2 (6.8, 10.6) n = 280 | 0.3319 |
| Peak RER | 1.16 (1.10, 1.23) n = 553 | 1.15 (1.11, 1.23) n = 204 | 1.16 (1.10, 1.23) n = 349 | 0.6836 |
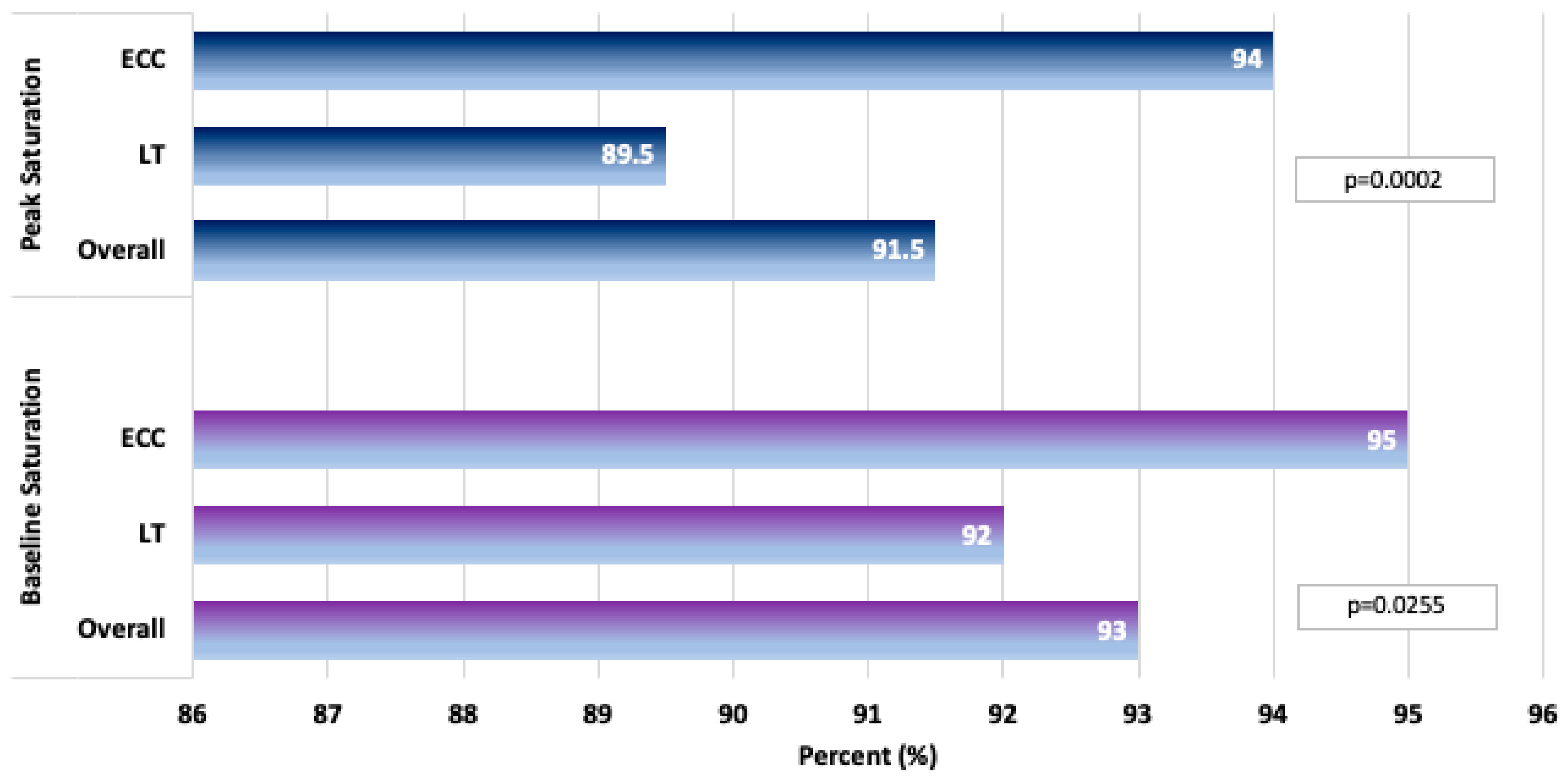
| Peak saturation (%) | 91 (88, 95) n = 564 | 90 (87, 94) n = 201 | 92 (88, 95) n = 363 | 0.0011 |
| Baseline/Pre-exercise saturation (%) | 94 (91, 96) n = 552 | 92 (90, 95) n = 201 | 94 (91, 97) n = 351 | <0.0001 |
| VE/VCO2 slope | 35.0 (31.0, 39.8) n = 503 | 34.7 (31.0, 40.0) n = 179 | 35.0 (31.0, 39.5) n = 324 | 0.5758 |
| Peak VO2 (mL/kg/min) | 27.7 (22.2, 33.0) n = 638 | 27.0 (22.0, 31.1) n = 223 | 28.2 (22.4, 34.2) n = 415 | 0.0237 |
| Percent of predicted peak VO2 (%) | 64 (53, 76) n = 570 | 62 (53, 72) n = 199 | 65 (53, 77) n = 371 | 0.0992 |
| VO2 at VAT (mL/kg/min) | 17.6 (14.1, 21.9) n = 475 | 16.3 (13.4, 19.3) n = 177 | 19.0 (14.5, 22.6) n = 298 | <0.0001 |
| Peak work rate (Watts) | 106 (85, 141) n = 387 | 112 (84, 145) n = 146 | 104 (85, 137) n = 241 | 0.4825 |
| Percent of predicted work rate (%) | 70.0 (59.0, 81.0) n = 305 | 68.0 (55.0, 77.0) n = 129 | 72.0 (60.5, 82.5) n = 176 | 0.0187 |
| Age at CPET Assessment (Years) | LT | ECC | SMD |
|---|---|---|---|
| Peak heart rate (beats/minute) | 16.2 (13.6, 19.0) n = 235 | 16.3 (13.6, 18.7) n = 235 | 0.0274 |
| Percent of predicted peak heart rate sample (%) | 16.1 (13.5, 18.9) n = 226 | 16.1 (13.5, 18.5) n = 226 | 0.0301 |
| Baseline/Pre-exercise heart rate (beats/minute) | 16.1 (13.5, 18.9) n = 234 | 16.2 (13.5, 18.5) n = 234 | 0.0297 |
| Heart rate at VAT (beats/minute) | 16.3 (13.6, 18.9) n = 157 | 16.3 (13.6, 18.3) n = 157 | 0.0724 |
| Peak O2 pulse (mL O2/beat) | 16.4 (13.8, 19.3) n = 170 | 16.4 (13.8, 18.5) n = 170 | 0.0665 |
| Peak RER sample | 16.4 (13.9, 19.3) n = 202 | 16.4 (13.9, 19.1) n = 202 | 0.0274 |
| Peak saturation (%) | 15.8 (13.3, 18.8) n = 199 | 15.8 (13.3, 18.8) n = 199 | 0.0177 |
| Baseline/Pre-exercise saturation (%) | 16.1 (13.4, 18.8) n = 198 | 15.9 (13.4, 18.8) n = 198 | 0.0199 |
| VE/VCO2 slope | 16.4 (14.0, 19.4) n = 177 | 16.4 (14.0, 18.8) n = 177 | 0.0461 |
| Peak VO2 (mL/kg/min) | 16.3 (13.8, 18.9) n = 221 | 16.3 (13.8, 18.8) n = 221 | 0.0199 |
| Percent of predicted peak VO2 (%) | 16.3 (13.8, 19.3) n = 197 | 16.3 (13.8, 19.3) n = 197 | 0.0155 |
| VO2 at VAT (mL/kg/min) | 16.4 (13.8, 18.9) n = 168 | 16.4 (13.8, 18.1) n = 168 | 0.0697 |
| Peak work rate (Watt) | 15.6 (12.5, 18.0) n = 138 | 15.6 (12.5, 17.9) n = 138 | 0.0454 |
| Percent of predicted work rate (%) | 15.3 (12.5, 17.8) n = 111 | 15.4 (12.5, 17.3) n = 111 | 0.0619 |
| Overall | LT | ECC | p-Value | |
|---|---|---|---|---|
| Peak heart rate (beats/minute) | 171 (160, 181) n = 470 | 169 (153, 179) n = 235 | 174 (162, 184) n = 235 | 0.0008 |
| Percent of predicted peak heart rate sample (%) | 86 (80, 90) n = 452 | 85 (78, 90) n = 226 | 86 (81, 91) n = 226 | 0.0354 |
| Baseline/pre-exercise heart rate (beats/minute) | 86 (75, 97) n = 468 | 86 (76, 96) n = 234 | 86 (75, 97) n = 234 | 0.9036 |
| Heart rate at VAT (beats/minute) | 124 (107, 140) n = 314 | 119 (104, 134) n = 157 | 130 (111, 145) n = 157 | 0.0005 |
| Peak O2 pulse (mL O2/beat) | 9.0 (6.8, 11.0) n = 340 | 8.7 (6.5, 11.0) n = 170 | 9.0 (7.0, 11.0) n = 170 | 0.6015 |
| Peak RER sample | 1.16 (1.10, 1.23) n = 404 | 1.15 (1.11, 1.22) n = 202 | 1.17 (1.10, 1.23) n = 202 | 0.8397 |
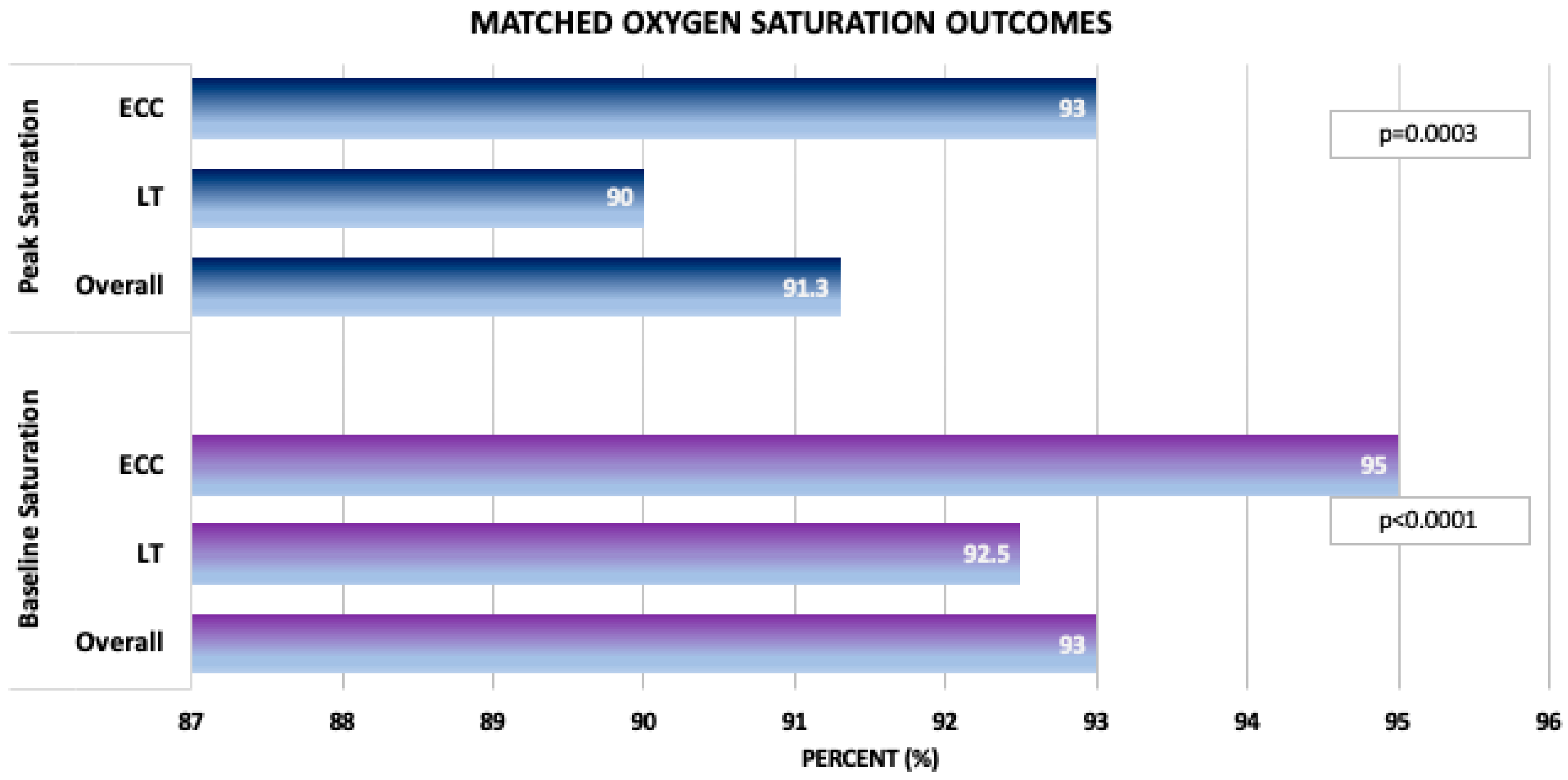
| Peak saturation (%) | 91.3 (88.0, 95.0) n = 398 | 90.0 (87.0, 94.0) n = 199 | 93.0 (89.0, 95.0) n = 199 | 0.0003 |
| Baseline/pre-exercise saturation (%) | 93.0 (91.0, 96.0) n = 396 | 92.5 (90.0, 95.0) n = 198 | 95.0 (91.0, 97.0) n = 198 | <0.0001 |
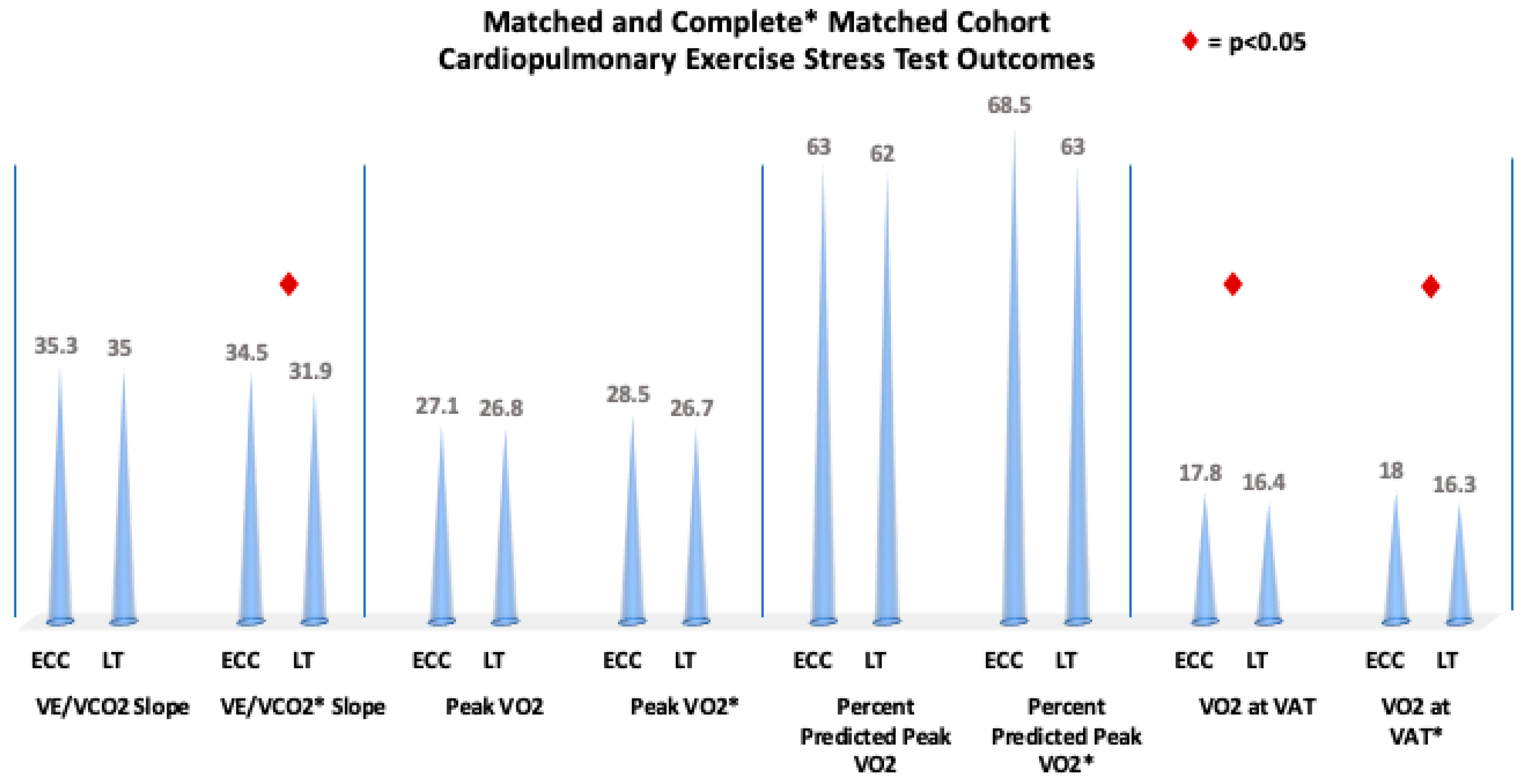
| VE/VCO2 slope | 35.0 (31.0, 40.0) n = 354 | 35.0 (31.0, 40.0) n = 177 | 35.3 (31.0, 39.8) n = 177 | 0.8438 |
| Peak VO2 (mL/kg/min) | 27.0 (21.8, 32.1) n = 442 | 26.8 (22.0, 30.8) n = 221 | 27.1 (21.7, 32.8) n = 221 | 0.5823 |
| Percent of predicted peak VO2 (%) | 62.5 (52.0, 73.0) n = 394 | 62.0 (53.0, 72.0) n = 197 | 63.0 (52.0, 74.0) n = 197 | 0.9619 |
| VO2 at VAT (mL/kg/min) | 16.8 (13.4, 20.6) n = 336 | 16.4 (13.4, 19.5) n = 168 | 17.8 (13.4, 22.0) n = 168 | 0.0123 |
| Peak work rate (Watt) | 112.0 (85.0, 144.0) n = 276 | 112.0 (83.0, 143.0) n = 138 | 112.5 (87.0, 144.0) n = 138 | 0.5080 |
| Percent of predicted work rate (%) | 69.5 (59.0, 82.0) n = 222 | 68.0 (56.0, 79.0) n = 111 | 71.0 (60.0, 83.0) n = 111 | 0.1325 |
| LT n = 235 | ECC n = 235 | p-Value | |
|---|---|---|---|
| Body mass index | 20.4 (18.5, 23.6) | 20.5 (17.8, 24.0) | 0.3730 |
| Ascending aorta, above the Stansel flow rate, indexed to BSA | 2.9 (2.5, 3.4) | 3.0 (2.5, 3.5) | 0.1678 |
| Fontan flow indexed on BSA | 1.6 (1.2, 2.0) | 1.5 (1.2, 1.9) | 0.0521 |
| Qp/Qs | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 0.5423 |
| Aortic or native aortic regurgitation fraction | 4.0 (2.0, 6.0) | 4.0 (2.0, 8.0) | 0.9568 |
| Mitral or common atrioventricular valve regurgitation fraction | 10.0 (6.0, 15.0) | 15.0 (5.0, 23.0) | 0.0983 |
| Pulmonary or neo-aortic regurgitation fraction | 5.0 (3.0, 6.0) | 5.0 (2.0, 11.0) | 0.4670 |
| Tricuspid regurgitation fraction | 17.0 (12.0, 25.0) | 14.0 (7.0, 27.0) | 0.1942 |
| Single ventricle end diastolic volume indexed on BSA | 105.6 (85.9, 128.8) | 96.5 (78.7, 121.0) | 0.0017 |
| Single ventricle ejection fraction | 51.2 (44.9, 55.9) | 51.7 (45.4, 57.5) | 0.1739 |
| Single ventricle end systolic volume indexed on BSA | 51.8 (39.0, 66.5) | 46.8 (34.9, 62.7) | 0.0065 |
| Single ventricle mass indexed on BSA | 52.6 (41.2, 66.2) | 50.6 (38.6, 67.2) | 0.2554 |
| Visible aortopulmonary collaterals | 47 (25.7) | 79 (20.4) | 0.1569 |
| Visible veno-venous collaterals | 27 (15.3) | 57 (14.0) | 0.7009 |
| Overall (n = 148) | LT (n = 74) | ECC (n = 74) | p-Value | |
|---|---|---|---|---|
| Peak heart rate (beats/minute) | 166 (157, 179) | 166 (153, 178) | 171 (160, 181) | 0.1221 |
| Percent of predicted peak heart rate sample (%) | 86.0 (79.5, 91.0) | 85.5 (76.0, 90.0) | 87.0 (81.0, 92.0) | 0.1939 |
| Baseline/pre-exercise heart rate (beats/minute) | 87 (78, 97) | 89 (79, 97) | 86.5 (78, 98) | 0.7386 |
| Heart rate at VAT (beats/minute) | 118.5 (105.0, 136.0) | 116.0 (103.0, 131.0) | 125.5 (111.0, 140.0) | 0.0291 |
| Peak O2 pulse (mL O2/beat) | 8.2 (7.0, 11.0) | 8.0 (6.6, 10.1) | 9.0 (7.0, 11.0) | 0.0769 |
| Peak RER sample | 1.19 (1.12, 1.27) | 1.14 (1.11, 1.22) | 1.23 (1.15, 1.30) | 0.0032 |
| Peak saturation (%) | 91.5 (88.0, 94.5) | 89.5 (86.0, 93.0) | 94.0 (90.0, 96.0) | 0.0002 |
| Baseline/pre-exercise saturation (%) | 93 (90, 97) | 92 (90, 95) | 95 (91, 97) | 0.0255 |
| VE/VCO2 slope | 33.0 (29.2, 37.4) | 34.5 (31.0, 40.0) | 31.9 (28.8, 35.3) | 0.0071 |
| Peak VO2 (mL/kg/min) | 27.4 (22.5, 32.7) | 26.7 (22.1, 31.3) | 28.5 (23.8, 34.5) | 0.1208 |
| Percent of predicted peak VO2 (%) | 65.0 (57.0, 77.0) | 63.0 (55.0, 75.0) | 68.5 (59.0, 79.0) | 0.1300 |
| VO2 at VAT (mL/kg/min) | 16.7 (13.8, 20.7) | 16.3 (13.4, 18.7) | 18.0 (14.7, 22.4) | 0.0044 |
| Peak work rate (Watt) | 113.5 (86.5, 143.5) | 112.0 (83.0, 139.0) | 118.5 (88.0, 149.0) | 0.0443 |
| Percent of predicted work rate (%) | 70.0 (59.5, 83.0) | 66.5 (56.0, 77.0) | 72.5 (63.0, 87.0) | 0.0141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seese, L.; Schiff, M.; Olivieri, L.; Da Fonseca Da Silva, L.; Da Silva, J.P.; Christopher, A.; Harris, T.H.; Morell, V.; Castro Medina, M.; Rathod, R.H.; et al. Differences in Exercise Performance in Fontan Patients with Extracardiac Conduit and Lateral Tunnel: A FORCE Fontan Registry Study. J. Clin. Med. 2025, 14, 4067. https://doi.org/10.3390/jcm14124067
Seese L, Schiff M, Olivieri L, Da Fonseca Da Silva L, Da Silva JP, Christopher A, Harris TH, Morell V, Castro Medina M, Rathod RH, et al. Differences in Exercise Performance in Fontan Patients with Extracardiac Conduit and Lateral Tunnel: A FORCE Fontan Registry Study. Journal of Clinical Medicine. 2025; 14(12):4067. https://doi.org/10.3390/jcm14124067
Chicago/Turabian StyleSeese, Laura, Mary Schiff, Laura Olivieri, Luciana Da Fonseca Da Silva, Jose P. Da Silva, Adam Christopher, Tyler H. Harris, Victor Morell, Mario Castro Medina, Rahul H. Rathod, and et al. 2025. "Differences in Exercise Performance in Fontan Patients with Extracardiac Conduit and Lateral Tunnel: A FORCE Fontan Registry Study" Journal of Clinical Medicine 14, no. 12: 4067. https://doi.org/10.3390/jcm14124067
APA StyleSeese, L., Schiff, M., Olivieri, L., Da Fonseca Da Silva, L., Da Silva, J. P., Christopher, A., Harris, T. H., Morell, V., Castro Medina, M., Rathod, R. H., Kreutzer, J., Diaz Castrillon, C., Viegas, M., Alsaied, T., & the FORCE Investigators. (2025). Differences in Exercise Performance in Fontan Patients with Extracardiac Conduit and Lateral Tunnel: A FORCE Fontan Registry Study. Journal of Clinical Medicine, 14(12), 4067. https://doi.org/10.3390/jcm14124067





