A Brief Overview of Uveal Melanoma Treatment Methods with a Focus on the Latest Advances
Abstract
1. Introduction
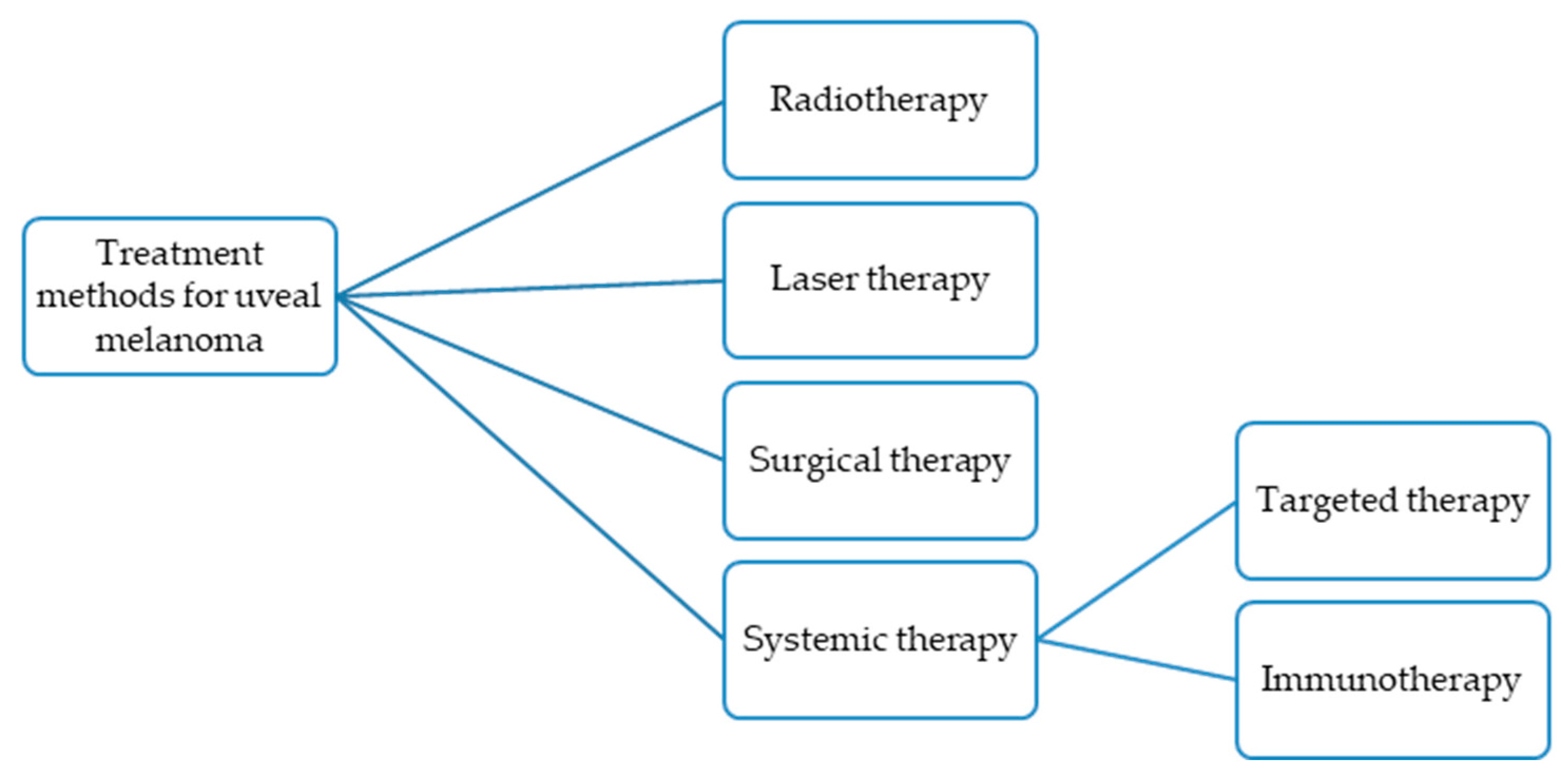
2. Established Treatment Methods for Uveal Melanoma
3. Tebentafusp
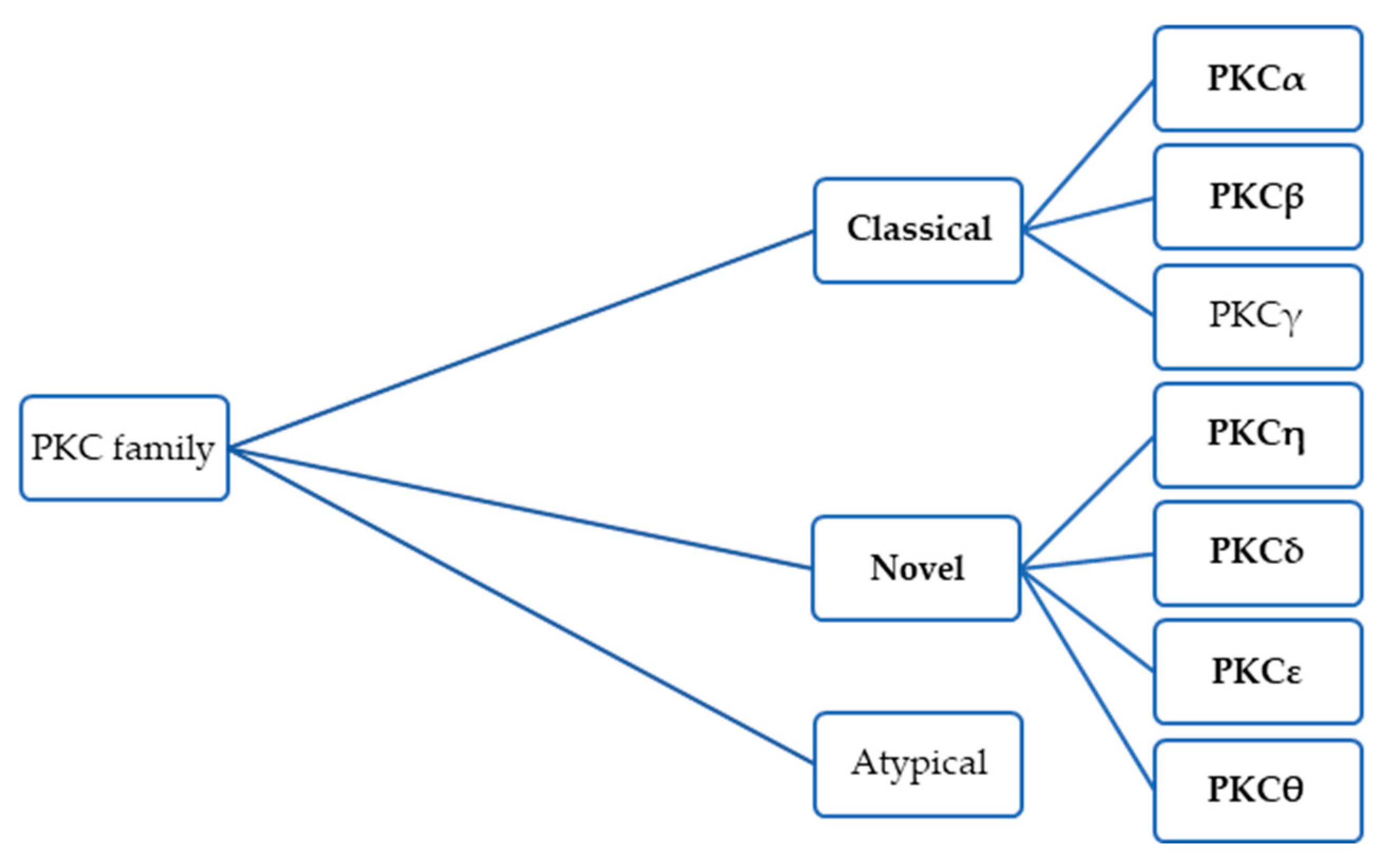
4. Darovasertib
5. Melphalan
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brănișteanu, D.E.; Porumb-Andrese, E.; Stărică, A.; Munteanu, A.C.; Toader, M.P.; Zemba, M.; Porumb, V.; Cozmin, M.; Moraru, A.D.; Nicolescu, A.C.; et al. Differences and Similarities in Epidemiology and Risk Factors for Cutaneous and Uveal Melanoma. Medicina 2023, 59, 943. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Rokohl, A.C.; Li, X.; Guo, Y.; Ju, X.; Fan, W.; Heindl, L.M. Global incidence and prevalence in uveal melanoma. Adv. Ophthalmol. Pract. Res. 2024, 4, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yavuzyigitoglu, S.; Brosens, E.; Ramdas, W.D.; Kiliç, E.; Rotterdam Ocular Melanoma Study Group (ROMS). Worldwide Incidence of Ocular Melanoma and Correlation with Pigmentation-Related Risk Factors. Investig. Ophthalmol. Vis. Sci. 2023, 64, 45. [Google Scholar] [CrossRef]
- Weinberger, Y.; Bena, J.; Singh, A.D. Uveal Melanoma: 5 Year Update on Incidence, Treatment, and Survival (SEER 1975–2020). Ocul. Oncol. Pathol. 2024, 11, 30–36. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 8 Regs Research Data, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, Released November 2020, Based on the 2020 Census. Available online: https://www.seer.cancer.gov (accessed on 12 March 2025).
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Primers 2020, 6, 24, Erratum in Nat. Rev. Dis. Primers 2022, 8, 4. https://doi.org/10.1038/s41572-022-00339-9. [Google Scholar] [CrossRef]
- Singh, L.; Chinnaswamy, G.; Meel, R.; Radhakrishnan, V.; Madan, R.; Kulkarni, S.; Sasi, A.; Kaur, T.; Dhaliwal, R.S.; Bakhshi, S. Epidemiology, Diagnosis and Genetics of Retinoblastoma: ICMR Consensus Guidelines. Indian J. Pediatr. 2024, 91, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, Y.; Kim, I.K. Epidemiology and management of uveal melanoma. Hematol. Oncol. Clin. N. Am. 2012, 26, 1169–1184. [Google Scholar] [CrossRef]
- Rishi, P.; Koundanya, V.V.; Shields, C.L. Using risk factors for detection and prognostication of uveal melanoma. Indian J. Ophthalmol. 2015, 63, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ramos, G.M.; Thomas, F.; VanderWalde, A.; King, B.; Wilson, M.; Pallera, A.M. Risk factors, clinical outcomes, and natural history of uveal melanoma: A single-institution analysis. Med. Oncol. 2019, 36, 17. [Google Scholar] [CrossRef]
- Yu, G.-P.; Hu, D.-N.; McCormick, S.; Finger, P.T. Conjunctival melanoma: Is it increasing in the United States? Am. J. Ophthalmol. 2003, 135, 800–806. [Google Scholar] [CrossRef]
- Hu, D.; Simon, J.D.; Sarna, T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem. Photobiol. 2008, 84, 639–644. [Google Scholar] [CrossRef]
- Salzano, S.; Vecchio, G.; Failla, M.; Russo, A.; Avitabile, T.; Longo, A.; Caltabiano, R.; Broggi, G. Metastases from uveal melanoma may lack S100 expression: A clinico-pathologic and immunohistochemical study with emphasis on potential causes and diagnostic implications. Ann. Diagn. Pathol. 2025, 76, 152464. [Google Scholar] [CrossRef]
- Mobuchon, L.; Derrien, A.C.; Houy, A.; Verrier, T.; Pierron, G.; Cassoux, N.; Milder, M.; Deleuze, J.F.; Boland, A.; Scelo, G.; et al. Different Pigmentation Risk Loci for High-Risk Monosomy 3 and Low-Risk Disomy 3 Uveal Melanomas. J. Natl. Cancer Inst. 2022, 114, 302–309. [Google Scholar] [CrossRef]
- Ferguson, R.; Vogelsang, M.; Ucisik-Akkaya, E.; Rai, K.; Pilarski, R.; Martinez, C.N.; Rendleman, J.; Kazlow, E.; Nagdimov, K.; Osman, I.; et al. Genetic markers of pigmentation are novel risk loci for uveal melanoma. Sci. Rep. 2016, 6, 31191. [Google Scholar] [CrossRef]
- Refaian, N.; Schlereth, S.L.; Koch, K.R.; Notara, M.; Hos, D.; Mescher, M.; Iden, S.; Bosch, J.J.; Jager, M.J.; Cursiefen, C.; et al. Comparing the Hem- and Lymphangiogenic Profile of Conjunctival and Uveal Melanoma Cell Lines. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5691–5697. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.M.; John, L.; Refaian, N.; Buettner, C.; Rottmar, T.; Sommer, J.; Bock, B.; Resheq, Y.J.; Ksander, B.R.; Heindl, L.M.; et al. Midkine Promotes Metastasis and Therapeutic Resistance via mTOR/RPS6 in Uveal Melanoma. Mol. Cancer Res. 2022, 20, 1320–1336. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Bosch, J.J.; Heindl, L.M. Current management of uveal melanoma: A review. Clin. Exp. Ophthalmol. 2023, 51, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Gelmi, M.C.; Jager, M.J. Uveal melanoma: Current evidence on prognosis, treatment and potential developments. Asia Pac. J. Ophthalmol. 2024, 13, 100060. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, D.A.; Brock, A.L. Radiation therapy for uveal melanoma: A review of treatment methods available in 2021. Curr. Opin. Ophthalmol. 2021, 32, 183–190. [Google Scholar] [CrossRef]
- Bolling, J.P.; Dagan, R.; Rutenberg, M.; Mamalui-Hunter, M.; Buskirk, S.J.; Heckman, M.G.; Hochwald, A.P.; Slopsema, R. Treatment of Uveal Melanoma With Radioactive Iodine 125 Implant Compared with Proton Beam Radiotherapy. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 6, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Arabi, A.; Siavashpour, Z.; Shahraki, T.; Ansari, I. Efficacy and complications of ruthenium-106 brachytherapy for uveal melanoma: A systematic review and meta-analysis. J. Contemp. Brachytherapy 2021, 13, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Finger, P.T. Regression patterns of choroidal melanoma: After palladium-103 (103Pd) plaque brachytherapy. Eur. J. Ophthalmol. 2018, 28, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Bellocq, D.; Roy, P.; Kodjikian, L.; Mathis, T.; Nguyen, A.M.; Herault, J.; Rivoire, M.; Négrier, S.; Thariat, J.; Grange, J.D. 20-year assessment of metastatic latency and subsequent time to death after proton therapy for uveal melanomas. Melanoma Res. 2020, 30, 272–278. [Google Scholar] [CrossRef]
- Wang, Z.; Nabhan, M.; Schild, S.E.; Stafford, S.L.; Petersen, I.A.; Foote, R.L.; Murad, M.H. Charged particle radiation therapy for uveal melanoma: A systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Semeniuk, O.; Yu, E.; Rivard, M.J. Current and Emerging Radiotherapy Options for Uveal Melanoma. Cancers 2024, 16, 1074. [Google Scholar] [CrossRef]
- Maheshwari, A.; Finger, P.T. Laser treatment for choroidal melanoma: Current concepts. Surv. Ophthalmol. 2023, 68, 211–224. [Google Scholar] [CrossRef]
- Blasi, M.A.; Laguardia, M.; Tagliaferri, L.; Scupola, A.; Villano, A.; Caputo, C.G.; Pagliara, M.M. BRACHYTHERAPY ALONE OR WITH NEOADJUVANT PHOTODYNAMIC THERAPY FOR AMELANOTIC CHOROIDAL MELANOMA: Functional Outcomes and Local Tumor Control. Retina 2016, 36, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, A.; Shields, C.L.; Rishi, P.; Atalay, H.T.; Pellegrini, M.; McLaughlin, J.P.; Patrick, K.A.; Morton, S.J.; Remmer, M.H.; Parendo, A.; et al. Primary transpupillary thermotherapy for choroidal melanoma in 391 cases: Importance of risk factors in tumor control. Ophthalmology 2015, 122, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Ahmed, I.I.K.; DiGiovanni, J.; Shields, C.L. Radiotherapeutic and surgical management of iris melanoma: A review. Surv. Ophthalmol. 2017, 62, 302–311. [Google Scholar] [CrossRef]
- Rusňák, Š.; Hecová, L.; Kasl, Z.; Sobotová, M.; Hauer, L. Uveal Melanoma Biopsy. A Review. Ceska Slov. Oftalmol. 2020, 76, 247–252. (In English) [Google Scholar] [CrossRef]
- Joussen, A.M.; Wong, D. Egress of large quantities of heavy liquids from exposed choroid: A route for possible tumor dissemination via vortex veins in endoresection of choroidal melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Hamza, H.S.; Elhusseiny, A.M. Choroidal Melanoma Resection. Middle E. Afr. J. Ophthalmol. 2018, 25, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Penel, N.; Delcambre, C.; Durando, X.; Clisant, S.; Hebbar, M.; Negrier, S.; Fournier, C.; Isambert, N.; Mascarelli, F.; Mouriaux, F. O-Mel-Inib: A Cancéro-pôle Nord-Ouest multicenter phase II trial of high-dose imatinib mesylate in metastatic uveal melanoma. Investig. New Drugs 2008, 26, 561–565. [Google Scholar] [CrossRef]
- Mahipal, A.; Tijani, L.; Chan, K.; Laudadio, M.; Mastrangelo, M.J.; Sato, T. A pilot study of sunitinib malate in patients with metastatic uveal melanoma. Melanoma Res. 2012, 22, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, M.E.; Orloff, M.; Sato, R.; Chervoneva, I.; Shields, C.L.; Shields, J.A.; Mastrangelo, M.J.; Sato, T. Adjuvant Sunitinib in High-Risk Patients with Uveal Melanoma: Comparison with Institutional Controls. Ophthalmology 2018, 125, 210–217. [Google Scholar] [CrossRef]
- Khan, S.; Lutzky, J.; Shoushtari, A.N.; Jeter, J.; Marr, B.; Olencki, T.E.; Cebulla, C.M.; Abdel-Rahman, M.; Harbour, J.W.; Sender, N.; et al. Adjuvant crizotinib in high-risk uveal melanoma following definitive therapy. Front. Oncol. 2022, 12, 976837. [Google Scholar] [CrossRef]
- Francis, J.H.; Kim, J.; Lin, A.; Folberg, R.; Iyer, S.; Abramson, D.H. Growth of Uveal Melanoma following Intravitreal Bevacizumab. Ocul. Oncol. Pathol. 2017, 3, 117–121. [Google Scholar] [CrossRef]
- Mahdjoubi, A.; Najean, M.; Lemaitre, S.; Dureau, S.; Dendale, R.; Levy, C.; Rouic, L.L.; Desjardins, L.; Cassoux, N. Intravitreal bevacizumab for neovascular glaucoma in uveal melanoma treated by proton beam therapy. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 411–420. [Google Scholar] [CrossRef]
- Hussain, R.N.; Heimann, H.; Damato, B. Neoadjuvant intravitreal ranibizumab treatment in high-risk ocular melanoma patients: A two-stage single-centre phase II single-arm study. Melanoma Res. 2020, 30, 102–106. [Google Scholar] [CrossRef]
- Mouriaux, F.; Servois, V.; Parienti, J.J.; Lesimple, T.; Thyss, A.; Dutriaux, C.; Neidhart-Berard, E.M.; Penel, N.; Delcambre, C.; Peyro Saint Paul, L.; et al. Sorafenib in metastatic uveal melanoma: Efficacy, toxicity and health-related quality of life in a multicentre phase II study. Br. J. Cancer 2016, 115, 20–24. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Schwartz, G.K.; Mann, H.; Smith, I.; Nathan, P.D. Study design and rationale for a randomised, placebo-controlled, double-blind study to assess the efficacy of selumetinib (AZD6244; ARRY-142886) in combination with dacarbazine in patients with metastatic uveal melanoma (SUMIT). BMC Cancer 2015, 15, 467. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Piperno-Neumann, S.; Kapiteijn, E.; Chapman, P.B.; Frank, S.; Joshua, A.M.; Piulats, J.M.; Wolter, P.; Cocquyt, V.; Chmielowski, B.; et al. Selumetinib in Combination with Dacarbazine in Patients with Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J. Clin. Oncol. 2018, 36, 1232–1239. [Google Scholar] [CrossRef]
- Zimmer, L.; Vaubel, J.; Mohr, P.; Hauschild, A.; Utikal, J.; Simon, J.; Garbe, C.; Herbst, R.; Enk, A.; Kämpgen, E.; et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-Naïve patients with metastatic uveal melanoma. PLoS ONE 2015, 10, e0118564. [Google Scholar] [CrossRef] [PubMed]
- Pelster, M.S.; Gruschkus, S.K.; Bassett, R.; Gombos, D.S.; Shephard, M.; Posada, L.; Glover, M.S.; Simien, R.; Diab, A.; Hwu, P.; et al. Nivolumab and Ipilimumab in Metastatic Uveal Melanoma: Results from a Single-Arm Phase II Study. J. Clin. Oncol. 2021, 39, 599–607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rapisuwon, S.; Carvajal, R.D.; Ansstas, G.; Tsai, K.K.; Hernandez-Aya, L.F.; Khan, S.; Chandra, S.; Harbour, J.W.; Sosman, J.A.; Daud, A.; et al. Phase II multi-center study of adjuvant nivolumab in combination with ipilimumab in patients with high-risk uveal melanoma (HCRN MEL17-309). J. Clin. Oncol. 2024, 42, 9509. [Google Scholar] [CrossRef]
- Montazeri, K.; Pattanayak, V.; Sullivan, R.J. Tebentafusp in the Treatment of Metastatic Uveal Melanoma: Patient Selection and Special Considerations. Drug Des. Dev. Ther. 2023, 17, 333–339. [Google Scholar] [CrossRef]
- Hassel, J.C.; Piperno-Neumann, S.; Rutkowski, P.; Baurain, J.F.; Schlaak, M.; Butler, M.O.; Sullivan, R.J.; Dummer, R.; Kirkwood, J.M.; Orloff, M.; et al. Three-Year Overall Survival with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2023, 389, 2256–2266. [Google Scholar] [CrossRef]
- Harper, J.; Adams, K.J.; Bossi, G.; Wright, D.E.; Stacey, A.R.; Bedke, N.; Martinez-Hague, R.; Blat, D.; Humbert, L.; Buchanan, H.; et al. An approved in vitro approach to preclinical safety and efficacy evaluation of engineered T cell receptor anti-CD3 bispecific (ImmTAC) molecules. PLoS ONE 2018, 13, e0205491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eteghadi, A.; Ebrahimi, M.; Keshel, S.H. New immunotherapy approaches as the most effective treatment for uveal melanoma. Crit. Rev. Oncol. Hematol. 2024, 194, 104260. [Google Scholar] [CrossRef]
- Kozani, P.S.; Kozani, P.S.; Sheikhi, A. Tebentafusp: The first FDA-approved monoclonal antibody for cancer treatment in 2022. Trends Med. Sci. 2021, 1, e123546. [Google Scholar]
- Middleton, M.R.; Steven, N.M.; Evans, T.J.; Infante, J.R.; Sznol, M.; Mulatero, C.; Hamid, O.; Shoushtari, A.N.; Shingler, W.; Johnson, A.; et al. Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific t cell redirector with solid tumour activity: Results from the FIH study in melanoma. J. Clin. Oncol. 2016, 34, 3016. [Google Scholar] [CrossRef]
- Sacco, J.; Carvajal, R.; Butler, M.; Shoushtari, A.; Hassel, J.; Ikeguchi, A.; Hernandez-Aya, L.; Nathan, P.; Hamid, O.; Rodriguez, J.P.; et al. 64MO A phase (ph) II, multi-center study of the safety and efficacy of tebentafusp (tebe)(IMCgp100) in patients (pts) with metastatic uveal melanoma (mUM). Ann. Oncol. 2020, 31, S1442–S1443. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Papillon, J.P.N.; Luzzio, M.; LaMarche, M.J.; Fan, J.; Michael, W.; Wang, D.; Zhang, A.; Straub, C.; Mathieu, S.; et al. Discovery of Darovasertib (NVP-LXS196), a Pan-PKC Inhibitor for the Treatment of Metastatic Uveal Melanoma. J. Med. Chem. 2024, 67, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Lietman, C.D.; McKean, M. Targeting GNAQ/11 through PKC inhibition in uveal melanoma. Cancer Gene Ther. 2022, 29, 1809–1813. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, S.; Sun, R.; Ashby, C.R., Jr.; Wei, L.; Huang, Z.; Chen, Z.S. Darovasertib, a novel treatment for metastatic uveal melanoma. Front. Pharmacol. 2023, 14, 1232787. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Khan, S.; Komatsubara, K.; Feun, L.; Acquavella, N.; Singh-Kandah, S.; Negri, T.; Nesson, A.; Abbate, K.; Cremers, S.; et al. A Phase Ib Study of Sotrastaurin, a PKC Inhibitor, and Alpelisib, a PI3K? Inhibitor, in Patients with Metastatic Uveal Melanoma. Cancers 2021, 13, 5504. [Google Scholar] [CrossRef]
- Malik, A.U.; Karapetsas, A.; Nirujogi, R.S.; Chatterjee, D.; Phung, T.K.; Wightman, M.; Gourlay, R.; Morrice, N.; Mathea, S.; Knapp, S.; et al. PKC isoforms activate LRRK1 kinase by phosphorylating conserved residues (Ser1064, Ser1074 and Thr1075) within the CORB GTPase domain. Biochem. J. 2022, 479, 1941–1965. [Google Scholar] [CrossRef]
- Koch, E.A.T.; Heppt, M.V.; Berking, C. The Current State of Systemic Therapy of Metastatic Uveal Melanoma. Am. J. Clin. Dermatol. 2024, 25, 691–700. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Carlino, M.S.; Boni, V.; Loirat, D.; Speetjens, F.M.; Park, J.J.; Calvo, E.; Carvajal, R.D.; Nyakas, M.; Gonzalez-Maffe, J.; et al. A phase I trial of LXS196, a protein kinase C (PKC) inhibitor, for metastatic uveal melanoma. Br. J. Cancer 2023, 128, 1040–1051. [Google Scholar] [CrossRef]
- Joshua, A.M.; O′Day, R.; Glasson, W.; Sia, D.; McGrath, L.; Ameratunga, M.; Cosman, R.; Cherepanoff, S.; O′Quigley, M.; Beaupre, D.M.; et al. A phase 2 safety and efficacy study of neoadjuvant/adjuvant darovasertib for localized ocular melanoma. J. Clin. Oncol. 2024, 42, 9510. [Google Scholar] [CrossRef]
- Hiong, A.; O’Day, R.; Fog, L.S.; McKay, D.; McKenzie, J.; Ameratunga, M.; Joshua, A.M.; Shackleton, M. Globe Salvage and Vision Preservation by Neoadjuvant Darovasertib and Crizotinib in Uveal Melanoma. Ophthalmol. Retin. 2024, 8, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Cosman, R.; Joshua, A.M. LXS196 for Metastatic Uveal Melanoma—Finally some progress. Br. J. Cancer 2023, 128, 1791–1793. [Google Scholar] [CrossRef]
- Tarin, M.; Némati, F.; Decaudin, D.; Canbezdi, C.; Marande, B.; Silva, L.; Derrien, H.; Jochemsen, A.G.; Gardrat, S.; Piperno-Neumann, S.; et al. FAK Inhibitor-Based Combinations with MEK or PKC Inhibitors Trigger Synergistic Antitumor Effects in Uveal Melanoma. Cancers 2023, 15, 2280. [Google Scholar] [CrossRef] [PubMed]
- Arang, N.; Lubrano, S.; Ceribelli, M.; Rigiracciolo, D.C.; Saddawi-Konefka, R.; Faraji, F.; Ramirez, S.I.; Kim, D.; Tosto, F.A.; Stevenson, E.; et al. High-throughput chemogenetic drug screening reveals PKC-RhoA/PKN as a targetable signaling vulnerability in GNAQ-driven uveal melanoma. Cell Rep. Med. 2023, 4, 101244. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT05987332 (accessed on 30 April 2025).
- Carvajal, R.D.; Sacco, J.J.; Jager, M.J.; Eschelman, D.J.; Bagge, R.O.; Harbour, J.W.; Chieng, N.D.; Patel, S.P.; Joshua, A.M.; Piperno-Neumann, S. Advances in the clinical management of uveal melanoma. Nature reviews. Clin. Oncol. 2023, 20, 99–115. [Google Scholar] [CrossRef]
- Prescribing Information for the HEPZATO KIT Hepatic Delivery System. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/201848s000lbl.pdf (accessed on 10 March 2025).
- Sajan, A.; Fordyce, S.; Sideris, A.; Liou, C.; Toor, Z.; Filtes, J.; Krishnasamy, V.; Ahmad, N.; Reis, S.; Brejt, S.; et al. Minimally Invasive Treatment Options for Hepatic Uveal Melanoma Metastases. Diagnostics 2023, 13, 1836. [Google Scholar] [CrossRef]
- The Medical Letter, Inc. In brief: Melphalan (Hepzato) for uveal melanoma. Med. Lett. Drugs Ther. 2023, 65, e148. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Pharmaceuticals. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100A.) MELPHALAN. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304320/ (accessed on 5 February 2025).
- Bagge, R.O.; Nelson, A.; Shafazand, A.; All-Eriksson, C.; Cahlin, C.; Elander, N.; Helgadottir, H.; Kiilgaard, J.F.; Kinhult, S.; Ljuslinder, I.; et al. Isolated Hepatic Perfusion with Melphalan for Patients with Isolated Uveal Melanoma Liver Metastases: A Multicenter, Randomized, Open-Label, Phase III Trial (the SCANDIUM Trial). J. Clin. Oncol. 2023, 41, 3042–3050. [Google Scholar] [CrossRef]
- Zager, J.S.; Orloff, M.; Ferrucci, P.F.; Choi, J.; Eschelman, D.J.; Glazer, E.S.; Ejaz, A.; Howard, J.H.; Richtig, E.; Ochsenreither, S.; et al. Efficacy and Safety of the Melphalan/Hepatic Delivery System in Patients with Unresectable Metastatic Uveal Melanoma: Results from an Open-Label, Single-Arm, Multicenter Phase 3 Study. Ann. Surg. Oncol. 2024, 31, 5340–5351. Erratum in Ann. Surg. Oncol. 2024, 31, 8262–8263. https://doi.org/10.1245/s10434-024-15886-6. [CrossRef] [PubMed] [PubMed Central]
- Bethlehem, M.S.; Katsarelias, D.; Bagge, R.O. Meta-Analysis of Isolated Hepatic Perfusion and Percutaneous Hepatic Perfusion as a Treatment for Uveal Melanoma Liver Metastases. Cancers 2021, 13, 4726. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Cutaneous Melanoma | Uveal Melanoma |
|---|---|---|
| Incidence rate | 12.2 to 48.1 cases per 100,000 individuals | 5.6 cases per million individuals |
| Typical age at diagnosis | Generally younger, with a median age of 57 years | Generally older, with peak incidence at 70–75 years |
| Prevalence among melanomas | Around 90% of all primary melanoma cases | Around 5% of all primary melanoma cases |
| Association with UV exposure | Strongly linked to UV radiation | Varies depending on tumor location |
| Common genetic mutations | Typically involves mutations in BRAF or NRAS genes | Most often involves GNAQ or GNA11 gene mutations |
| S100 immunoreactivity | Common in metastatic CM | Common in primary UM, rare in metastatic UM |
| 5-year survival rate | ~94%; ~32% in metastatic disease | ~85%; ~15% in metastatic disease |
| Treatment Modality | Indications | Efficacy | Complications |
|---|---|---|---|
| Brachytherapy | Small to medium-sized tumors; vision-sparing approach | High local tumor control; outcomes comparable to surgery for medium-sized tumors | Radiation retinopathy, optic neuropathy, cataract |
| Charged particle therapy | Larger tumors or tumors near optic nerve | High precision and effective local control | Limited availability; radiation damage to nearby critical structures |
| Stereotactic radiotherapy | Select tumors unsuitable for other radiotherapy approaches | Effective in selected cases | Possible collateral tissue damage |
| Photodynamic therapy/transpupillary thermotherapy | Mainly used as neoadjuvant/adjuvant therapy | Limited as monotherapy; can improve outcomes when combined with other treatments | Retinal damage, limited penetration in deeper tumors |
| Local resection | Small to medium-sized tumors | Organ-sparing; variable success based on tumor location and surgeon experience | Risk of recurrence; surgical complications |
| Enucleation | Large tumors, advanced disease | Definitive local control | Loss of eye; psychological impact |
| Exenteration | Orbital invasion by tumor | Only option in very advanced cases | Highly disfiguring; functional and psychological burden |
| Systemic therapy (targeted & immunotherapy) | Metastatic or unresectable disease; mutation- or immune-guided therapy | Generally limited efficacy; combination immunotherapy and targeted agents shows some promise | Systemic toxicity, immune-related adverse events, incomplete or short-lived response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wdowiak, K.; Dolar-Szczasny, J.; Rejdak, R.; Drab, A.; Maciocha, A. A Brief Overview of Uveal Melanoma Treatment Methods with a Focus on the Latest Advances. J. Clin. Med. 2025, 14, 4058. https://doi.org/10.3390/jcm14124058
Wdowiak K, Dolar-Szczasny J, Rejdak R, Drab A, Maciocha A. A Brief Overview of Uveal Melanoma Treatment Methods with a Focus on the Latest Advances. Journal of Clinical Medicine. 2025; 14(12):4058. https://doi.org/10.3390/jcm14124058
Chicago/Turabian StyleWdowiak, Krystian, Joanna Dolar-Szczasny, Robert Rejdak, Agnieszka Drab, and Agnieszka Maciocha. 2025. "A Brief Overview of Uveal Melanoma Treatment Methods with a Focus on the Latest Advances" Journal of Clinical Medicine 14, no. 12: 4058. https://doi.org/10.3390/jcm14124058
APA StyleWdowiak, K., Dolar-Szczasny, J., Rejdak, R., Drab, A., & Maciocha, A. (2025). A Brief Overview of Uveal Melanoma Treatment Methods with a Focus on the Latest Advances. Journal of Clinical Medicine, 14(12), 4058. https://doi.org/10.3390/jcm14124058





