Non-Traumatic Lower-Limb Amputations: Outcome, Sex-Differences, Comorbidity Patterns and Temporal Trends from 2006 to 2022
Abstract
1. Introduction
2. Methods
2.1. Study Population and Outcome
2.2. Statistics
3. Results
3.1. Baseline Characteristics and Comorbidities
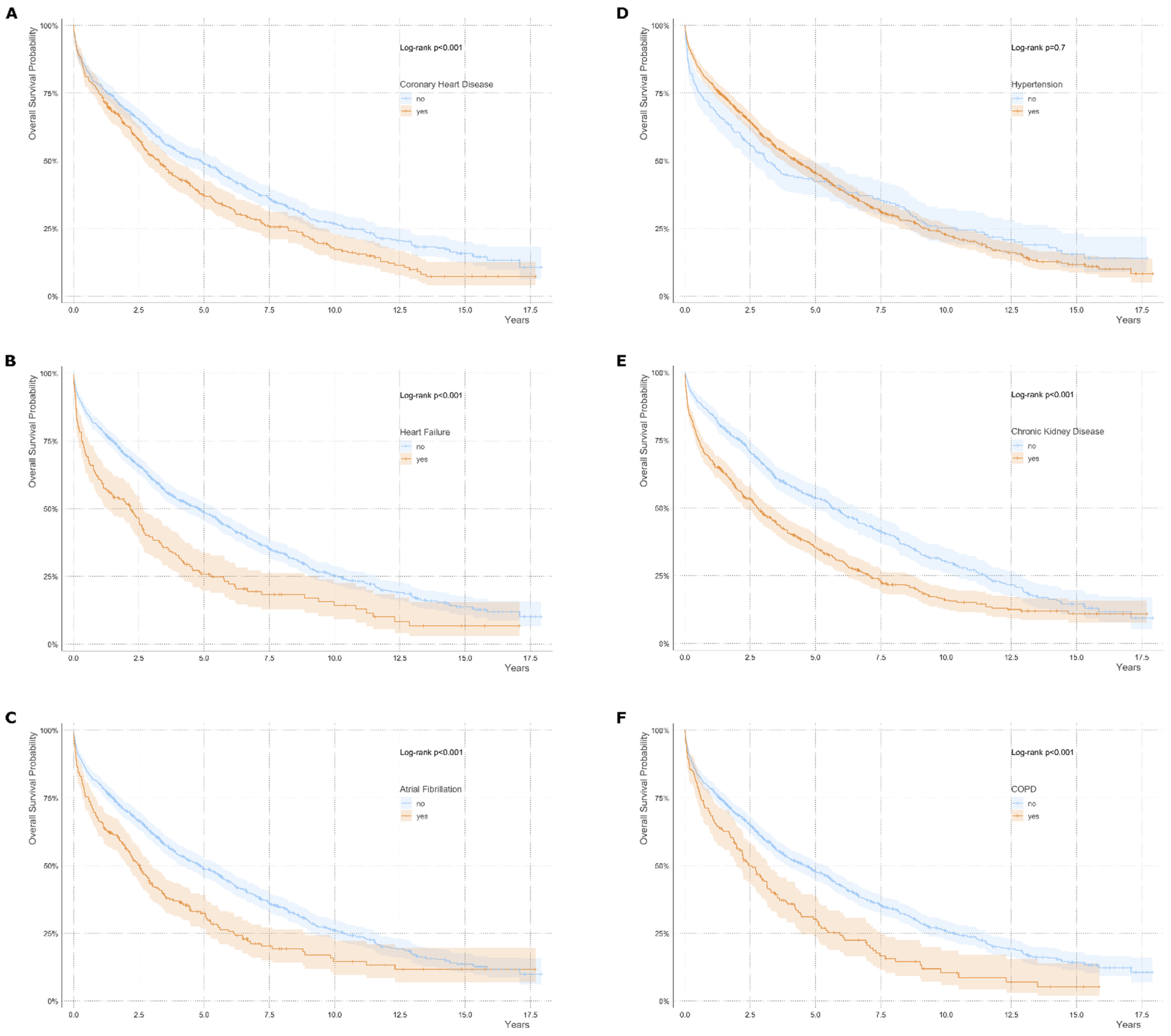
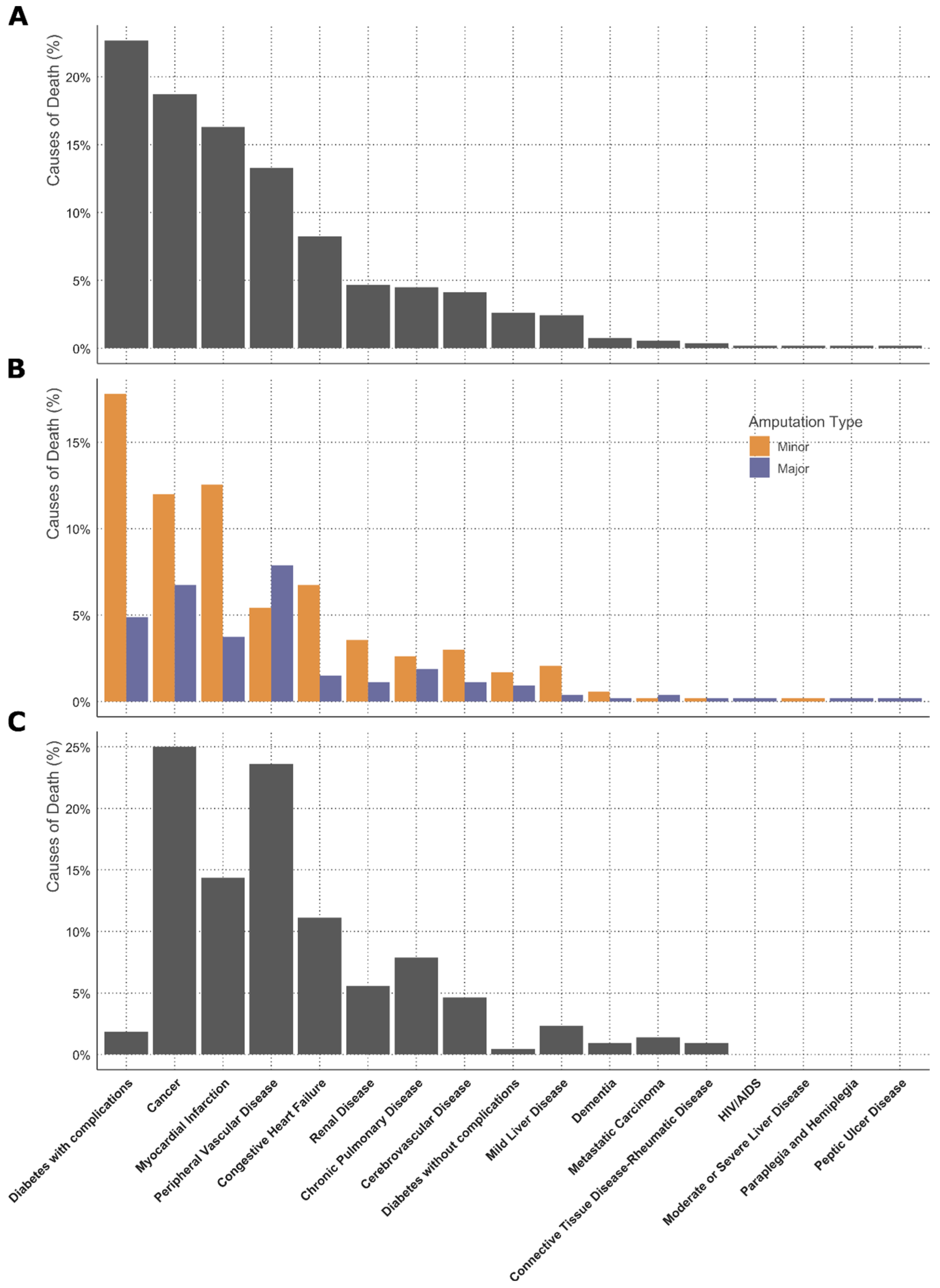
3.2. Survival Analysis and Causes of Death
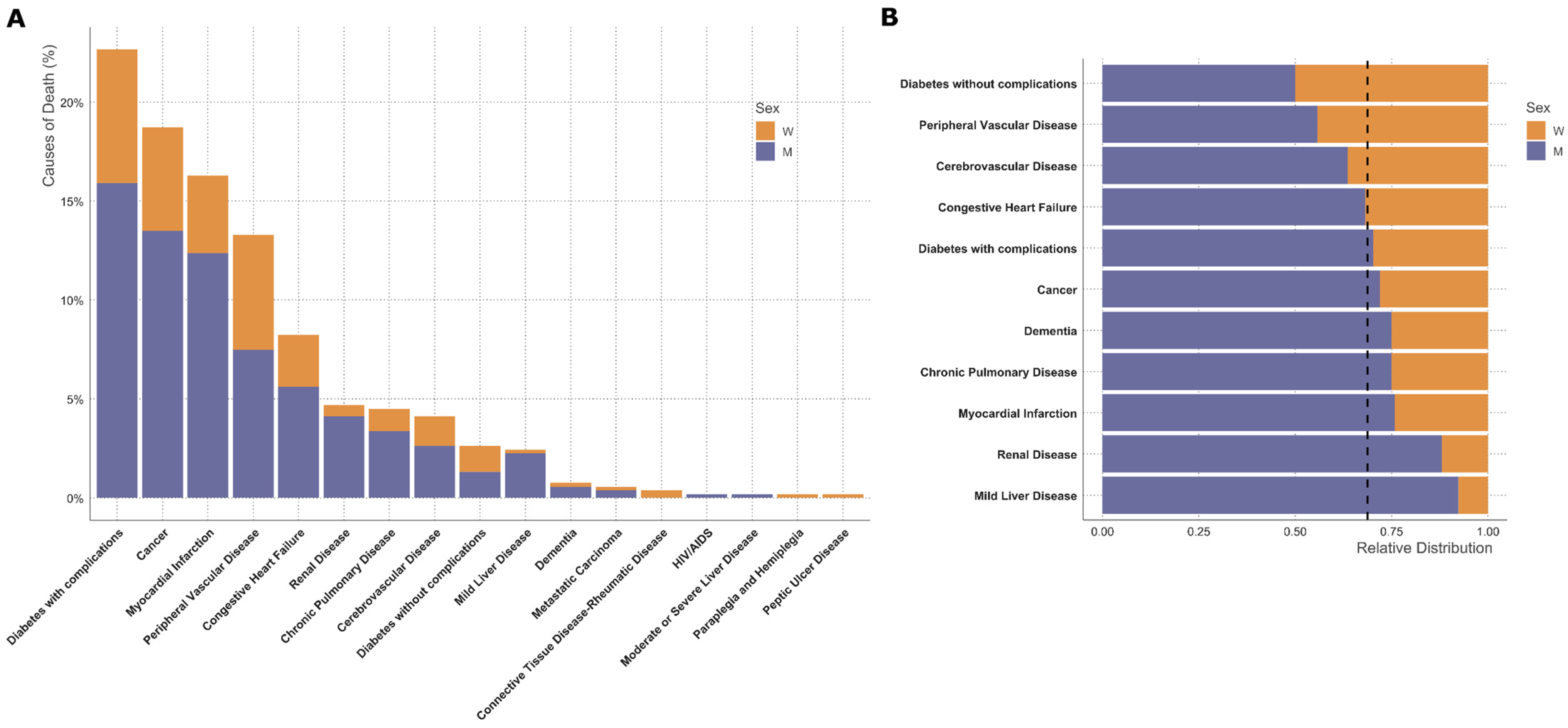
3.3. Sex-Specific Analysis
4. Discussion
5. Conclusions
Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Creager, M.A.; Matsushita, K.; Arya, S.; Beckman, J.A.; Duval, S.; Goodney, P.P.; Gutierrez, J.A.T.; Kaufman, J.A.; Maddox, K.E.J.; Pollak, A.W.; et al. Reducing Nontraumatic Lower-Extremity Amputations by 20% by 2030: Time to Get to Our Feet: A Policy Statement from the American Heart Association. Circulation 2021, 143, e875–e891. [Google Scholar] [CrossRef]
- Heikkila, K.; Loftus, I.M.; Mitchell, D.C.; Johal, A.S.; Waton, S.; Cromwell, D.A. Population-based study of mortality and major amputation following lower limb revascularization. Br. J. Surg. 2018, 105, 1145–1154. [Google Scholar] [CrossRef]
- Li, Q.; Birmpili, P.; Atkins, E.; Johal, A.S.; Waton, S.; Williams, R.; Boyle, J.R.; Harkin, D.W.; Pherwani, A.D.; Cromwell, D.A. Illness Trajectories After Revascularization in Patients with Peripheral Artery Disease: A Unified Approach to Understanding the Risk of Major Amputation and Death. Circulation 2024, 150, 261–271. [Google Scholar] [CrossRef]
- Qaarie, M.Y. Life Expectancy and Mortality After Lower Extremity Amputation: Overview and Analysis of Literature. Cureus 2023, 15, e38944. [Google Scholar] [CrossRef]
- Gyldenkerne, C.; Olesen, K.K.W.; Thrane, P.G.; Hansen, M.K.; Stodkilde-Jorgensen, N.; Sorensen, H.T.; Thomsen, R.W.; Maeng, M. Trends in Peripheral Artery Disease, Lower Extremity Revascularization, and Lower Extremity Amputation in Incident Type 2 Diabetes: A Danish Population-Based Cohort Study. Diabetes Care 2025, 48, 76–83. [Google Scholar] [CrossRef]
- Lopez-de-Andres, A.; Jimenez-Garcia, R.; Hernandez-Barrera, V.; de Miguel-Diez, J.; de Miguel-Yanes, J.M.; Omana-Palanco, R.; Carabantes-Alarcon, D. Trends of Non-Traumatic Lower-Extremity Amputation and Type 2 Diabetes: Spain, 2001–2019. J. Clin. Med. 2022, 11, 1246. [Google Scholar] [CrossRef]
- Aziz, F.; Reichardt, B.; Sourij, C.; Dimai, H.P.; Reichart, D.; Kohler, G.; Brodmann, M.; Sourij, H. Epidemiology of major lower extremity amputations in individuals with diabetes in Austria, 2014–2017: A retrospective analysis of health insurance database. Diabetes Res. Clin. Pract. 2020, 170, 108477. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Leutner, M.; Harreiter, J. Sex differences in type 2 diabetes. Diabetologia 2023, 66, 986–1002. [Google Scholar] [CrossRef]
- Emerging Risk Factors, C.; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Huxley, R.; Barzi, F.; Woodward, M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. BMJ 2006, 332, 73–78. [Google Scholar] [CrossRef]
- Norhammar, A. Diabetes and cardiovascular mortality: The impact of sex. Lancet Diabetes Endocrinol. 2018, 6, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies, C.; Asia Pacific Cohort Studies, C. Sex-specific relevance of diabetes to occlusive vascular and other mortality: A collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes Endocrinol. 2018, 6, 538–546. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.; Cheng, S.; Altin, S.E.; Sethi, S.S.; Nelson, M.D.; Moreau, K.L.; Hamburg, N.; Hess, C.N. Sex Differences in Peripheral Artery Disease. Circ. Res. 2022, 130, 496–511. [Google Scholar] [CrossRef]
- Sigvant, B.; Lundin, F.; Nilsson, B.; Bergqvist, D.; Wahlberg, E. Differences in presentation of symptoms between women and men with intermittent claudication. BMC Cardiovasc. Disord. 2011, 11, 39. [Google Scholar] [CrossRef]
- Hiramoto, J.S.; Katz, R.; Weisman, S.; Conte, M. Gender-specific risk factors for peripheral artery disease in a voluntary screening population. J. Am. Heart Assoc. 2014, 3, e000651. [Google Scholar] [CrossRef]
- Tunstall-Pedoe, H.; Peters, S.A.E.; Woodward, M.; Struthers, A.D.; Belch, J.J.F. Twenty-Year Predictors of Peripheral Arterial Disease Compared with Coronary Heart Disease in the Scottish Heart Health Extended Cohort (SHHEC). J. Am. Heart Assoc. 2017, 6, e005967. [Google Scholar] [CrossRef]
- Chase-Vilchez, A.Z.; Chan, I.H.Y.; Peters, S.A.E.; Woodward, M. Diabetes as a risk factor for incident peripheral arterial disease in women compared to men: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2020, 19, 151. [Google Scholar] [CrossRef]
- Anand, S.S.; Islam, S.; Rosengren, A.; Franzosi, M.G.; Steyn, K.; Yusufali, A.H.; Keltai, M.; Diaz, R.; Rangarajan, S.; Yusuf, S.; et al. Risk factors for myocardial infarction in women and men: Insights from the INTERHEART study. Eur. Heart J. 2008, 29, 932–940. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Ohman, E.M.; Hirsch, A.T.; Ikeda, Y.; Mas, J.L.; Goto, S.; Liau, C.S.; Richard, A.J.; Rother, J.; et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006, 295, 180–189. [Google Scholar] [CrossRef]
- Bavry, A.A.; Anderson, R.D.; Gong, Y.; Denardo, S.J.; Cooper-Dehoff, R.M.; Handberg, E.M.; Pepine, C.J. Outcomes Among hypertensive patients with concomitant peripheral and coronary artery disease: Findings from the INternational VErapamil-SR/Trandolapril STudy. Hypertension 2010, 55, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Shaw, P.A.; Townsend, R.R.; Anderson, A.H.; Xie, D.; Wang, X.; Nessel, L.C.; Mohler, E.R.; Sozio, S.M.; Jaar, B.G.; et al. Sex Differences in the Incidence of Peripheral Artery Disease in the Chronic Renal Insufficiency Cohort. Circ. Cardiovasc. Qual. Outcomes 2016, 9 (Suppl. S1), S86–S93. [Google Scholar] [CrossRef] [PubMed]
- Eraso, L.H.; Fukaya, E.; Mohler, E.R., 3rd; Xie, D.; Sha, D.; Berger, J.S. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur. J. Prev. Cardiol. 2014, 21, 704–711. [Google Scholar] [CrossRef]
- Haine, A.; Kavanagh, S.; Berger, J.S.; Hess, C.N.; Norgren, L.; Fowkes, F.G.R.; Katona, B.G.; Mahaffey, K.W.; Blomster, J.I.; Patel, M.R.; et al. Sex-Specific Risks of Major Cardiovascular and Limb Events in Patients with Symptomatic Peripheral Artery Disease. J. Am. Coll. Cardiol. 2020, 75, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.H.; Arya, S.; Bryce, Y.; Gornik, H.L.; Long, C.A.; McDermott, M.M.; West Pollak, A.; Rowe, V.L.; Sullivan, A.E.; Whipple, M.O.; et al. Sex Differences in Peripheral Vascular Disease: A Scientific Statement from the American Heart Association. Circulation 2025, 151, e877–e904. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- R Core Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Subirana, I.; Sanz, H.; Vila, J. Building Bivariate Tables: The compareGroups Package for R. J. Stat. Softw. 2014, 57, 16. [Google Scholar] [CrossRef]
- TM, T. A Package for Survival Analysis in R. 2024. Available online: https://cran.r-project.org/web/packages/survival/vignettes/survival.pdf (accessed on 2 June 2025).
- Wasey, J.O.L.M.; R Core Team. icd: Comorbidity Calculations and Tools for ICD-9 and ICD-10 Codes; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Sarroca, N.; Valero, J.; Deus, J.; Casanova, J.; Luesma, M.J.; Lahoz, M. Quality of life, body image and self-esteem in patients with unilateral transtibial amputations. Sci. Rep. 2021, 11, 12559. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.P.A.D. Global burden of peripheral artery disease and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Glob. Health 2023, 11, e1553–e1565. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021.
- Cherla, A.; Kyriopoulos, I.; Pearcy, P.; Tsangalidou, Z.; Hajrulahovic, H.; Theodorakis, P.; Andersson, C.E.; Mehra, M.R.; Mossialos, E. Trends in avoidable mortality from cardiovascular diseases in the European Union, 1995–2020: A retrospective secondary data analysis. Lancet Reg. Health Eur. 2024, 47, 101079. [Google Scholar] [CrossRef]
- Chesnaye, N.C.; Carrero, J.J.; Hecking, M.; Jager, K.J. Differences in the epidemiology, management and outcomes of kidney disease in men and women. Nat. Rev. Nephrol. 2024, 20, 7–20. [Google Scholar] [CrossRef]
- Inker, L.A.; Levey, A.S.; Tighiouart, H.; Shafi, T.; Eckfeldt, J.H.; Johnson, C.; Okparavero, A.; Post, W.S.; Coresh, J.; Shlipak, M.G. Performance of glomerular filtration rate estimating equations in a community-based sample of Blacks and Whites: The multiethnic study of atherosclerosis. Nephrol. Dial. Transplant. 2018, 33, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Bull, C.; Robinson, J.; Deanfield, J.E. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J. Am. Coll. Cardiol. 1994, 24, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; Hildreth, K.L.; Meditz, A.L.; Deane, K.D.; Kohrt, W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012, 97, 4692–4700. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Mattei, P.; Sudano, I.; Bernini, G.; Pinto, S.; Salvetti, A. Menopause is associated with endothelial dysfunction in women. Hypertension 1996, 28, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Kavurma, M.M.; Boccanfuso, L.; Cutmore, C.; Passam, F.; Patel, S.; Hennessy, A.; Loa, J.; Figtree, G.A.; Golledge, J.; Robinson, D.A.; et al. A hidden problem: Peripheral artery disease in women. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 342–350. [Google Scholar] [CrossRef]
- Kozak, M.; Poredos, P.; Blinc, A.; Kaja Jezovnik, M.; Poredos, P. Peripheral arterial disease in women. Vasa 2024, 53, 366–370. [Google Scholar] [CrossRef]
- Altin, S.E.; Castro-Dominguez, Y.S.; Kennedy, K.F.; Orion, K.C.; Lanksy, A.J.; Abbott, J.D.; Aronow, H.D. Predictors of Underutilization of Medical Therapy in Patients Undergoing Endovascular Revascularization for Peripheral Artery Disease. JACC Cardiovasc. Interv. 2020, 13, 2911–2918. [Google Scholar] [CrossRef]
- Sourij, H.; Azhar, K.; Aziz, F.; Kojzar, H.; Sourij, C.; Fasching, P.; Clodi, M.; Ludvik, B.; Mader, J.K.; Resl, M.; et al. Metabolic risk factor targets in relation to clinical characteristics and comorbidities among individuals with type 2 diabetes treated in primary care—The countrywide cross-sectional AUSTRO-PROFIT study. Diabetes Obes. Metab. 2025, 27, 111–122. [Google Scholar] [CrossRef]
- Ramirez-Morros, A.; Franch-Nadal, J.; Real, J.; Miro-Catalina, Q.; Bundo, M.; Vlacho, B.; Mauricio, D. Clinical characteristics and degree of cardiovascular risk factor control in patients with newly-diagnosed type 2 diabetes in Catalonia. Front. Endocrinol. 2024, 15, 1339879. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Nault, P.; Giugliano, R.P.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Low-Density Lipoprotein Cholesterol Lowering with Evolocumab and Outcomes in Patients with Peripheral Artery Disease: Insights from the FOURIER Trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk). Circulation 2018, 137, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.; Mathisen, A.; Thomsen, T.R.; Rossing, P.; Kirketerp-Moller, K. Glucagon-like peptide-1 treatment reduces the risk of diabetes-type 2 related amputations: A cohort study in Denmark. Diabetes Res. Clin. Pract. 2023, 202, 110799. [Google Scholar] [CrossRef] [PubMed]
- Wisman, P.P.; Tangelder, M.J.; van Hattum, E.S.; de Borst, G.J.; Moll, F.L. Young women with PAD are at high risk of cardiovascular complications. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 441–445. [Google Scholar] [CrossRef]
- Pawlik, A.; Januszek, R.; Ruzsa, Z.; Orias, V.; Kleczynski, P.; Wojtasik-Bakalarz, J.; Arif, S.; Nyerges, A.; Chyrchel, M.; Stanek, A.; et al. Gender differences and long-term clinical outcomes in patients with chronic total occlusions of infrainguinal lower limb arteries treated from retrograde access with peripheral vascular interventions. Adv. Med. Sci. 2020, 65, 197–201. [Google Scholar] [CrossRef]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2007, 25, 1105–1187. [Google Scholar] [CrossRef]


| [ALL] | 2006–2010 | 2011–2015 | 2016–2019 | 2020–2022 | p.overall | p.trend | |
|---|---|---|---|---|---|---|---|
| N = 1107 | N = 361 | N = 290 | N = 250 | N = 206 | |||
| Age (years) | 73.6 [64.7; 81.5] | 72.0 [62.7; 80.5] | 73.9 [66.0; 82.0] | 73.4 [64.1; 81.4] | 75.3 [66.4; 82.1] | 0.071 | 0.033 |
| <Median | 553 (50.0%) | 192 (53.2%) | 140 (48.3%) | 127 (50.8%) | 94 (45.6%) | 0.328 | 0.194 |
| >Median | 554 (50.0%) | 169 (46.8%) | 150 (51.7%) | 123 (49.2%) | 112 (54.4%) | ||
| Female Sex | 345 (31.2%) | 135 (37.4%) | 92 (31.7%) | 59 (23.6%) | 59 (28.6%) | 0.006 | 0.007 |
| BMI (kg/m2) | 24.8 [22.0; 28.5] | 25.0 [21.8; 28.0] | 24.4 [21.6; 27.9] | 26.4 [22.9; 29.6] | 24.4 [22.0; 28.2] | 0.004 | 0.471 |
| Risk factors | |||||||
| Smoking | 279 (28.0%) | 83 (31.7%) | 83 (29.5%) | 58 (23.2%) | 55 (27.0%) | <0.001 | 0.011 |
| Diabetes | 608 (55.0%) | 224 (62.2%) | 134 (46.2%) | 142 (56.8%) | 108 (52.4%) | 0.001 | 0.124 |
| LDL-C (mg/dL) | 79.0 [59.0; 102] | 87.5 [68.0; 117] | 77.0 [57.0; 98.0] | 78.0 [59.0; 99.0] | 74.0 [52.0; 98.5] | 0.001 | 0.003 |
| Hypertension | 856 (77.5%) | 259 (72.1%) | 210 (72.4%) | 208 (83.2%) | 179 (86.9%) | <0.001 | <0.001 |
| Statin usage | 563 (50.9%) | 124 (34.3%) | 158 (54.5%) | 159 (63.6%) | 122 (59.2%) | <0.001 | <0.001 |
| Comorbidities | |||||||
| CHD | 401 (36.3%) | 123 (34.2%) | 81 (27.9%) | 102 (40.8%) | 95 (46.1%) | 0.001 | 0.003 |
| Heart failure | 182 (16.5%) | 59 (16.5%) | 35 (12.1%) | 53 (21.2%) | 35 (17.0%) | 0.071 | 0.330 |
| CKD | 478 (43.9%) | 157 (44.4%) | 134 (46.5%) | 114 (46.0%) | 73 (36.5%) | 0.143 | 0.197 |
| Creatinine (mg/dL) | 1.07 [0.81; 1.62] | 1.06 [0.80; 1.66] | 1.09 [0.82; 1.72] | 1.14 [0.86; 1.53] | 1.00 [0.77; 1.41] | 0.141 | 0.272 |
| eGFR (mL/min/1.73 m2) | 0.118 | 0.068 | |||||
| <30 | 174 (16.0%) | 66 (18.6%) | 50 (17.4%) | 33 (13.3%) | 25 (12.5%) | ||
| 30–60 | 304 (27.9%) | 91 (25.7%) | 84 (29.2%) | 81 (32.7%) | 48 (24.0%) | ||
| >60 | 612 (56.1%) | 197 (55.6%) | 154 (53.5%) | 134 (54.0%) | 127 (63.5%) | ||
| Atrial Fibrillation | 267 (24.2%) | 65 (18.1%) | 62 (21.4%) | 80 (32.1%) | 60 (29.1%) | 0.001 | <0.001 |
| COPD | 177 (16.0%) | 45 (12.5%) | 45 (15.5%) | 46 (18.5%) | 41 (20.0%) | 0.107 | 0.020 |
| Amputation Type | 0.263 | 0.230 | |||||
| Minor | 752 (67.9%) | 240 (66.5%) | 188 (64.8%) | 181 (72.4%) | 143 (69.4%) | ||
| Major | 355 (32.1%) | 121 (33.5%) | 102 (35.2%) | 69 (27.6%) | 63 (30.6%) |
| All N = 1107 | Females N = 345 | Males N = 762 | p-Value | |
|---|---|---|---|---|
| Age (years) | 73.6 [64.7; 81.5] | 78.9 [70.3; 86.8] | 71.4 [63.0; 79.3] | <0.001 |
| <Median | 553 (50.0%) | 115 (33.3%) | 438 (57.5%) | <0.001 |
| >Median | 554 (50.0%) | 230 (66.7%) | 324 (42.5%) | |
| BMI (kg/m2) | 24.8 [22.0; 28.5] | 23.9 [20.2; 28.6] | 25.1 [22.6; 28.5] | 0.002 |
| Risk factors | ||||
| Smoking | 279 (28.0%) | 65 (21.2%) | 214 (31.0%) | 0.002 |
| Diabetes | 608 (55.0%) | 167 (48.4%) | 441 (58.0%) | 0.005 |
| LDL-C (mg/dL) | 79.0 [59.0; 102] | 88.0 [63.0; 115] | 77.0 [57.0; 98.0] | 0.002 |
| Hypertension | 856 (77.5%) | 266 (77.1%) | 590 (77.6%) | 0.906 |
| Statin usage | 563 (50.9%) | 142 (41.2%) | 421 (55.2%) | <0.001 |
| Comorbidities | ||||
| CHD | 401 (36.3%) | 94 (27.2%) | 307 (40.3%) | <0.001 |
| Heart failure | 182 (16.5%) | 51 (14.8%) | 131 (17.3%) | 0.399 |
| CKD | 478 (43.9%) | 176 (51.6%) | 302 (40.3%) | 0.001 |
| Creatinine (mg/dL) | 1.07 [0.81; 1.62] | 0.98 [0.73; 1.50] | 1.12 [0.85; 1.65] | <0.001 |
| eGFR (mL/min/1.73 m2) | 0.003 | |||
| <30 | 174 (16.0%) | 64 (18.8%) | 110 (14.7%) | |
| 30–60 | 304 (27.9%) | 112 (32.8%) | 192 (25.6%) | |
| >60 | 612 (56.1%) | 165 (48.4%) | 447 (59.7%) | |
| Atrial Fibrillation | 267 (24.2%) | 77 (22.3%) | 190 (25.0%) | 0.399 |
| COPD | 177 (16.0%) | 36 (10.4%) | 141 (18.6%) | 0.002 |
| Amputation Type | <0.001 | |||
| Minor | 752 (67.9%) | 187 (54.2%) | 565 (74.1%) | |
| Major | 355 (32.1%) | 158 (45.8%) | 197 (25.9%) |
| [ALL] | 2006–2010 | 2011–2015 | 2016–2019 | 2020–2022 | p.overall | p.trend | |
|---|---|---|---|---|---|---|---|
| N = 345 | N = 135 | N = 92 | N = 59 | N = 59 | |||
| Age (years) | 78.9 [70.3; 86.8] | 79.3 [69.0; 85.5] | 80.8 [72.9; 89.3] | 77.1 [70.1; 86.5] | 78.3 [68.3; 86.9] | 0.678 | 0.893 |
| <Median | 115 (33.3%) | 46 (34.1%) | 27 (29.3%) | 22 (37.3%) | 20 (33.9%) | 0.774 | 0.893 |
| >Median | 230 (66.7%) | 89 (65.9%) | 65 (70.7%) | 37 (62.7%) | 39 (66.1%) | ||
| BMI (kg/m2) | 23.9 [20.2; 28.6] | 23.4 [19.8; 26.9] | 24.4 [19.3; 28.7] | 26.0 [21.2; 30.0] | 23.2 [21.7; 26.6] | 0.459 | 0.423 |
| Risk factors | |||||||
| Smoking | 65 (21.2%) | 24 (24.0%) | 19 (21.3%) | 8 (13.6%) | 14 (24.1%) | 0.090 | 0.423 |
| Diabetes | 167 (48.4%) | 70 (51.9%) | 41 (44.6%) | 30 (50.8%) | 26 (44.1%) | 0.710 | 0.671 |
| LDL-C (mg/dL) | 88.0 [63.0; 115] | 88.5 [61.8; 124] | 77.0 [63.0; 100] | 94.0 [70.0; 110] | 92.0 [57.0; 116] | 0.658 | 0.893 |
| Hypertension | 266 (77.1%) | 99 (73.3%) | 63 (68.5%) | 53 (89.8%) | 51 (86.4%) | 0.032 | 0.052 |
| Statin usage | 142 (41.2%) | 37 (27.4%) | 42 (45.7%) | 36 (61.0%) | 27 (45.8%) | 0.001 | 0.007 |
| Comorbidities | |||||||
| CHD | 94 (27.2%) | 42 (31.1%) | 18 (19.6%) | 15 (25.4%) | 19 (32.2%) | 0.468 | 0.962 |
| Heart Failure | 51 (14.8%) | 21 (15.6%) | 12 (13.2%) | 11 (18.6%) | 7 (11.9%) | 0.767 | 0.893 |
| CKD | 176 (51.6%) | 71 (53.4%) | 47 (51.6%) | 33 (55.9%) | 25 (43.1%) | 0.678 | 0.613 |
| Creatinine (mg/dL) | 0.98 [0.73; 1.50] | 1.01 [0.74; 1.63] | 0.98 [0.71; 1.41] | 1.01 [0.78; 1.21] | 0.84 [0.69; 1.41] | 0.678 | 0.423 |
|
eGFR (mL/min/1.73 m2) | 0.459 | 0.423 | |||||
| <30 | 64 (18.8%) | 30 (22.6%) | 17 (18.7%) | 6 (10.2%) | 11 (19.0%) | ||
| 30–60 | 112 (32.8%) | 41 (30.8%) | 30 (33.0%) | 27 (45.8%) | 14 (24.1%) | ||
| >60 | 165 (48.4%) | 62 (46.6%) | 44 (48.4%) | 26 (44.1%) | 33 (56.9%) | ||
| Atrial Fibrillation | 77 (22.3%) | 26 (19.3%) | 18 (19.6%) | 17 (28.8%) | 16 (27.1%) | 0.606 | 0.423 |
| COPD | 36 (10.4%) | 10 (7.41%) | 10 (10.9%) | 5 (8.47%) | 11 (18.6%) | 0.459 | 0.243 |
| Amputation Type | 0.476 | 0.701 | |||||
| Minor | 187 (54.2%) | 71 (52.6%) | 46 (50.0%) | 39 (66.1%) | 31 (52.5%) | ||
| Major | 158 (45.8%) | 64 (47.4%) | 46 (50.0%) | 20 (33.9%) | 28 (47.5%) |
| [ALL] | 2006–2010 | 2011–2015 | 2016–2019 | 2020–2022 | p.overall | p.trend | |
|---|---|---|---|---|---|---|---|
| N = 762 | N = 226 | N = 198 | N = 191 | N = 147 | |||
| Age (years) | 71.4 [63.0; 79.3] | 68.1 [60.1; 76.6] | 71.7 [64.5; 77.9] | 72.3 [63.0; 80.7] | 73.5 [66.0; 81.2] | 0.001 | <0.001 |
| <Median | 438 (57.5%) | 146 (64.6%) | 113 (57.1%) | 105 (55.0%) | 74 (50.3%) | 0.065 | 0.010 |
| >Median | 324 (42.5%) | 80 (35.4%) | 85 (42.9%) | 86 (45.0%) | 73 (49.7%) | ||
| BMI (kg/m2) | 25.1 [22.6; 28.5] | 25.6 [23.1; 28.6] | 24.4 [21.8; 27.7] | 26.6 [23.1; 28.9] | 24.5 [22.3; 29.0] | 0.007 | 0.779 |
| Risk factors | |||||||
| Smoking | 214 (31.0%) | 59 (36.4%) | 64 (33.3%) | 50 (26.2%) | 41 (28.1%) | 0.001 | 0.010 |
| Diabetes | 441 (58.0%) | 154 (68.4%) | 93 (47.0%) | 112 (58.6%) | 82 (55.8%) | 0.001 | 0.098 |
| LDL-C (mg/dL) | 77.0 [57.0; 98.0] | 87.5 [68.0; 107] | 76.5 [53.8; 98.0] | 75.0 [56.8; 95.5] | 70.0 [52.0; 89.0] | 0.001 | <0.001 |
| Hypertension | 590 (77.6%) | 160 (71.4%) | 147 (74.2%) | 155 (81.2%) | 128 (87.1%) | 0.003 | <0.001 |
| Statin usage | 421 (55.2%) | 87 (38.5%) | 116 (58.6%) | 123 (64.4%) | 95 (64.6%) | <0.001 | <0.001 |
| Comorbidities | |||||||
| CHD | 307 (40.3%) | 81 (36.0%) | 63 (31.8%) | 87 (45.5%) | 76 (51.7%) | 0.001 | 0.001 |
| Heart failure | 131 (17.3%) | 38 (17.0%) | 23 (11.6%) | 42 (22.0%) | 28 (19.0%) | 0.075 | 0.261 |
| CKD | 302 (40.3%) | 86 (38.9%) | 87 (44.2%) | 81 (42.9%) | 48 (33.8%) | 0.272 | 0.537 |
| Creatinine (mg/dL) | 1.12 [0.85; 1.65] | 1.10 [0.82; 1.71] | 1.15 [0.88; 1.87] | 1.19 [0.87; 1.59] | 1.04 [0.81; 1.40] | 0.110 | 0.365 |
| eGFR (mL/min/1.73 m2) | 0.385 | 0.261 | |||||
| <30 | 110 (14.7%) | 36 (16.3%) | 33 (16.8%) | 27 (14.3%) | 14 (9.86%) | ||
| 30–60 | 192 (25.6%) | 50 (22.6%) | 54 (27.4%) | 54 (28.6%) | 34 (23.9%) | ||
| >60 | 447 (59.7%) | 135 (61.1%) | 110 (55.8%) | 108 (57.1%) | 94 (66.2%) | ||
| Atrial Fibrillation | 190 (25.0%) | 39 (17.3%) | 44 (22.2%) | 63 (33.2%) | 44 (29.9%) | 0.002 | 0.001 |
| COPD | 141 (18.6%) | 35 (15.6%) | 35 (17.7%) | 41 (21.6%) | 30 (20.5%) | 0.423 | 0.193 |
| Amputation Type | 0.806 | 0.756 | |||||
| Minor | 565 (74.1%) | 169 (74.8%) | 142 (71.7%) | 142 (74.3%) | 112 (76.2%) | ||
| Major | 197 (25.9%) | 57 (25.2%) | 56 (28.3%) | 49 (25.7%) | 35 (23.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaser, S.; Radlinger, B.; Blasinger, J.; Koellenberger, N.; Streitberger, V.; Kopp, L.; Bifano, E.; Aziz, F.; Sourij, H.; Goebel, G.; et al. Non-Traumatic Lower-Limb Amputations: Outcome, Sex-Differences, Comorbidity Patterns and Temporal Trends from 2006 to 2022. J. Clin. Med. 2025, 14, 4030. https://doi.org/10.3390/jcm14124030
Kaser S, Radlinger B, Blasinger J, Koellenberger N, Streitberger V, Kopp L, Bifano E, Aziz F, Sourij H, Goebel G, et al. Non-Traumatic Lower-Limb Amputations: Outcome, Sex-Differences, Comorbidity Patterns and Temporal Trends from 2006 to 2022. Journal of Clinical Medicine. 2025; 14(12):4030. https://doi.org/10.3390/jcm14124030
Chicago/Turabian StyleKaser, Susanne, Bernhard Radlinger, Jana Blasinger, Nicolas Koellenberger, Verena Streitberger, Lena Kopp, Elena Bifano, Faisal Aziz, Harald Sourij, Georg Goebel, and et al. 2025. "Non-Traumatic Lower-Limb Amputations: Outcome, Sex-Differences, Comorbidity Patterns and Temporal Trends from 2006 to 2022" Journal of Clinical Medicine 14, no. 12: 4030. https://doi.org/10.3390/jcm14124030
APA StyleKaser, S., Radlinger, B., Blasinger, J., Koellenberger, N., Streitberger, V., Kopp, L., Bifano, E., Aziz, F., Sourij, H., Goebel, G., & Klocker, J. (2025). Non-Traumatic Lower-Limb Amputations: Outcome, Sex-Differences, Comorbidity Patterns and Temporal Trends from 2006 to 2022. Journal of Clinical Medicine, 14(12), 4030. https://doi.org/10.3390/jcm14124030








