Endometriosis and Nutrition: Therapeutic Perspectives
Abstract
1. Introduction
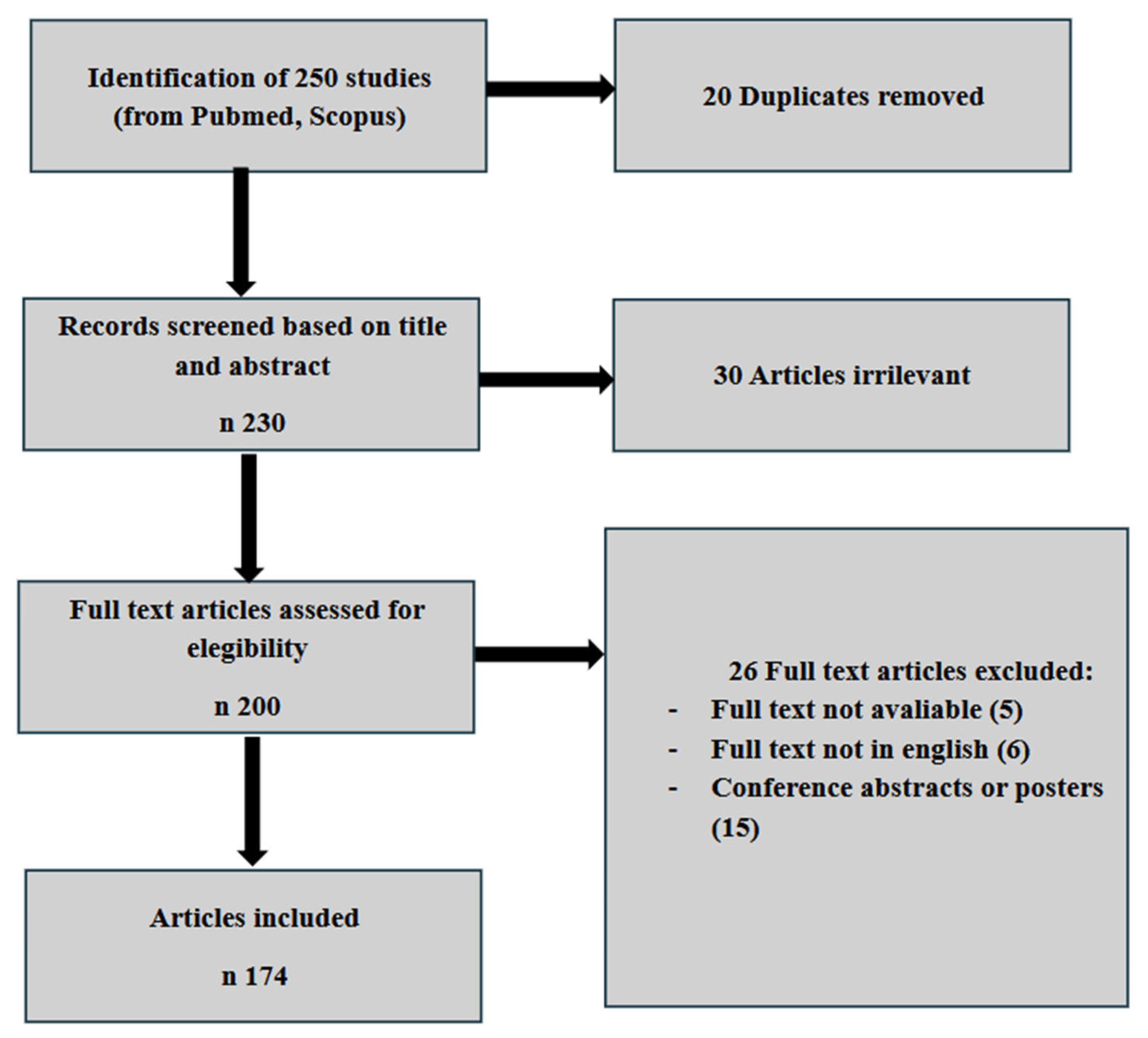
2. Materials and Methods
3. Epidemiology and Risk Factors
- Socioeconomic status
- Family history
- Gynecological factors
- Contraception
- Diet
- Physical activity
- Smoke
- Comorbidities
4. Pathogenesis of Endometriosis
5. Posterior DIE and Bowel Functional Symptoms
6. Discussion: Nutrition as a Therapeutic Strategy for Endometriosis
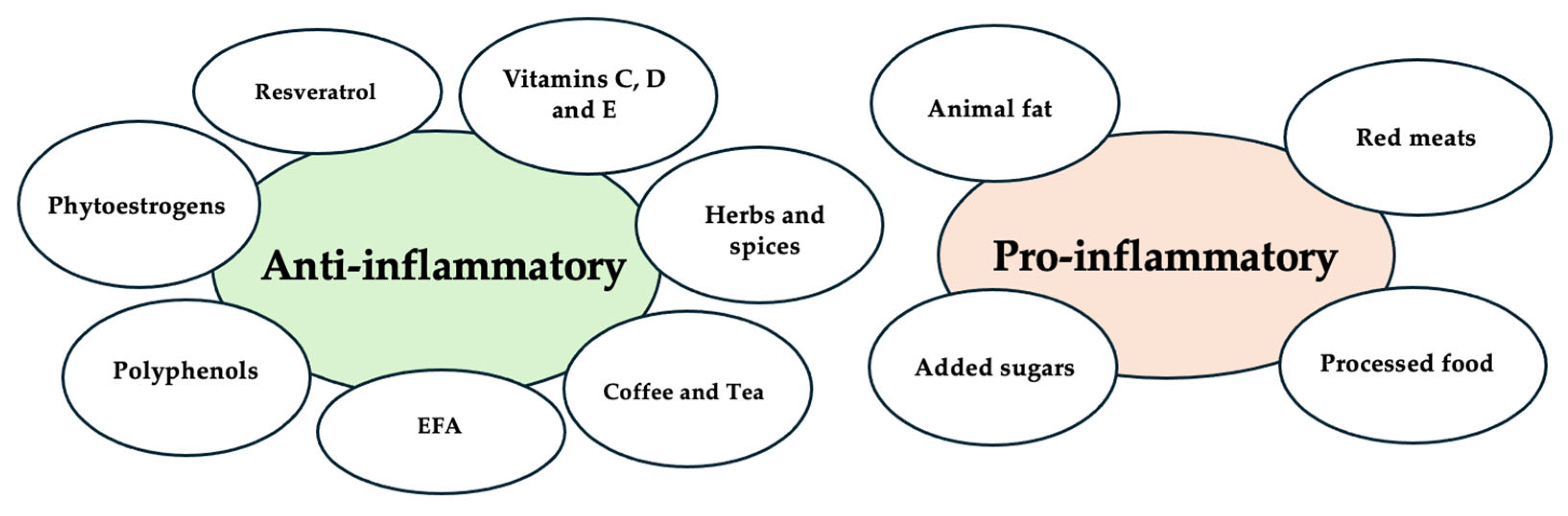
6.1. Nutritional Factor and Inflammation
- Polyunsaturated Fatty Acids (PUFAs): Omega-3 fatty acids (e.g., EPA, DHA) have anti-inflammatory properties, while omega-6 fatty acids, found in animal products, are considered pro-inflammatory. Omega-3s have been shown to reduce cardiovascular risk, rheumatoid arthritis, and cancer cachexia [125,126,127,128,129]. Supplementation with omega-3 fatty acids has shown benefits in reducing inflammatory markers and increasing body mass, although evidence remains moderate, with weak recommendations for their use in cancer [130]. Omega-3 supplementation also helps reduce the risk of coronary heart disease [125,126].
- Saturated and Trans Fatty Acids: Trans fatty acids, derived from partially hydrogenated oils, are linked to pro-inflammatory effects and increase oxidative stress [131]. The role of saturated fatty acids in inflammation remains debated, but long-chain saturated fats may promote inflammation, while short-chain fatty acids could have anti-inflammatory effects [124].
- Fiber: Fiber has recognized anti-inflammatory effects [132]. It is fermented by gut microbiota into short-chain fatty acids (SCFAs), which activate immune-regulating pathways, reducing inflammation by inhibiting NF-κB and promoting PPAR-γ [124]. Although direct evidence remains limited, the known anti-inflammatory properties of SCFAs may prove beneficial in attenuating pelvic inflammation and gastrointestinal comorbidities associated with endometriosis [133,134,135].
- Added Sugars: High consumption of added sugars amplifies pro-inflammatory effects. Elevated blood glucose levels from sugary foods can form advanced glycation end products (AGEs), which trigger oxidative stress, inflammation, and cell death. The binding of AGEs to the AGE receptor (RAGE) activates NF-κB, modulating gene expression and promoting inflammation. AGEs are implicated in chronic diseases like atherosclerosis and diabetes [137,138].
6.2. Nutritional Factor and Functional Bowel Symptoms
6.3. Nutritional Factor and Endometriosis
- Vitamins C and E: These antioxidants may act synergistically to reduce oxidative stress. Vitamin C plays roles in neutralizing free radicals, supporting enzymatic activity, collagen synthesis, and the production of catecholamines and vasopressin [162,163,164,165]. Vitamin E is known for its antioxidant, anti-inflammatory, and anti-angiogenic properties [166,167]. While some studies report no significant association between vitamin E levels and endometriosis [34], others have shown lower serum levels in affected individuals, possibly due to increased antioxidant demand [35]. Reduced vitamin C levels in follicular fluid have also been linked to endometriosis [36]. In animal models, vitamin C supplementation has significantly reduced the size and severity of lesions [37,168]. In humans, combined supplementation with vitamins C and E has been associated with reduced pain, inflammation, and oxidative stress [38,39].
- Polyphenols: They are bioactive compounds abundantly found in fruits, vegetables, and other plant-based foods, known for their potent antioxidant, anti-inflammatory, anticancer, and cardioprotective properties [124,137,169,170]. They exert their anti-inflammatory effects through multiple mechanisms, including the neutralization of reactive oxygen species (ROS), modulation of key inflammatory pathways such as NF-κB and MAPK, and inhibition of cyclooxygenases (COXs) [169]. Additionally, polyphenols contribute to gut health by promoting the growth of beneficial microbial populations, further supporting systemic anti-inflammatory activity [135]. Given these properties, polyphenols may play a valuable role in symptom management and disease modulation in endometriosis [40].
- Resveratrol: A polyphenol found in grapes, berries, and red wine, resveratrol has anti-inflammatory and anti-proliferative properties [172]. Laboratory studies show its ability to suppress inflammatory and growth-related pathways [42,43]. Preliminary clinical research combining resveratrol with hormonal treatments has shown symptom reduction, especially pelvic pain [45], although further studies are needed [45].
- Essential fatty acids: Omega-3s—found in fatty fish, nuts, and seeds—are well known for their anti-inflammatory properties. Higher omega-3 levels have been associated with reduced endometriosis risk or symptoms [49]. The role of omega-6 is more complex, and maintaining a proper balance may be key [50].
- Red meat and processed foods: High consumption of red meat and processed foods has been associated with increased risk of endometriosis in observational studies. Additionally, such dietary patterns have been linked in the broader literature to elevated levels of inflammatory markers and adverse hormonal profiles, supporting recommendations to limit these foods in favor of plant-based proteins and whole grains [52].
6.4. Comparison with Previous Studies
6.5. Limitations and Strengths of the Study
6.6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Martire, F.G.; Costantini, E.; D’Abate, C.; Schettini, G.; Sorrenti, G.; Centini, G.; Zupi, E.; Lazzeri, L. Endometriosis and Adenomyosis: From Pathogenesis to Follow-Up. Curr. Issues Mol. Biol. 2025, 47, 298. [Google Scholar] [CrossRef]
- Vercellini, P.; Bandini, V.; Viganò, P.; Di Stefano, G.; Merli, C.E.M.; Somigliana, E. Proposal for targeted, neo-evolutionary-oriented, secondary prevention of early-onset endometriosis and adenomyosis. Part I: Pathogenic aspects. Hum. Reprod. 2024, 39, 1–17. [Google Scholar] [CrossRef]
- Vercellini, P.; Bandini, V.; Viganò, P.; Ambruoso, D.; Cetera, G.E.; Somigliana, E. Proposal for targeted, neo-evolutionary-oriented secondary prevention of early-onset endometriosis and adenomyosis. Part II: Medical interventions. Hum. Reprod. 2024, 39, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Martire, F.G.; Giorgi, M.; D’abate, C.; Colombi, I.; Ginetti, A.; Cannoni, A.; Fedele, F.; Exacoustos, C.; Centini, G.; Zupi, E.; et al. Deep Infiltrating Endometriosis in Adolescence: Early Diagnosis and Possible Prevention of Disease Progression. J. Clin. Med. 2024, 13, 550. [Google Scholar] [CrossRef]
- Neri, B.; Russo, C.; Mossa, M.; Martire, F.G.; Selntigia, A.; Mancone, R.; Calabrese, E.; Rizzo, G.; Exacoustos, C.; Biancone, L. High frequency of deep infiltrating endometriosis in patients with inflammatory bowel disease: A nested case-control study. Dig. Dis. 2023, 41, 719–728. [Google Scholar] [CrossRef]
- Colombi, I.; Ginetti, A.; Cannoni, A.; Cimino, G.; d’Abate, C.; Schettini, G.; Giorgi, M.; Raimondo, D.; Martire, F.G.; Lazzeri, L.; et al. Combine Surgery and In Vitro Fertilization (IVF) in Endometriosis-Related Infertility: When and Why. J. Clin. Med. 2024, 13, 7349. [Google Scholar] [CrossRef]
- Cozzolino, M.; Cosentino, M.; Loiudice, L.; Martire, F.G.; Galliano, D.; Pellicer, A.; Exacoustos, C. Impact of adenomyosis on in vitro fertilization outcomes in women undergoing donor oocyte transfers: A prospective observational study. Fertil. Steril. 2024, 121, 480–488. [Google Scholar] [CrossRef]
- Martire, F.G.; D’abate, C.; Schettini, G.; Cimino, G.; Ginetti, A.; Colombi, I.; Cannoni, A.; Centini, G.; Zupi, E.; Lazzeri, L. Adenomyosis and Adolescence: A Challenging Diagnosis and Complex Management. Diagnostics 2024, 14, 2344. [Google Scholar] [CrossRef]
- Exacoustos, C.; Morosetti, G.; Conway, F.; Camilli, S.; Martire, F.G.; Lazzeri, L.; Piccione, E.; Zupi, E. New Sonographic Classification of Adenomyosis: Do Type and Degree of Adenomyosis Correlate to Severity of Symptoms? J. Minim. Invasive Gynecol. 2020, 27, 1308–1315. [Google Scholar] [CrossRef]
- Exacoustos, C.; Lazzeri, L.; Martire, F.G.; Russo, C.; Martone, S.; Centini, G.; Piccione, E.; Zupi, E. Ultrasound findings of adenomyosis in adolescents: Type and grade of the disease. J. Minim. Invasive Gynecol. 2021, 29, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Viganò, P.; Candiani, M.; Fedele, L. Diet and endometriosis risk: A literature review. Reprod. Biomed. Online 2013, 26, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Abbiati, A.; Vigano, P.; Somigliana, E.; Daguati, R.; Meroni, F.; Crosignani, P. Asymmetry in distribution of dia-phragmatic endometriotic lesions: Evidence in favour of the menstrual reflux theory. Hum. Reprod. 2007, 22, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Horne, A.W.; Missmer, S.A. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef]
- Martire, F.G.; Russo, C.; Selntigia, A.; Nocita, E.; Soreca, G.; Lazzeri, L.; Zupi, E.; Exacoustos, C. Early noninvasive diagnosis of endometriosis: Dysmenorrhea and specific ultrasound findings are important indicators in young women. Fertil. Steril. 2023, 119, 455–464. [Google Scholar] [CrossRef]
- Weiss, E.A.; Gandhi, M. Preferential cyclooxygenase 2 inhibitors as a nonhormonal method of emergency contraception: A look at the evidence. J. Pharm. Pract. 2016, 29, 160–164. [Google Scholar] [CrossRef]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels with inflammatory processes. Endocr. Rev. 2018, 40, 369–416. [Google Scholar] [CrossRef]
- Berbic, M.; Schulke, L.; Markham, R.; Tokushige, N.; Russell, P.; Fraser, I.S. Macrophage expression in endometrium of women with and without endometriosis. Hum. Reprod. 2009, 24, 325–332. [Google Scholar] [CrossRef]
- Zulfikaroglu, E.; Kılıc, S.; Islimye, M.; Aydin, M.; Zergeroglu, S.; Batioglu, S. Efficacy of anti-tumor necrosis factor therapy on endometriosis in an experimental rat model. Arch. Gynecol. Obstet. 2011, 283, 799–804. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis-based diagnosis and treatment of endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Gomel, V.; Martin, D.C. Peritoneal fluid progesterone and progesterone resistance in super-ficial endometriosis lesions. Hum. Reprod. 2022, 37, 203–211. [Google Scholar]
- Gantenbein, K.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Argento, F.R.; Becatti, M.; Fiorillo, C.; Coccia, M.E.; Fatini, C. Mediterranean Diet and Oxidative Stress: A Relationship with Pain Perception in Endometriosis. Int. J. Mol. Sci. 2023, 24, 14601. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.S.; Gibson, P.R.; Perry, R.E.; Burgell, R.E. Endometriosis in patients with irritable bowel syndrome: Specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Chang, W.-P.; Chang, Y.-H.; Li, C.-P.; Chuang, C.-M. The risk of irritable bowel syndrome in patients with endometriosis during a 5-year fol-low-up: A nationwide population-based cohort study. Int. J. Color. Dis. 2015, 30, 907–912. [Google Scholar] [CrossRef]
- Chiaffarino, F.; Cipriani, S.; Ricci, E.; Mauri, P.A.; Esposito, G.; Barretta, M.; Vercellini, P.; Parazzini, F. Endometriosis and irritable bowel syndrome: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021, 303, 17–25. [Google Scholar] [CrossRef]
- Heard, M.E.; Melnyk, S.B.; Simmen, F.A.; Yang, Y.; Pabona, J.M.P.; Simmen, R.C.M. High-fat diet promotion of endometriosis in an immunocompetent mouse model is associated with altered peripheral and ectopic lesion redox and inflammatory status. Endocrinology 2016, 157, 2870–2882. [Google Scholar] [CrossRef]
- Della Corte, L.; Di Filippo, C.; Gabrielli, O.; Reppuccia, S.; La Rosa, V.L.; Ragusa, R.; Fichera, M.; Commodari, E.; Bifulco, G.; Giampaolino, P. The burden of endometriosis on women’s lifespan: A narrative overview on quality of life and psychosocial wellbeing. Int. J. Environ. Res. Public Health 2020, 17, 4683. [Google Scholar] [CrossRef]
- Jurkiewicz-Przondziono, J.; Lemm, M.; Kwiatkowska-Pamuła, A.; Ziółko, E.; Wójtowicz, M.K. Influence of diet on the risk of developing endometriosis. Ginekol. Pol. 2017, 88, 96–102. [Google Scholar] [CrossRef]
- Trabert, B.; Peters, U.; De Roos, A.J.; Scholes, D.; Holt, V.L. Diet and risk of endometriosis in a population-based case-control study. Br. J. Nutr. 2011, 105, 459–467. [Google Scholar] [CrossRef]
- Heilier, J.-F.; Donnez, J.; Nackers, F.; Rousseau, R.; Verougstraete, V.; Rosenkranz, K.; Donnez, O.; Grandjean, F.; Lison, D.; Tonglet, R. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: A matched case-control study. Environ. Res. 2007, 103, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, M.G.; Jordão Jr, A.A.; Ferriani, R.A.; Navarro, P.A. Oocyte Oxidative DNA Damage May Be Involved in Minimal/Mild Endometriosis-Related Infertility. Mol. Reprod. Dev. 2018, 85, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Ekici, E.I.; Güney, M.; Nazıroğlu, M. Protective Effect of Cabergoline on Mitochondrial Oxidative Stress-Induced Apoptosis Is Mediated by Modulations of TRPM2 in Neutrophils of Patients with Endometriosis. J. Bioenerg. Biomembr. 2020, 52, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wu, Z.; Wang, M.; Cheng, W. Effects of Vitamin C on the Outcome of in Vitro Fertilization–Embryo Transfer in Endome-triosis: A Randomized Controlled Study. J. Int. Med. Res. 2018, 46, 4624–4633. [Google Scholar] [CrossRef]
- Hoorsan, H.; Simbar, M.; Tehrani, F.R.; Fathi, F.; Mosaffa, N.; Riazi, H.; Akradi, L.; Nasseri, S.; Bazrafkan, S. The Effectiveness of Antioxidant Therapy (Vitamin C) in an Experimentally Induced Mouse Model of Ovarian Endometriosis. Womens Health 2022, 18, 174550572210962. [Google Scholar] [CrossRef]
- Amini, L.; Chekini, R.; Nateghi, M.R.; Haghani, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res. Manag. 2021, 2021, 5. [Google Scholar] [CrossRef]
- Santanam, N.; Kavtaradze, N.; Murphy, A.; Dominguez, C.; Parthasarathy, S. Antioxidant Supplementation Reduces Endometriosis-Related Pelvic Pain in Humans. Transl. Res. 2013, 161, 189–195. [Google Scholar] [CrossRef]
- Dull, A.-M.; Moga, M.A.; Dimienescu, O.G.; Sechel, G.; Burtea, V.; Anastasiu, C.V. Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways. Molecules 2019, 24, 667. [Google Scholar] [CrossRef]
- Bartiromo, L.; Schimberni, M.; Villanacci, R.; Ottolina, J.; Dolci, C.; Salmeri, N.; Viganò, P.; Candiani, M. Endometriosis and Phytoestrogens: Friends or Foes? A Systematic Review. Nutrients 2021, 13, 2532. [Google Scholar] [CrossRef] [PubMed]
- Cenksoy, P.O.; Oktem, M.; Erdem, O.; Karakaya, C.; Cenksoy, C.; Erdem, A.; Guner, H.; Karabacak, O. A Potential Novel Treatment Strategy: Inhibition of Angiogenesis and Inflammation by Resveratrol for Regression of Endometriosis in an Experimental Rat Model. Gynecol. Endocrinol. 2014, 31, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; Aydin, N.; Celik, O.; Yilmaz, E.; Ozerol, E.; Tanbek, K. Resveratrol Successfully Treats Experimental Endometriosis through Modulation of Oxida-tive Stress and Lipid Peroxidation. J. Cancer Res. Ther. 2014, 10, 324–329. [Google Scholar] [CrossRef]
- Maia Jr, H.; Haddad, C.; Pinheiro, N.; Casoy, J. Advantages of the Association of Resveratrol with Oral Contraceptives for Man-agement of Endometriosis-Related Pain. Int. J. Women’s Health 2012, 4, 543–549. [Google Scholar] [CrossRef]
- Meresman, G.F.; Götte, M.; Laschke, M.W. Plants as Source of New Therapies for Endometriosis: A Review of Preclinical and Clinical Studies. Hum. Reprod. Update 2020, 27, 367–392. [Google Scholar] [CrossRef]
- Signorile, P.G.; Viceconte, R.; Baldi, A. Novel Dietary Supplement Association Reduces Symptoms in Endometriosis Patients. J. Cell Physiol. 2018, 233, 5920–5925. [Google Scholar] [CrossRef]
- Fadin, M.; Nicoletti, M.C.; Pellizzato, M.; Accardi, M.; Baietti, M.G.; Fratter, A. Effectiveness of the Integration of Quercetin, Turmeric, and N-Acetylcysteine in Reducing Inflammation and Pain Associated with Endometriosis. In-Vitro and In-Vivo Studies. Minerva Ginecol. 2020, 72, 285–291. [Google Scholar] [CrossRef]
- Kechagias, K.S.; Triantafyllidis, K.K.; Kyriakidou, M.; Giannos, P.; Kalliala, I.; Veroniki, A.A.; Paraskevaidi, M.; Kyrgiou, M. The Relation Between Caffeine Consumption and Endometriosis: An Updated Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3457. [Google Scholar] [CrossRef] [PubMed]
- Hopeman, M.M.; Riley, J.K.; Frolova, A.I.; Jiang, H.; Jungheim, E.S. Serum Polyunsaturated Fatty Acids and Endometriosis. Reprod. Sci. 2014, 22, 1083–1087. [Google Scholar] [CrossRef]
- Pereira, F.E.X.G.; Medeiros, F.d.C.; Rocha, H.A.L.; da Silva, K.S. Effects of Omega-6/3 and Omega-9/6 Nutraceuticals on Pain and Fertility in Peritoneal Endometriosis in Rats. Acta Cir. Bras. 2019, 34, e201900405. [Google Scholar] [CrossRef]
- Ghanavatinejad, A.; Rashidi, N.; Mirahmadian, M.; Rezania, S.; Mosalaei, M.; Ghasemi, J.; Zarnani, A.-H. Vitamin D3 Controls TLR4- and TLR2-Mediated Inflammatory Re-sponses of Endometrial Cells. Gynecol. Obstet. Investig. 2021, 86, 139–148. [Google Scholar] [CrossRef]
- Yamamoto, A.; Harris, H.R.; Vitonis, A.F.; Chavarro, J.E.; Missmer, S.A. A Prospective Cohort Study of Meat and Fish Consumption and Endometriosis Risk. Am. J. Obstet. Gynecol. 2018, 219, 178.e1–178.e10. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.; Kulkarni, M.T.; Missmer, S.A. Is endometriosis more common and more severe than it was 30 years ago? J. Minim. Invasive Gynecol. 2020, 27, 452–461. [Google Scholar] [CrossRef]
- Parazzini, F.; Roncella, E.; Cipriani, S.; Trojano, G.; Barbera, V.; Herranz, B.; Colli, E. The frequency of endometriosis in the general and selected populations: A systematic review. J. Endometr. Pelvic Pain Disord. 2020, 12, 176–189. [Google Scholar] [CrossRef]
- Sarria-Santamera, A.; Orazumbekova, B.; Terzic, M.; Issanov, A.; Chaowen, C.; Asúnsolo-Del-Barco, A. Systematic review and me-ta-analysis of incidence and prevalence of endometriosis. Healthcare 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Martire, F.G.; Russo, C.; Selntigia, A.; Siciliano, T.; Lazzeri, L.; Piccione, E.; Zupi, E.; Exacoustos, C. Transvaginal ultrasound evaluation of the pelvis and symptoms after laparoscopic partial cystectomy for bladder endometriosis. J. Turk. Ger. Gynecol. Assoc. 2022, 23, 145. [Google Scholar] [CrossRef] [PubMed]
- Martire, F.G.; Zupi, E.; Lazzeri, L.; Morosetti, G.; Conway, F.; Centini, G.; Solima, E.; Pietropolli, A.; Piccione, E.; Exacoustos, C. Transvaginal Ultrasound Findings After Laparoscopic Rectosigmoid Segmental Resection for Deep Infiltrating Endometriosis. J. Ultrasound Med. 2021, 40, 1219–1228. [Google Scholar] [CrossRef]
- Guo, S.W.; Wang, Y. The prevalence of endometriosis in women with chronic pelvic pain. Gynecol. Obstet. Investig. 2006, 62, 121–130. [Google Scholar] [CrossRef]
- Cramer, D.W.; Wilson, E.; Stillman, R.J.; Berger, M.J.; Belisle, S.; Schiff, I.; Albrecht, B.; Gibson, M.; Stadel, B.V.; Schoenbaum, S.C. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 1986, 255, 1904–1908. [Google Scholar] [CrossRef]
- Signorello, L.B.; Harlow, B.L.; Cramer, D.W.; Spiegelman, D.; Hill, J.A. Epidemiologic determinants of endometriosis: A hospital-based case–control study. Ann. Epidemiol. 1997, 7, 267–274. [Google Scholar] [CrossRef]
- Marmot, M.; Feeney, A. General explanations for social inequalities in health. IARC Sci. Publ. 1997, 138, 207–228. [Google Scholar]
- Vigano, P.; Somigliana, E.; Vignali, M.; Busacca, M.; Di Blasio, A.M. Genetics of endometriosis: Current status and prospects. Front. Biosci. 2007, 12, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Candiani, G.B.; Danesino, V.; Gastaldi, A.; Parazzini, F.; Ferraroni, M. Reproductive and menstrual factors and risk of peritoneal and ovarian endometriosis. Fertil. Steril. 1991, 56, 230–234. [Google Scholar] [CrossRef]
- Parazzini, F.; Ferraroni, M.; Fedele, L.; Bocciolone, L.; Rubessa, S.; Riccardi, A. Pelvic endometriosis: Reproductive and menstrual risk factors at different stages in Lombardy, northern Italy. J. Epidemiol. Community Health 1995, 49, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Chavarro, J.E.; Malspeis, S.; Bertone-Johnson, E.R.; Hornstein, M.D.; Spiegelman, D.; Barbieri, R.L.; Willett, W.C.; Hankinson, S.E. A prospective study of dietary fat consumption and endometriosis risk. Hum. Reprod. 2010, 25, 1528–1535. [Google Scholar] [CrossRef]
- Parazzini, F.; Chiaffarino, F.; Surace, M.; Chatenoud, L.; Cipriani, S.; Chiantera, V.; Benzi, G.; Fedele, L. Selected food intake and risk of endometriosis. Hum. Reprod. 2004, 19, 1755–1759. [Google Scholar] [CrossRef]
- Friberg, E.; Wallin, A.; Wolk, A. Sucrose, high-sugar foods, and risk of endometrial cancer—A population-based cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1831–1837. [Google Scholar] [CrossRef]
- Wu, M.H.; Shoji, Y.; Chuang, P.C.; Tsai, S.J. Endometriosis: Disease pathophysiology and the role of prostaglandins. Expert Rev. Mol. Med. 2007, 9, 1–20. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Bijon, A.; Clavel-Chapelon, F.; Mesrine, S.; Boutron-Ruault, M.C. Childhood and adolescent exposures and the risk of endometriosis. Epidemiology 2013, 24, 261–269. [Google Scholar] [CrossRef]
- Vitonis, A.F.; Hankinson, S.E.; Hornstein, M.D.; Missmer, S.A. Adult physical activity and endometriosis risk. Epidemiology 2010, 21, 16–23. [Google Scholar] [CrossRef]
- Gonçalves, R.B.; Coletta, R.D.; Silvério, K.G.; Benevides, L.; Casati, M.Z.; da Silva, J.S.; Nociti, F.H. Impact of smoking on inflammation: Overview of molecular mechanisms. Inflamm. Res. 2011, 60, 409–424. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A high-risk population for major chronic diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.M.; Jorgensen, K.T.; Pedersen, B.V.; Rostgaard, K.; Frisch, M. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjogren syndrome. Hum. Reprod. 2011, 26, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Costenbader, K.H.; Mu, F.; Kvaskoff, M.; Malspeis, S.; Karlson, E.W.; Missmer, S.A. Endometriosis and the risks of systemic lupus erythematosus and rheumatoid arthritis in the Nurses’ Health Study II. Ann. Rheum. Dis. 2015, 75, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, J.; Arendt-Nielsen, L. Evolutionary considerations in the development of chronic pelvic pain. Am. J. Obstet. Gynecol. 2016, 215, 201.e1–201.e4. [Google Scholar] [CrossRef]
- Jarrell, J. The significance and evolution of menstruation. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 50, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Hsu, T.F.; Jiang, L.Y.; Chan, I.S.; Shih, Y.C.; Chang, Y.H.; Wang, P.H.; Chen, Y.J. Maintenance therapy for preventing endometrio-ma recurrence after endometriosis resection surgery—A systematic review and network meta-analysis. J. Minim. Invasive Gynecol. 2022, 29, 602–612. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Ve-nous Circulation. Am. J. Pathol. 1927, 3, 93–110. [Google Scholar]
- Porpora, M.G.; Scaramuzzino, S.; Sangiuliano, C.; Piacenti, I.; Bonanni, V.; Piccioni, M.G.; Ostuni, R.; Masciullo, L.; Benedetti Panici, P.L. High prevalence of autoimmune diseases in women with endometriosis: A case-control study. Gynecol. Endocrinol. 2020, 36, 356–359. [Google Scholar] [CrossRef]
- Greenbaum, H.; Weil, C.; Chodick, G.; Shalev, V.; Eisenberg, V.H. Evidence for an association between endometriosis, fibromyalgia, and autoimmune diseases. Am. J. Reprod. Immunol. 2019, 81, e13095. [Google Scholar] [CrossRef]
- Leyendecker, G.; Kunz, G.; Noe, M.; Herbertz, M.; Mall, G. Endometriosis: A dysfunction and disease of the archimetra. Hum. Reprod. Update 1998, 4, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P.; Mall, G.; Böttcher, B.; Wildt, L. Adenomyosis and endometrio-sis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2014, 291, 917–932. [Google Scholar] [CrossRef]
- Cousins, F.L.; Gargett, C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 27–38. [Google Scholar] [CrossRef]
- Ibrahim, M.G.; Sillem, M.; Plendl, J.; Chiantera, V.; Sehouli, J.; Mechsner, S. Myofibroblasts Are Evidence of Chronic Tissue Microtrauma at the Endometrial-Myometrial Junctional Zone in Uteri with Adenomyosis. Reprod. Sci. 2017, 24, 1410–1418. [Google Scholar] [CrossRef]
- Gulino, F.A.; Dilisi, V.; Capriglione, S.; Cannone, F.; Catania, F.; Martire, F.G.; Tuscano, A.; Gulisano, M.; D’urso, V.; Di Stefano, A.; et al. Anti-Mullerian Hormone (AMH) and adenomyosis: Mini-review of literature of the last 5 years. Front. Endocrinol. 2022, 13, 1014519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometri-osis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Barlow, D.; Kennedy, S. Implantation versus infiltration: The Sampson versus the endometriotic disease theory. Gynecol. Obstet. Investig. 1999, 47 (Suppl. S1), 3–9. [Google Scholar] [CrossRef]
- Koninckx, P.R. Is mild endometriosis a condition occurring intermittently in all women? Hum. Reprod. 1994, 9, 2202–2205. [Google Scholar] [CrossRef]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef]
- Krakauer, D.C.; Plotkin, J.B. Redundancy, antiredundancy, and the robustness of genomes. Proc. Natl. Acad. Sci. USA 2002, 99, 1405–1409. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The pathogenesis of endometriosis: Mo-lecular and cell biology insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef]
- Wilson, D.; Bordoni, B. Embryology, Mullerian Ducts (Paramesonephric Ducts). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fuldeore, M.J.; Soliman, A.M. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: National estimates from a cross-sectional survey of 59,411 women. Gynecol. Obstet. Investig. 2017, 82, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.; Sadatmahalleh, S.J.; Akhoond, M.R.; Talebi, M. Evaluation of risk factors associated with endometriosis in infer-tile women. Int. J. Fertil. Steril. 2016, 10, 11–21. [Google Scholar] [PubMed]
- Peterson, C.M.; Johnstone, E.B.; Hammoud, A.O.; Stanford, J.B.; Varner, M.W.; Kennedy, A.; Chen, Z.; Sun, L.; Fujimoto, V.Y.; Hediger, M.L.; et al. Risk factors associated with endometriosis: Importance of study population for characterizing disease in the ENDO Study. Am. J. Obstet. Gynecol. 2013, 208, 451.e1–451.e11. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Marshall, L.M.; Hunter, D.J. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am. J. Epidemiol. 2004, 160, 784–796. [Google Scholar] [CrossRef]
- Saha, R.; Marions, L.; Tornvall, P. Validity of self-reported endometriosis and endometriosis-related questions in a Swedish female twin cohort. Fertil. Steril. 2017, 107, 174–178.e2. [Google Scholar] [CrossRef]
- Gulisano, M.; Gulino, F.A.; Incognito, G.; Cimino, M.; Dilisi, M.; Di Stefano, A.; D’Urso, V.; Cannone, F.; Martire, F.G.; Palumbo, M. Role of hysteroscopy on infertility: The eternal dilemma. Clin. Exp. Obstet. Gynecol. 2023, 50, 99. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T.; Study, W.E.R.F.G. Impact of endometriosis on quality of life and work productivity: A multi-center study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef]
- Miller-Matero, L.R.; Saulino, C.; Clark, S.; Bugenski, M.; Eshelman, A.; Eisenstein, D. When treating the pain is not enough: A multidisciplinary approach for chron-ic pelvic pain. Arch. Womens Ment. Health 2016, 19, 349–354. [Google Scholar] [CrossRef]
- Nowakowski, A.C. Chronic inflammation and quality of life in older adults: A cross-sectional study using biomarkers to predict emotional and relational outcomes. Health Qual. Life Outcomes 2014, 12, 141. [Google Scholar] [CrossRef]
- Moradi, M.; Parker, M.; Sneddon, A.; Lopez, V.; Ellwood, D. Impact of endometriosis on women’s lives: A qualitative study. BMC Womens Health 2014, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Selntigia, A.; Exacoustos, C.; Ortoleva, C.; Russo, C.; Monaco, G.; Martire, F.G.; Rizzo, G.; Della-Morte, D.; Mercuri, N.B.; Albanese, M. Correlation between endometriosis and migraine features: Results from a prospective case-control study. Cephalalgia 2024, 44, 33310. [Google Scholar] [CrossRef] [PubMed]
- Machairiotis, N.; Stylianaki, A.; Dryllis, G.; Zarogoulidis, P.; Kouroutou, P.; Tsiamis, N.; Katsikogiannis, N.; Sarika, E.; Courcoutsakis, N.; Tsiouda, T.; et al. Extrapelvic endometriosis: A rare entity or an underdiagnosed condition? Diagn. Pathol. 2013, 8, 194. [Google Scholar] [CrossRef]
- Martire, F.G.; Lazzeri, L.; Conway, F.; Siciliano, T.; Pietropolli, A.; Piccione, E.; Solima, E.; Centini, G.; Zupi, E.; Exacoustos, C. Adolescence and Endometriosis: Symptoms, Ultrasound Signs, and Early Diagnosis. Fertil. Steril. 2020, 114, 1049–1057. [Google Scholar] [CrossRef]
- Martire, F.G.; Piccione, E.; Exacoustos, C.; Zupi, E. Endometriosis and Adolescence: The Impact of Dysmenorrhea. J. Clin. Med. 2023, 12, 5624. [Google Scholar] [CrossRef]
- DiVasta, A.D.; Vitonis, A.F.; Laufer, M.R.; Missmer, S.A. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. Am. J. Obstet. Gynecol. 2018, 218, 324.e1–324.e11. [Google Scholar] [CrossRef]
- Lazzeri, L.; Andersson, K.L.; Angioni, S.; Arena, A.; Arena, S.; Bartiromo, L.; Berlanda, N.; Bonin, C.; Candiani, M.; Centini, G.; et al. How to Manage Endometriosis in Adolescence: The Endometriosis Treatment Italian Club Approach. J. Minim. Invasive Gynecol. 2023, 30, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Canis, M.; Pouly, J.L.; Rabischong, B.; Botchorishvili, R.; Mage, G. Relationship between delay of surgical diag-nosis and severity of disease in patients with symptomatic deep infiltrating endometriosis. Fertil. Steril. 2006, 86, 1314–1316. [Google Scholar] [CrossRef]
- Hudelist, G.; Fritzer, N.; Thomas, A.; Niehues, C.; Oppelt, P.; Haas, D.; Tammaa, A.; Salzer, H. Diagnostic delay for endome-triosis in Austria and Germany: Causes and possible consequences. Hum. Reprod. 2012, 27, 3412–3416. [Google Scholar] [CrossRef]
- Vercellini, P.; Chapron, C.; Fedele, L.; Gattei, U.; Daguati, R.; Crosignani, P.G. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG An Int. J. Obstet. Gynaecol. 2004, 111, 1213–1217. [Google Scholar] [CrossRef]
- Nezhat, C.; Li, A.; Falik, R.; Copeland, D.; Razavi, G.; Shakib, A.; Mihailide, C.; Bamford, H.; DiFrancesco, L.; Tazuke, S.; et al. Bowel endometriosis: Diagnosis and management. Am. J. Obstet. Gynecol. 2018, 218, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P. Endometriosis: What a pain it is. Semin. Reprod. Endocrinol. 1997, 15, 251–261. [Google Scholar] [CrossRef]
- Roman, H.; Ness, J.; Suciu, N.; Bridoux, V.; Gourcerol, G.; Leroi, A.M.; Tuech, J.J.; Ducrotté, P.; Savoye-Collet, C.; Savoye, G. Are digestive symptoms in women presenting with pelvic endometriosis specific to lesion localizations? A preliminary prospective study. Hum. Reprod. 2012, 27, 3440–3449. [Google Scholar] [CrossRef]
- Trencheva, K.; Morrissey, K.P.; Wells, M.; Mancuso, C.A.; Lee, S.W.; Sonoda, T.; Michelassi, F.; Charlson, M.E.; Milsom, J.W. Identifying important predictors for anastomotic leak after colon and rectal resection: Prospective study on 616 patients. Ann. Surg. 2013, 257, 108–113. [Google Scholar] [CrossRef]
- Vercellini, P.; Carmignani, L.; Rubino, T.; Barbara, G.; Abbiati, A.; Fedele, L. Surgery for deep endometriosis: A pathogenesis-oriented approach. Gynecol. Obstet. Investig. 2009, 68, 88–103. [Google Scholar] [CrossRef]
- Morley, J.E.; Thomas, D.R.; Wilson, M.M. Cachexia: Pathophysiology and clinical relevance. Am. J. Clin. Nutr. 2006, 83, 735–743. [Google Scholar] [CrossRef]
- Preiser, J.C.; Ichai, C.; Orban, J.C.; Groeneveld, A.B. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Muller, N.A.; Kaegi-Braun, N.; Durmisi, M.; Gressies, C.; Tribolet, P.; Stanga, Z.; Mueller, B.; Schuetz, P. Low T3 syndrome up-on admission and response to nutritional support in malnourished medical inpatients. J. Clin. Endocrinol. Metab. 2022, 108, e240–e248. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, O.; Preiser, J.C. Role of Nutrition Support in Inflammatory Conditions. Nutr. Clin. Pract. 2017, 32, 310–317. [Google Scholar] [CrossRef]
- Kuhlmann, M.K.; Levin, N.W. Potential interplay between nutrition and inflammation in dialysis patients. Contrib. Nephrol. 2008, 161, 76–82. [Google Scholar]
- Oner-Iyidogan, Y.; Gurdol, F.; Kocak, H.; Oner, P.; Cetinalp-Demircan, P.; Caliskan, Y.; Kocak, T.; Turkmen, A. Appetite-regulating hormones in chronic kidney disease patients. J. Ren. Nutr. 2011, 21, 316–321. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Mul-ler, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Ajabnoor, S.M.; O’Brien, A.T.; Winstanley, L.E.; Donaldson, D.H.; et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD011094. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Bäck, M.; Hansson, G.K. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. Faseb. J. 2019, 33, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Raad, T.; Griffin, A.; George, E.S.; Larkin, L.; Fraser, A.; Kennedy, N.; Tierney, A.C. Dietary Interventions with or without Omega-3 Supplementation for the Management of Rheumatoid Arthritis: A Systematic Review. Nutrients 2021, 13, 3506. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Okugawa, Y.; Hishida, A.; Ogawa, A.; Okamoto, K.; Shintani, M.; Morimoto, Y.; Nishikawa, R.; Yokoe, T.; Tanaka, K.; et al. Fish oil-enriched nutrition combined with systemic chemotherapy for gastrointestinal cancer patients with cancer cachexia. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Solís-Martínez, O.; Plasa-Carvalho, V.; Phillips-Sixtos, G.; Trujillo-Cabrera, Y.; Hernández-Cuellar, A.; Queipo-García, G.E.; Meaney-Mendiolea, E.; Ceballos-Reyes, G.M.; Fuchs-Tarlovsky, V. Effect of Eicosapentaenoic Acid on Body Composition and Inflammation Markers in Patients with Head and Neck Squamous Cell Cancer from a Public Hospital in Mexico. Nutr. Cancer 2018, 70, 663–670. [Google Scholar] [CrossRef]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taladrid, D.; Rebollo-Hernanz, M.; Martin-Cabrejas, M.A.; Moreno-Arribas, M.V.; Bartolomé, B. Grape Pomace as a Cardiometabolic Health-Promoting Ingredient: Activity in the Intestinal Environment. Antioxidants 2023, 12, 979. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Chang, S.K.; Kris-Etherton, P.M.; Sullivan, V.K.; Petersen, K.S.; Guasch-Ferré, M.; Jenkins, D.J.A. Dried Fruits: Bioactives, Effects on Gut Microbiota, and Possible Health Benefits-An Update. Nutrients 2023, 15, 1611. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Stanišić, N.; Stajić, S.B.; Dmitrić, M.; Živković, S.; Kurćubić, L.V.; Živković, V.; Jakovljević, V.; Mašković, P.Z.; Mašković, J. Valorizing Grape Pomace: A Review of Applications, Nutritional Benefits, and Potential in Functional Food Development. Foods 2024, 13, 4169. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Kuzan, A. Toxicity of advanced glycation end products (Review). Biomed. Rep. 2021, 14, 46. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide prevalence and burden of func-tional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology 2021, 160, 99–114. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Black, C.J.; EBurr, N.; Camilleri, M.; Earnest, D.L.; Quigley, E.M.; Moayyedi, P.; AHoughton, L.; Ford, A.C. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: Systematic review and network meta-analysis. Gut 2020, 69, 74–82. [Google Scholar] [CrossRef]
- Black, C.J.; Burr, N.E.; Ford, A.C. Relative efficacy of tegaserod in a systematic review and network meta-analysis of licensed therapies for irritable bowel syndrome with constipation. Clin. Gastroenterol. Hepatol. 2020, 18, 1238–1239. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Moayyedi, P. Meta-analysis: Factors affecting placebo response rate in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2010, 32, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Moayyedi, P.; Chey, W.D.; Harris, L.A.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am. J. Gastroenterol. 2018, 113 (Suppl. S2), 1–18. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Lahner, E.; Bellentani, S.; Bastiani, R.D.; Tosetti, C.; Cicala, M.; Esposito, G.; Arullani, P.; Annibale, B. A survey of pharmacological and nonpharmaco-logical treatment of functional gastrointestinal disorders. United Eur. Gastroenterol. J. 2013, 1, 385–393. [Google Scholar] [CrossRef]
- Shepherd, S.; Parker, F.; Muir, J.; Gibson, P. Dietary triggers of abdominal symptoms in patients with irritable bowel syn-drome: Randomized placebo-controlled evidence. Clin. Gastroenterol. Hepatol. 2008, 6, 765–767. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syn-drome. Gastroenterology 2014, 146, 67–75. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. Am. J. Gastroenterol. 2018, 113, 129. [Google Scholar] [CrossRef]
- van Lanen, A.S.; de Bree, A.; Greyling, A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: A systematic re-view and meta-analysis. Eur. J. Nutr. 2021, 60, 3505–3522. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; APaine, P.; Black, C.J.; AHoughton, L.; AEveritt, H.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- Hookway, C.; Buckner, S.; Crosland, P.; Longson, D. Irritable bowel syndrome in adults in primary care: Summary of updated NICE guidance. BMJ 2015, 350, h701. [Google Scholar] [CrossRef]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H.; Gulia, P.; Horobin, J.; O’Sullivan, N.A.; Pettitt, C.; Reeves, L.B.; Seamark, L.; Williams, M.; et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016, 29, 549–575. [Google Scholar] [CrossRef] [PubMed]
- O’keeffe, M.; Jansen, C.; Martin, L.; Williams, M.; Seamark, L.; Staudacher, H.M.; Irving, P.M.; Whelan, K.; Lomer, M.C. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13154. [Google Scholar] [CrossRef]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Morganti, R.; Pancetti, A.; Bertani, L.; De Bortoli, N.; Costa, F.; Mosca, M.; Marchi, S.; et al. A low-FODMAP diet for irritable bowel syndrome: Some answers to the doubts from a long-term follow-up. Nutrients 2020, 12, 2360. [Google Scholar] [CrossRef] [PubMed]
- ECrichton, G.; Howe, P.R.; Buckley, J.D.; Coates, A.M.; Murphy, K.J.; Bryan, J. Long-term dietary intervention trials: Critical issues and challenges. Trials 2012, 13, 111. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Popli, P.; Ambati, C.R.; Tycksen, E.; Han, S.J.; EBulun, S.; Putluri, N.; Biest, S.W.; Kommagani, R. Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci. Alliance 2021, 4, e202101224. [Google Scholar] [CrossRef]
- Shan, J.; Ni, Z.; Cheng, W.; Zhou, L.; Zhai, D.; Sun, S.; Yu, C. Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch. Gynecol. Obstet. 2021, 304, 1363–1373. [Google Scholar] [CrossRef]
- Britton, J.A.; Westhoff, C.; Howe, G.; Gammon, M.D. Diet and benign ovarian tumors (United States). Cancer Causes Control CCC 2000, 11, 389–401. [Google Scholar] [CrossRef]
- Hondal, R.J. Selenium Vitaminology: The Connection between Selenium, Vitamin C, Vitamin E, and Ergothioneine. Curr. Opin. Chem. Biol. 2023, 75, 102328. [Google Scholar] [CrossRef]
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.; Levine, M. Vitamin C: The Known and the Unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-Van Straaten, H.M. Vitamin C: Should we supplement? Curr. Opin. Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Han, S. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Dai, Y.; Lin, X.; Xu, W.; Lin, X.; Huang, Q.; Shi, L.; Pan, Y.; Zhang, Y.; Zhu, Y.; Li, C.; et al. MiR-210-3p Protects Endometriotic Cells from Oxidative Stress-Induced Cell Cycle Arrest by Targeting BARD1. Cell Death Dis. 2019, 10, 144. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenol-Enriched Extracts of Prunus spinosa Fruits: Anti-Inflammatory and Antioxidant Effects in Human Immune Cells Ex Vivo in Relation to Phytochemical Profile. Molecules 2022, 27, 1691. [Google Scholar] [CrossRef]
- Youseflu, S.; Sadatmahalleh, S.J.; Mottaghi, A.; Kazemnejad, A. Dietary Phytoestrogen Intake and The Risk of Endometriosis in Iranian Women: A Case-Control Study. Int. J. Fertil. Steril. 2019, 13, 296–300. [Google Scholar]
- Novakovic, R.; Rajkovic, J.; Gostimirovic, M.; Gojkovic-Bukarica, L.; Radunovic, N. Resveratrol and Reproductive Health. Life 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and Spices—Biomarkers of Intake Based on Human Intervention Studies—A Systematic Review. Genes Nutr. 2019, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Hay, E.; Lucariello, A.; Contieri, M.; Esposito, T.; De Luca, A.; Guerra, G.; Perna, A. Therapeutic Effects of Turmeric in Several Diseases: An Overview. Chem Interact. 2019, 310, 108729. [Google Scholar] [CrossRef]
- Anaeigoudari, A.; Safari, H.; Khazdair, M.R. Effects of Nigella sativa, Camellia sinensis, and Allium sativum as Food Addi-tives on Metabolic Disorders, a Literature Review. Front. Pharmacol. 2021, 12, 762182. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Vitale, S.G.; Frangež, H.B.; Vrtačnik-Bokal, E.; D’Anna, R. Vitamin D in Human Reproduction: The More, The Better? An Evidence-Based Critical Appraisal. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4243–4251. [Google Scholar]
| Author/Title | Study Type | Nutritional Therapy | Mechanism | Improvement on Symptoms/Disease | Level of Recommendation |
|---|---|---|---|---|---|
| [26] Moore JS, Gibson PR, Perry RE, et al. Endometriosis in patients with irritable bowel syndrome: Specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust N Z J Obstet Gynaecol 2017;57:201–205. | Observational study | Low FODMAP diet | Symptom relief for IBS in endometriosis patients | Yes | B |
| [27] Wu CY, Chang WP, Chang YH, et al. The risk of irritable bowel syndrome in patients with endometriosis during a 5-year follow-up: a nationwide population-based cohort study. Int J Colorectal Dis 2015;30:907–912. | Population-based cohort study | None | Epidemiological association between IBS and endometriosis | No | B |
| [28] Chiaffarino F, Cipriani S, Ricci E, et al. Endometriosis and irritable bowel syndrome: a systematic review and meta-analysis. Arch Gynecol Obstet 2021;303:17–25. | Systematic review and meta-analysis | None | Evaluates overlap and comorbidity of endometriosis and IBS | No | A |
| [29] Heard ME, Melnyk SB, Simmen FA, et al. High-fat diet promotion of endometriosis in an immunocompetent mouse model is associated with altered peripheral and ectopic lesion redox and inflammatory status. Endocrinology 2016;157:2870–2882. | Experimental animal study | High-fat diet | Increased inflammation and oxidative stress | No | C |
| [30] Della Corte L, Di Filippo C, Gabrielli O, et al. The burden of endometriosis on women’s lifespan: a narrative overview on quality of life and psychosocial wellbeing. Int J Environ Res Public Health 2020;17:4683. | Narrative review | None | Impact on quality of life and psychological wellbeing | No | C |
| [31] Jurkiewicz-Przondziono J, Lemm M, Kwiatkowska-Pamuła A, et al. Influence of diet on the risk of developing endometriosis. Ginekol Pol 2017;88:96–102. | Review | General dietary influences | Dietary patterns may influence inflammation and hormone levels | Yes | C |
| [32] Trabert B, Peters U, De Roos AJ, et al. Diet and risk of endometriosis in a population-based case-control study. Br J Nutr 2011; 105: 459–467. | Case-control study | Dietary fat and omega-3 | High trans fats increase risk; omega-3 may reduce it | Yes | B |
| [33] Heilier JF, Donnez J, Nackers F, et al. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: a matched case-control study. Environ Res 2007; 103: 121–129. | Case-control study | Diet/environmental exposure | Link between diet/environmental toxins and endometriosis | No | B |
| [34] Da Broi MG, Jordão-Jr AA, Ferriani RA, Navarro PA. Oocyte Oxidative DNA Damage May Be Involved in Minimal/Mild Endometriosis-Related Infertility. Mol Reprod Dev 2018; 85: 128–136. | Experimental study | None | Oxidative DNA damage in oocytes linked to infertility in endometriosis | No | C |
| [35] Ekici EI, Guney M, Nazıroğlu M. Protective Effect of Cabergoline on Mitochondrial Oxidative Stress-Induced Apoptosis Is Mediated by Modulations of TRPM2 in Neutrophils of Patients with Endometriosis. J Bioenerg Biomembr 2020; 52: 131–142. | Experimental study | None (pharmacological) | Reduction of oxidative stress in immune cells | Yes | C |
| [36] Lu X, Wu Z, Wang M, Cheng W. Effects of Vitamin C on the Outcome of in Vitro Fertilization–Embryo Transfer in Endometriosis: A Randomized Controlled Study. J Int Med Res 2018; 46: 4624–4633. | Randomized Controlled Trial | Vitamin C | Improves IVF outcomes via antioxidant activity | Yes | A |
| [37] Hoorsan H, Simbar M, Tehrani FR, et al. The Effectiveness of Antioxidant Therapy (Vitamin C) in an Experimentally Induced Mouse Model of Ovarian Endometriosis. Womens Health 2022; 18: 174550572210962. | Experimental animal study | Vitamin C | Reduces oxidative stress and lesion size | Yes | C |
| [38] Amini L, Chekini R, Nateghi MR, et al. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res Manag 2021; 2021: 5529741. | Randomized Controlled Trial | Vitamin C + E | Reduction of oxidative stress markers and pelvic pain | Yes | A |
| [39] Santanam N, Kavtaradze N, Murphy A, et al. Antioxidant Supplementation Reduces Endometriosis-Related Pelvic Pain in Humans. Transl Res 2013; 161: 189–195. | Clinical trial | Antioxidant supplementation | Reduction of inflammation and pain | Yes | B |
| [40] Dull A-M, Moga MA, Dimienescu OG, et al. Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways. Molecules 2019; 24: 667. | Review | Resveratrol | Anti-inflammatory and anti-angiogenic pathways | Yes | C |
| [41] Bartiromo L, Schimberni M, Villanacci R, et al. Endometriosis and Phytoestrogens: Friends or Foes? A Systematic Review. Nutrients 2021; 13: 2532. | Systematic review | Phytoestrogens | Hormonal modulation and symptom relief | Yes | A |
| [42] Cenksoy PO, Oktem M, Erdem O, et al. A Potential Novel Treatment Strategy: Inhibition of Angiogenesis and Inflammation by Resveratrol for Regression of Endometriosis in an Experimental Rat Model. Gynecol Endocrinol 2014; 31: 219–224. | Experimental animal study | Resveratrol | Inhibits angiogenesis and inflammation in endometriosis model | Yes | C |
| [43] Yavuz S, Aydin N, Celik O, et al. Resveratrol Successfully Treats Experimental Endometriosis through Modulation of Oxidative Stress and Lipid Peroxidation. J Cancer Res Ther 2014; 10: 324–329. | Experimental animal study | Resveratrol | Reduces oxidative stress and lipid peroxidation | Yes | C |
| [44] Maia H Jr, DA Silva DM, Haddad C, et al. Advantages of the Association of Resveratrol with Oral Contraceptives for Management of Endometriosis-Related Pain. Int J Women’s Health 2012; 4: 543–549. | Clinical trial | Resveratrol + oral contraceptives | Synergistic reduction of inflammation and pain | Yes | B |
| [45] Meresman GF, Götte M, Laschke MW. Plants as Source of New Therapies for Endometriosis: A Review of Preclinical and Clinical Studies. Hum Reprod Update 2020; 27: 367–392. | Review | Plant-based compounds | Anti-inflammatory and anti-angiogenic properties | Yes | C |
| [46] Signorile PG, Viceconte R, Baldi A. Novel Dietary Supplement Association Reduces Symptoms in Endometriosis Patients. J Cell Physiol 2018; 233: 5920–5925. | Clinical study | Multicomponent dietary supplement | Reduction in inflammation and symptom severity | Yes | B |
| [47] Fadin M, Nicoletti MC, Pellizzato M, et al. Effectiveness of the Integration of Quercetin, Turmeric, and N-Acetylcysteine in Reducing Inflammation and Pain Associated with Endometriosis. In-Vitro and In-Vivo Studies. Minerva Ginecol 2020; 72: 285–291. | In-vitro and in-vivo studies | Quercetin, turmeric, NAC | Anti-inflammatory and antioxidant activity | Yes | C |
| [48] Kechagias KS, Triantafyllidis KK, Kyriakidou M, et al. The Relation Between Caffeine Consumption and Endometriosis: An Updated Systematic Review and Meta-Analysis. Nutrients 2021; 13: 3457. | Systematic review and meta-analysis | Caffeine | Investigates relationship between caffeine and endometriosis risk | No | A |
| [49] Hopeman MM, Riley JK, Frolova AI, et al. Serum Polyunsaturated Fatty Acids and Endometriosis. Reprod Sci 2014; 22: 1083–1087. | Observational study | PUFAs | Association between fatty acid profile and endometriosis | No | B |
| [50] Pereira FEXG, Medeiros FDC, Rocha HAL, Da Silva KS. Effects of Omega-6/3 and Omega-9/6 Nutraceuticals on Pain and Fertility in Peritoneal Endometriosis in Rats. Acta Cir Bras 2019; 34: e201900405. | Experimental animal study | Omega-6/3 and 9/6 | Improved pain and fertility parameters in rats | Yes | C |
| [51] Ghanavatinejad A, Rashidi N, Mirahmadian M, et al. Vitamin D3 Controls TLR4- and TLR2-Mediated Inflammatory Responses of Endometrial Cells. Gynecol Obstet Investig 2021; 86: 139–148. | Cellular study | Vitamin D3 | Regulates inflammation via TLR pathways | Yes | C |
| [52] Yamamoto A, Harris HR, Vitonis AF, Chavarro JE, Missmer SA. A Prospective Cohort Study of Meat and Fish Consumption and Endometriosis Risk. Am J Obstet Gynecol 2018; 219: 178.e1–178.e10. [CrossRef] [PubMed]. | Prospective cohort study | Meat and fish intake | Red meat associated with increased risk; fish protective | No | B |
| Dietary Element | Mechanism/Properties | Potential Effects on Endometriosis | Future Research Focus |
|---|---|---|---|
| Vitamins C and E | Antioxidant properties; anti-inflammatory and anti-angiogenic effects | Reduce oxidative stress and pain, and improve inflammation markers | Optimal dosages and long-term effects in endometriosis management |
| Polyphenols | Antioxidant, anti-inflammatory, anti-cancer, and cardiovascular benefits | Alleviate symptoms through reduced inflammation | Identifying specific polyphenol compounds most beneficial for endometriosis |
| Phytoestrogens | Plant-derived compounds mimicking estrogen; support hormonal balance | Potential protective effect, reducing risk and alleviating inflammation | Long-term impact on disease progression and hormonal balance |
| Resveratrol | Anti-inflammatory, anti-proliferative properties | Suppresses cell growth and inflammation, potential symptom relief (e.g., pelvic pain) | Confirming clinical efficacy, especially in combination with hormonal treatments |
| Herbs and Spices | Anti-inflammatory compounds (e.g., curcumin, ginger, chili) | Reducing inflammation and managing symptoms | Optimal dosages and delivery methods for curcumin and other spices |
| Coffee and Tea | Antioxidants like catechins (especially in green/white tea) | Combat oxidative stress and inflammation | Exploring caffeine’s effects on disease progression and symptom management |
| Essential Fatty Acids | Anti-inflammatory effects (especially omega-3s) | Reducing inflammation and alleviating symptoms | Investigating optimal omega-3/omega-6 ratio for inflammation reduction |
| Vitamin D | Supports immune function and inflammation regulation | Reduces pain and other symptoms of endometriosis | Assessing vitamin D supplementation’s effects on endometriosis symptoms |
| Red Meat and Processed Foods | High in pro-inflammatory components (trans fats, hormones) | Worsens inflammation and hormonal imbalances | Impact of reducing red meat and processed foods on disease progression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martire, F.G.; Costantini, E.; d’Abate, C.; Capria, G.; Piccione, E.; Andreoli, A. Endometriosis and Nutrition: Therapeutic Perspectives. J. Clin. Med. 2025, 14, 3987. https://doi.org/10.3390/jcm14113987
Martire FG, Costantini E, d’Abate C, Capria G, Piccione E, Andreoli A. Endometriosis and Nutrition: Therapeutic Perspectives. Journal of Clinical Medicine. 2025; 14(11):3987. https://doi.org/10.3390/jcm14113987
Chicago/Turabian StyleMartire, Francesco Giuseppe, Eugenia Costantini, Claudia d’Abate, Giovanni Capria, Emilio Piccione, and Angela Andreoli. 2025. "Endometriosis and Nutrition: Therapeutic Perspectives" Journal of Clinical Medicine 14, no. 11: 3987. https://doi.org/10.3390/jcm14113987
APA StyleMartire, F. G., Costantini, E., d’Abate, C., Capria, G., Piccione, E., & Andreoli, A. (2025). Endometriosis and Nutrition: Therapeutic Perspectives. Journal of Clinical Medicine, 14(11), 3987. https://doi.org/10.3390/jcm14113987






