Sutureless Scleral-Fixated Soleko Fil Carlevale Intraocular Lens and Associated Pars Plana Vitrectomy in Aphakia Management: A National Multicenter Audit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Surgical Technique
2.5. Statistical Analysis
3. Results
3.1. Visual and Refractive Outcomes
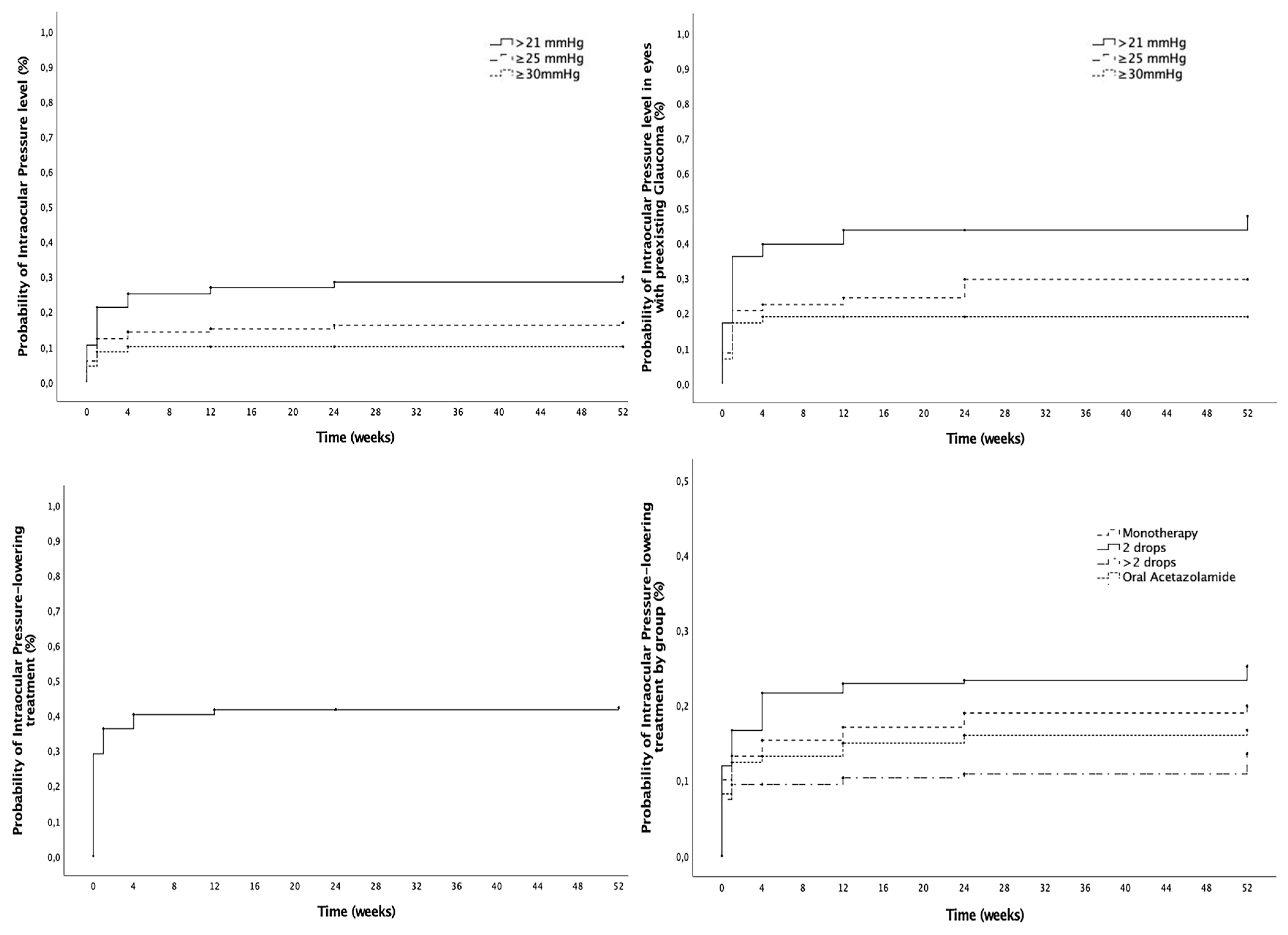
3.2. Intraocular Pressure Outcomes
3.3. Macular Edema Development and Management
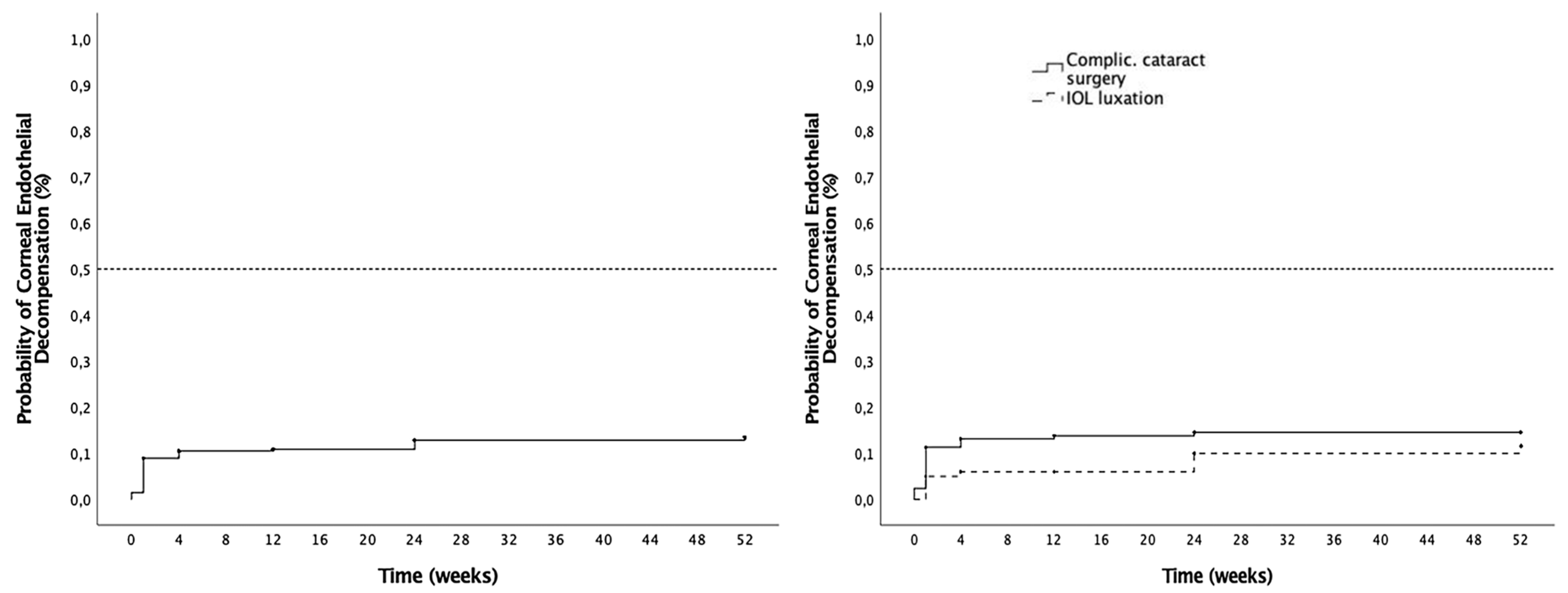
3.4. Corneal Complications
3.5. Other Complications
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSF | Sutureless scleral-fixated |
| SC-IOL | Soleko Fil Carlevale intraocular lens |
| PPV | Pars plana vitrectomy |
| BCVA | Best-corrected visual acuity |
| IOP | Intraocular pressure |
| ME | Macular edema |
Appendix A
- Sutureless Scleral-Fixated Soleko Fil Carlevale IOL Spanish study group
- (centers and principal investigators):
- Hospital Clínic of Barcelona, University of Barcelona, Barcelona, Spain
- Javier Zarranz-Ventura, Lorena Ferrer-Alapont, Carolina Bernal-Morales, Manuel J. Navarro
- Hospital Ramón y Cajal, Madrid, Spain
- Diego Ruiz-Casas, Pablo de Arriba, Carolina Arruabarrena
- Hospital Universitari Vall d’Hebron, Barcelona, Spain
- Claudia García-Arumí, Olaia Subirá, Laura Sanchez-Vela, Jose García-Arumí
- Hospital La Arruzafa, Córdoba, Spain
- Juan Manuel Cubero-Parra, Consuelo Spínola, Mercedes Giménez de Azcárate, Juan Manuel Laborda
- Hospital Universitario La Paz, Madrid, Spain
- Jose Vicente Dabad-Moreno, Mónica Asencio-Duran, Miguel Del Piñal Alvarez de Buergo, Adriana de la Hoz Polo, María Capote Diaz, Almudena del Hierro Zarzuelo, Lucas San Juan Riera, Javier Coca Robinot, Ana Boto de los Bueis, Oriana D’Anna Mardero, Mireia Ariadna Roca Cabau, Maria del Pino Cidad, Felix Armadá-Maresca
- Clínica Villoria, Pontevedra, Spain
- Daniel Velázquez-Villoria, Alvaro Velazquez-Villoria, Rolando Herrera-Vasquez, Alejandra María Parra-Morales
- Complejo Hospitalario Universitario de Santiago de Compostela, A Coruña, Spain
- Joaquin Marticorena-Salinero, Maria Jose Blanco Teijeiro, Purificación Mera Yáñez
- Hospital de Sagunto, Valencia, Spain
- Julian Zarco-Bosquet, Hector Mascaros Mena, Raquel Burggraaf Sanchez de las Matas
- Hospital Universitario San Francisco de Asis, Madrid, Spain
- Felix Armadá-Maresca, Ana Boto de los Bueis
- Hospital Universitario de Donostia, Donostia, Spain
- Cristina Irigoyen-Laborra, Jorge Sánchez-Molina
- Hospital Universitario de Bellvitge, Barcelona, Spain
- Juan Francisco Santamaría-Alvarez, Lluis Arias-Barquet, Josep María Caminal, Estefanía Cobos, Pere García-Bru, Rahul Morwani, Daniel Lorenzo-Parra
- Centro de Ojos de A Coruña, A Coruña, Spain
- Pablo Carnota-Méndez, Carlos Méndez Vázquez, Carlos Ignacio Torres Borrego
- Hospital de La Princesa, Madrid, Spain
- Idaira Sanchez-Santos, Muxima Acebes García
- Complejo Hospitalario Universitario de Ferrol, A Coruña, Spain
- Nuria Olivier-Pascual, Rosa Arroyo Castillo
- Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain
- Francisco Javier Ascaso, Olivia Esteban Floria
References
- Liu, Y.-C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Dick, H.B.; Augustin, A.J. Lens Implant Selection with Absence of Capsular Support. Curr. Opin. Ophthalmol. 2001, 12, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Por, Y.M.; Lavin, M.J. Techniques of Intraocular Lens Suspension in the Absence of Capsular/Zonular Support. Surv. Ophthalmol. 2005, 50, 429–462. [Google Scholar] [CrossRef]
- Dajee, K.P.; Abbey, A.M.; Williams, G.A. Management of Dislocated Intraocular Lenses in Eyes with Insufficient Capsular Support. Curr. Opin. Ophthalmol. 2016, 27, 191–195. [Google Scholar] [CrossRef]
- Castaldelli, G.B.; Firmino, G.d.C.; Castaldelli, V.A.; Costa, R.d.S.; Ribeiro, J.C. Use of Techniques for Scleral and Iris Fixation in Secondary Implantation of Intraocular Lenses. Ophthalmic Res. 2021, 64, 1–9. [Google Scholar] [CrossRef]
- Carlà, M.M.; Boselli, F.; Giannuzzi, F.; Caporossi, T.; Gambini, G.; Mosca, L.; Savastano, A.; Rizzo, S. Sutureless Scleral Fixation Carlevale IOL: A Review on the Novel Designed Lens. Int. Ophthalmol. 2022, 43, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Ripa, M.; Angunawela, R.; Motta, L. Scleral Fixation of Carlevale Intraocular Lens: A Systematic Review And Meta-Analysis. Retina 2023, 43, 1750–1762. [Google Scholar] [CrossRef]
- Georgalas, I.; Spyropoulos, D.; Gotzaridis, S.; Papakonstantinou, E.; Kandarakis, S.; Kanakis, M.; Karamaounas, A.; Petrou, P. Scleral Fixation of Carlevale Intraocular Lens: A New Tool in Correcting Aphakia with No Capsular Support. Eur. J. Ophthalmol. 2022, 32, 527–533. [Google Scholar] [CrossRef]
- Barca, F.; Caporossi, T.; de Angelis, L.; Giansanti, F.; Savastano, A.; Di Leo, L.; Rizzo, S. Trans-Scleral Plugs Fixated IOL: A New Paradigm for Sutureless Scleral Fixation. J. Cataract Refract. Surg. 2020, 46, 716–720. [Google Scholar] [CrossRef]
- Veronese, C.; Maiolo, C.; Armstrong, G.W.; Primavera, L.; Torrazza, C.; Della Mora, L.; Ciardella, A.P. New Surgical Approach for Sutureless Scleral Fixation. Eur. J. Ophthalmol. 2020, 30, 612–615. [Google Scholar] [CrossRef]
- Rossi, T.; Iannetta, D.; Romano, V.; Carlevale, C.; Forlini, M.; Telani, S.; Imburgia, A.; Mularoni, A.; Fontana, L.; Ripandelli, G. A Novel Intraocular Lens Designed for Sutureless Scleral Fixation: Surgical Series. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, A.S.; Hoffer, K.J.; Greco, A.; Greco, A.; D’Amico, G.; Pasqualitto, V.; Carlevale, C.; Savini, G. Long-Term Outcomes and Complications of the New Carlevale Sutureless Scleral Fixation Posterior Chamber IOL. J. Refract. Surg. 2021, 37, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Mularoni, A.; Imburgia, A.; Forlini, M.; Rania, L.; Possati, G.L. In Vivo Evaluation of a 1-Piece Foldable Sutureless Intrascleral Fixation Intraocular Lens Using Ultrasound Biomicroscopy and Anterior Segment OCT. J. Cataract Refract. Surg. 2021, 47, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Seknazi, D.; Colantuono, D.; Tahiri, R.; Amoroso, F.; Miere, A.; Souied, E.H. Secondary Sutureless Posterior Chamber Lens Implantation with Two Specifically Designed IOLs: Iris Claw Lens versus Sutureless Trans-Scleral Plugs Fixated Lens. J. Clin. Med. 2021, 10, 2216. [Google Scholar] [CrossRef]
- D’Agostino, I.; Parrulli, S.; De Angelis, S.; Invernizzi, A.; Bottoni, F.; Staurenghi, G.; Cereda, M.G. Sutureless Scleral Fixation: Comparison between 3-Piece IOL and New Single-Piece Foldable IOL. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1365–1373. [Google Scholar] [CrossRef]
- Januschowski, K.; Boden, K.T.; Macek, A.M.; Szurman, P.; Bisorca-Gassendorf, L.; Hoogmartens, C.; Rickmann, A. Modified sutureless intrascleral fixation technique for secondary intraocular lens implantation. Retina 2023, 43, 1802–1806. [Google Scholar] [CrossRef] [PubMed]
- Fiore, T.; Messina, M.; Muzi, A.; Lupidi, M.; Reibaldi, M.; Giansanti, F.; Cagini, C. A Novel Approach for Scleral Fixation Using Carlevale Lens. Eur. J. Ophthalmol. 2021, 31, 2947–2954. [Google Scholar] [CrossRef]
- Rouhette, H.; Meyer, F.; Pommier, S.; Benzerroug, M.; Denion, E.; Guigou, S.; Lorenzi, U.; Mazit, C.; Mérité, P.-Y.; Rebollo, O. FIL-SSF Carlevale Intraocular Lens for Sutureless Scleral Fixation: 7 Recommendations from a Serie of 72 Cases. MICA Study (Multicentric Study of the Carlevale IOL). J. Fr. Ophtalmol. 2021, 44, 1038–1046. [Google Scholar] [CrossRef]
- Danese, C.; Lanzetta, P. Combined Transconjunctival Sutureless Three-Port Vitrectomy and Scleral Fixation of Intraocular Lens. Eur. J. Ophthalmol. 2022, 32, 1287–1290. [Google Scholar] [CrossRef]
- Caporossi, T.; Governatori, L.; Baldascino, A.; Mosca, L.; Scampoli, A.; D’Amico, G.; De Vico, U.; Rizzo, S. Modified Carlevale Intraocular Lens Fixation Technique: Two Vitrectomy Ports as Lens Plug Fixation Sites. Retina 2023, 43, 2034–2036. [Google Scholar] [CrossRef]
- Fiore, T.; Messina, M.; Muzi, A.; Tosi, G.; Lupidi, M.; Casini, G.; Marruso, V.; Cagini, C. Comparison of Two Different Scleral Fixation Techniques of Posterior Chamber Carlevale Lens. Medicine 2021, 100, e26728. [Google Scholar] [CrossRef] [PubMed]
- Gabai, A.; Zeppieri, M.; Toneatto, G.; Salati, C. Enhanced Surgical Technique for Sutureless Intrascleral Fixation of Intraocular Lenses. J. Cataract Refract. Surg. 2021, 47, e75–e79. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulos, G.; Siskou, E.; Koronis, S.; Tranos, P.; Gatzioufas, Z.; Balidis, M. Novel Sutureless Scleral Fixated IOL for Inadequate or Absent Capsular Support. J. Ophthalmol. 2022, 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dyrda, A.; Pighin, M.S.; Jürgens, I. Endoscope-Assisted Carlevale Lens Implantation in Patients Without Capsular Support: A Novel Surgical Approach to Ensure Correct Lens Positioning. Retina 2023, 43, 2084–2088. [Google Scholar] [CrossRef]
- Franco, F.; Serino, F.; Vicini, G.; Nicolosi, C.; Giansanti, F. Comparison of Visual and Aberrometric Outcomes in Suture-Free Scleral Fixation: Three-Piece Lenses versus Carlevale Lenses. J. Clin. Med. 2022, 12, 188. [Google Scholar] [CrossRef]
- Van Severen, V.; Maaijwee, K.J.M.; Pennekamp, C.W.A.; Feenstra, H.M.A.; van Dijk, E.H.C.; Lindstedt, E.W.; Bamonte, G. Comparison of Surgical Outcomes of Carlevale Sutureless Scleral Fixation and Artisan Aphakia Intraocular Lens. Acta Ophthalmol. 2024, 102, 491–496. [Google Scholar] [CrossRef]
- Bernal-Morales, C.; Hernández-Martínez, A.; Navarro-Angulo, M.J.; Ruiz-Miguel, M.; Rodriguez-Maqueda, M.; Velazquez-Villoria, D.; Cubero-Parra, J.M.; Marticorena, J.; Ruiz-Casas, D.; Adan, A.; et al. Retropupillary Iris-Claw Intraocular Lens and Pars Plana Vitrectomy in Aphakia Management. Retina 2021, 41, 2048–2058. [Google Scholar] [CrossRef]
- Obergassel, J.; Heiduschka, P.; Alten, F.; Eter, N.; Clemens, C.R. A Comparative Analysis of Carlevale IOL Versus Artisan IOL Implantation Using a Scleral Tunnel Incision Technique. J. Clin. Med. 2024, 13, 6964. [Google Scholar] [CrossRef]
- Bontemps, J.; Loria, O.; Sejournet, L.; Allignet, B.; Elbany, S.; Matonti, F.; Burillon, C.; Denis, P.; Kodjikian, L.; Mathis, T. Refractive Outcomes for Secondary Sutureless Posterior Chamber Lens Implantation: Sutureless Scleral Fixating Lens Carlevale® versus Retropupillary Iris-Claw Lens Artisan®. Graefe’s Arch. Clin. Exp. Ophthalmol. 2025, 263, 735–743. [Google Scholar] [CrossRef]
- Sánchez-Vela, L.; García-Arumí Fusté, C.; Castany-Aregall, M.; Subirà-González, O.; Ruiz-Casas, D.; de-Arriba-Palomero, P.; García-Arumí, J. Reverse Pupillary Block after Implantation of a Sutureless Scleral Fixation Carlevale Intraocular Lens. Ophthalmol. Retin. 2025, 9, 322–329. [Google Scholar] [CrossRef]
- Ilveskoski, L.; Taipale, C.; Holmström, E.J.; Tuuminen, R. Macular Edema after Cataract Surgery in Eyes with and without Pseudoexfoliation Syndrome. Eur. J. Ophthalmol. 2019, 29, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Shingleton, B.J.; Nguyen, B.-K.C.; Eagan, E.F.; Nagao, K.; O’Donoghue, M.W. Outcomes of Phacoemulsification in Fellow Eyes of Patients with Unilateral Pseudoexfoliation. J. Cataract Refract. Surg. 2008, 34, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, M.; Schmutz, L.; Gkaragkani, E.; Droutsas, K.; Kymionis, G.D. Simultaneous Penetrating Keratoplasty and Implantation of a New Scleral-Fixated, Sutureless, Posterior Chamber Intraocular Lens (Soleko, Carlevale): A Novel Technique. Cornea 2020, 39, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- de Angelis, L.; Barca, F.; Rizzo, S.; Di Leo, L.; Oliverio, L.; Caporossi, T. Trans-Scleral Plugs Scleral Fixation IOL and Penetrating Keratoplasty to Restore Vision in Vitrectomized Eyes. Eur. J. Ophthalmol. 2022, 32, NP67–NP70. [Google Scholar] [CrossRef]
- Kymionis, G.; Petrelli, M.; Schmutz, L.; Petrovic, A. New Sutureless, Scleral-Fixated Intraocular Lens (Carlevale, Soleko) Implantation Combined with Descemet Stripping Automated Endothelial Keratoplasty: An Innovative Surgical Approach. Cornea 2020, 39, 1460–1462. [Google Scholar] [CrossRef]


| Total | IOL Luxation | Complicated Cataract Surgery | p-Value | |
|---|---|---|---|---|
| N (%) | 268 | 62.7% (168/268) | 37.3% (100/268) | - |
| Gender | ||||
| Female (%) | 36.6 (98/268) | 35.7 (60/168) | 38.0 (38/100) | 0.77 |
| Laterality | ||||
| Right eye (%) | 53.4 (143/268) | 54.2 (91/168) | 52.0 (52/100) | 0.66 |
| Age | ||||
| Mean ± SD | 70.9 ± 16.6 | 72.5 ± 13.5 | 68.1 ± 20.5 | 0.06 |
| Median (IQR) | 75.0; 18.8 | 75.0; 15.5 | 75.0; 25.5 | |
| Preop VA (logMAR) | 0 | |||
| Mean ± SD | 0.9 ± 0.6 | 0.9 ± 0.6 | 1.0 ± 0.6 | 0.02 |
| Median (IQR) | 0.8; 1.2 | 0.7; 1.3 | 1.0; 1.2 | |
| IOP (mmHg) | ||||
| Mean ± SD | 17.3 ± 6.1 | 17.3 ± 5.1 | 17.2 ± 7.6 | 0.86 |
| Median (IQR) | 16.0; 6.0 | 16.6; 6.0 | 15.0; 4.5 | |
| Axial length (mm) | ||||
| Mean ± SD | 24.5 ± 2.6 | 25.1 ± 3.0 | 23.6 ± 1.5 | <0.01 |
| Median (IQR) | 23.7; 1.7 | 23.9; 2.3 | 23.5; 1.4 | |
| Anterior chamber depth (mm) | <0.01 | |||
| Mean ± SD | 3.9 ± 0.9 | 4.4 ± 0.8 | 3.3 ± 0.7 | |
| Median (IQR) | 3.9; 1.4 | 4.3; 1.0 | 3.1; 1.1 | |
| Preop macular edema | 10.4 (28/268) | 11.3 (19/168) | 9.0 (9/100) | 0.68 |
| Pseudoexfoliation | 27.6 (74/268) | 30.4 (51/168) | 23.0 (23/100) | 0.12 |
| Glaucoma | 21.6 (58/268) | 23.8 (10/168) | 18.0 (18/100) | 0.25 |
| Diabetes | 12.7 (34/268) | 11.9 (20/168) | 14.0 (14/100) | 0.64 |
| Diabetic retinopathy | 6.0 (16/268) | 7.1 (12/168) | 4.0 (4/100) | 0.17 |
| High myopia | 17.2 (46/268) | 22.0 (37/168) | 9.0 (9/100) | <0.01 |
| Uveitis | 6.0 (16/268) | 6.0 (10/168) | 6.0 (6/100) | 0.99 |
| Traumatism | 14.2 (38/268) | 12.5 (21/168) | 17.0 (17/100) | 0.32 |
| Baseline | 1 Month | 3 Months | 6 Months | 12 Months | |
|---|---|---|---|---|---|
| Data entries at individual timepoints | n = 268 | n = 206 | n = 177 | n = 139 | n = 121 |
| Visual acuity levels (logMAR) | |||||
| ≤0.3 (%) | 21.6 | 47.1 | 56.5 | 56.5 | 64.2 |
| ≤0.7 (%) | 44.8 | 73.9 | 78.3 | 80.9 | 86.7 |
| ≤1 (%) | 59.7 | 86.1 | 88.7 | 90.1 | 93.7 |
| n = 268 | n = 206 | n = 177 | n = 139 | n = 121 | |
| Cumulative probability of IOP levels (mmHg) | |||||
| >21 (%) | 10.4 | 25.1 | 26.9 | 28.5 | 29.8 |
| ≥25 (%) | 6.0 | 14.2 | 15.1 | 16.2 | 16.9 |
| ≥30 (%) | 4.5 | 10.1 | 10.1 | 10.1 | 10.1 |
| n = 204 | n = 202 | n = 165 | n = 123 | n = 100 | |
| Cumulative probability of IOP-lowering drops (%) | 21.0 | 40.3 | 41.6 | 42.3 | 42.3 |
| Monotherapy (%) | 10.1 | 15.3 | 17.1 | 18.9 | 19.9 |
| 2 drops (%) | 11.9 | 21.6 | 22.9 | 23.3 | 25.2 |
| >2 drops (%) | 7.5 | 9.4 | 10.3 | 10.8 | 13.5 |
| Oral acetazolamide (%) | 8.2 | 13.2 | 14.9 | 16.0 | 16.7 |
| n = 212 | n = 188 | n = 165 | n = 127 | n = 97 | |
| Cumulative probability of macular edema development (%) | 10.4 | 20.1 | 26.9 | 30.2 | 34.3 |
| Cumulative probability of postoperative macular edema development (%) | - | 10.8 | 18.3 | 22.1 | 26.6 |
| Cumulative probability of macular edema resolution (%) | - | 12.2 | 20.0 | 37.2 | 53.5 |
| n = 268 | n = 155 | n = 135 | n = 113 | n = 98 | |
| Cumulative probability of endothelial decompensation (%) | - | 10.5 | 10.9 | 12.8 | 13.4 |
| n = 182 | n = 156 | n = 121 | n = 94 |
| Total | |
|---|---|
| “T” Haptic rupture/IOL disenclavation | 8 (3.0%) |
| Iris/ciliary body hemorrhage | 5 (1.9%) |
| Vitreous hemorrhage | 5 (1.9%) |
| Retinal detachment | 4 (1.5%) |
| Choroidal hemorrhage | 3 (1.1%) |
| IOL rotation/IOL upside down | 3 (1.1%) |
| Hypotony | 2 (0.7%) |
| Retinal tear | 2 (0.7%) |
| Reverse pupillary block | 2 (0.7%) |
| IOL opacification | 2 (0.7%) |
| Haptic extrusion | 2 (0.7%) |
| Sclerotomy leak | 1 (0.4%) |
| Endophthalmitis | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer-Alapont, L.; Bernal-Morales, C.; Navarro, M.J.; Ruiz-Casas, D.; García-Arumí, C.; Cubero-Parra, J.M.; Dabad-Moreno, J.V.; Velázquez-Villoria, D.; Marticorena, J.; Zarco-Bosquet, J.; et al. Sutureless Scleral-Fixated Soleko Fil Carlevale Intraocular Lens and Associated Pars Plana Vitrectomy in Aphakia Management: A National Multicenter Audit. J. Clin. Med. 2025, 14, 3963. https://doi.org/10.3390/jcm14113963
Ferrer-Alapont L, Bernal-Morales C, Navarro MJ, Ruiz-Casas D, García-Arumí C, Cubero-Parra JM, Dabad-Moreno JV, Velázquez-Villoria D, Marticorena J, Zarco-Bosquet J, et al. Sutureless Scleral-Fixated Soleko Fil Carlevale Intraocular Lens and Associated Pars Plana Vitrectomy in Aphakia Management: A National Multicenter Audit. Journal of Clinical Medicine. 2025; 14(11):3963. https://doi.org/10.3390/jcm14113963
Chicago/Turabian StyleFerrer-Alapont, Lorena, Carolina Bernal-Morales, Manuel J. Navarro, Diego Ruiz-Casas, Claudia García-Arumí, Juan Manuel Cubero-Parra, Jose Vicente Dabad-Moreno, Daniel Velázquez-Villoria, Joaquín Marticorena, Julián Zarco-Bosquet, and et al. 2025. "Sutureless Scleral-Fixated Soleko Fil Carlevale Intraocular Lens and Associated Pars Plana Vitrectomy in Aphakia Management: A National Multicenter Audit" Journal of Clinical Medicine 14, no. 11: 3963. https://doi.org/10.3390/jcm14113963
APA StyleFerrer-Alapont, L., Bernal-Morales, C., Navarro, M. J., Ruiz-Casas, D., García-Arumí, C., Cubero-Parra, J. M., Dabad-Moreno, J. V., Velázquez-Villoria, D., Marticorena, J., Zarco-Bosquet, J., Armada-Maresca, F., Irigoyen, C., Santamaría-Álvarez, J.-F., Carnota-Méndez, P., Sánchez-Santos, I., Olivier-Pascual, N., Ascaso, F. J., & Zarranz-Ventura, J., on behalf of the Sutureless Scleral Fixated-Soleko Fil Carlevale-IOL Spanish Study Group. (2025). Sutureless Scleral-Fixated Soleko Fil Carlevale Intraocular Lens and Associated Pars Plana Vitrectomy in Aphakia Management: A National Multicenter Audit. Journal of Clinical Medicine, 14(11), 3963. https://doi.org/10.3390/jcm14113963






