Evaluating the Chorioretinal Microcirculation in Preeclampsia with OCT-Angiography: A Narrative Literature Review
Abstract
:1. Introduction
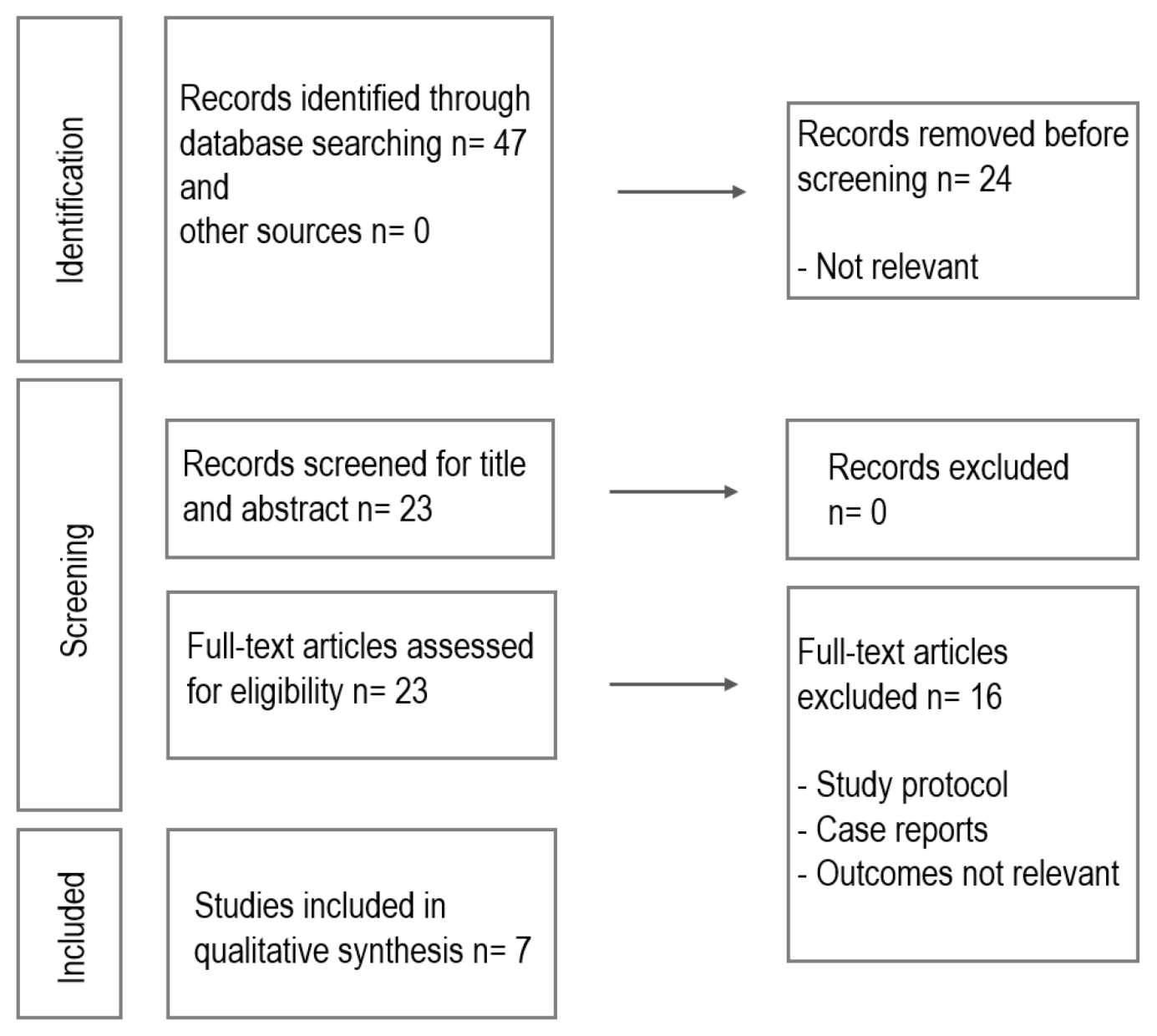
2. Materials and Methods
3. Results
3.1. Pathophysiology of Vasculature Changes in Pregnancy
3.2. Ocular Manifestations in Preeclampsia
3.3. OCTA Imaging Principles
3.4. Imaging Chorioretinal Microcirculatory Changes in Normal Pregnancy
3.5. Chorioretinal Microcirculation and Blood Flow Alterations in Preeclampsia
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Errera, M.H.; Kohly, R.P.; da Cruz, L. Pregnancy-associated retinal diseases and their management. Surv. Ophthalmol. 2013, 58, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.L.; Birnbaum, A.D.; Goldstein, D.A. Ocular disease in pregnancy. Curr. Opin. Ophthalmol. 2005, 16, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Khong, E.W.C.; Chan, H.H.L.; Watson, S.L.; Lim, L.L. Pregnancy and the eye. Curr. Opin. Ophthalmol. 2021, 32, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Barbazetto, I.A.; Pizzarello, L.D. Ocular changes during pregnancy. Compr. Ophthalmol. Update 2007, 8, 155–167. [Google Scholar]
- Abu Samra, K. The eye and visual system in the preeclampsia/eclampsia syndrome: What to expect? Saudi J. Ophthalmol. 2013, 27, 51–53. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Kızıltunç, P.B.; Varlı, B.; Büyüktepe, T.Ç.; Atilla, H. Ocular vascular changes during pregnancy: An optical coherence tomography angiography study. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 395–401. [Google Scholar] [CrossRef]
- Chanwimol, K.; Balasubramanian, S.; Nassisi, M.; Gaw, S.L.; Janzen, C.; Sarraf, D.; Sadda, S.R.; Tsui, I. Retinal Vascular Changes During Pregnancy Detected With Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2726–2732. [Google Scholar] [CrossRef]
- Hepokur, M.; Gönen, B.; Hamzaoğlu, K.; Tüten, A.; Sarici, A.M. Investigation of Retinal Vascular Changes during Pregnancy Using Optical Coherence Tomography Angiography. Semin. Ophthalmol. 2021, 36, 19–27. [Google Scholar] [CrossRef]
- Lin, B.R.; Lin, F.; Su, L.; Nassisi, M.; Sadda, S.R.; Gaw, S.L.; Tsui, I. Relative Postpartum Retinal Vasoconstriction Detected With Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2021, 10, 40. [Google Scholar] [CrossRef]
- Urfalıoglu, S.; Bakacak, M.; Özdemir, G.; Güler, M.; Beyoglu, A.; Arslan, G. Posterior ocular blood flow in preeclamptic patients evaluated with optical coherence tomography angiography. Pregnancy Hypertens. 2019, 17, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Ciloglu, E.; Okcu, N.T.; Dogan, N.Ç. Optical coherence tomography angiography findings in preeclampsia. Eye 2019, 33, 1946–1951. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.Y.; Bae, J.G.; Lee, J.K.; Kim, Y.C. Relationship between proteinuria and optical coherence tomographic features of the chorioretina in patients with pre-eclampsia. PLoS ONE 2021, 16, e0251933. [Google Scholar] [CrossRef] [PubMed]
- Özcan, Z.Ö.; Güngör, K.; Saygili, O.; Özcan, H.Ç. The role of optical coherence tomography angiography in patients with preeclampsia. Retina 2022, 42, 1931–1938. [Google Scholar] [CrossRef]
- Fayed, A.E.; Thabet, M.M.; Salama, M.M.; El Shazly, M. Diminished choroidal blood flow in hypertensive and preeclamptic third trimester pregnancies using optical coherence tomography angiography. PLoS ONE 2023, 18, e0285884. [Google Scholar] [CrossRef]
- Hoel, S.; Moe, K.; Sugulle, M.; Petrovski, G.; Veiby, N.C.B.B.; Staff, A.C. Retinal oximetry and microvascular assessment after hypertensive pregnancy complications. Acta Ophthalmol. 2024, 102, 653–661. [Google Scholar] [CrossRef]
- Erkan Pota, Ç.; Doğan, M.E.; Alkan Bülbül, G.; Sanhal, C.Y.; Pota, A. Optical coherence tomography angiography assessment of retinochoroidal microcirculation differences in preeclampsia. Photodiagn. Photodyn. Ther. 2024, 46, 104004. [Google Scholar] [CrossRef]
- Martinez-Velazquez, L.; Begaj, T.; Garg, I.; Zhou, P.; Hoyek, S.; Wai, K.M.; Miller, J.B.; Patel, N.A. Widefield Swept-Source OCTA Findings in HELLP Syndrome: Choroidal Infarcts. Retin. Cases Brief Rep. 2024, 2022, 10–1097. [Google Scholar] [CrossRef]
- Ouzounian, J.G.; Elkayam, U. Physiologic changes during normal pregnancy and delivery. Cardiol. Clin. 2012, 30, 317–329. [Google Scholar] [CrossRef]
- Greer, C.; Troughton, R.W.; Adamson, P.D.; Harris, S.L. Preterm birth and cardiac function in adulthood. Heart 2022, 108, 172–177. [Google Scholar] [CrossRef]
- de Haas, S.; Ghossein-Doha, C.; van Kuijk, S.M.J.; van Drongelen, J.; Spaanderman, M.E.A. Physiological adaptation of maternal plasma volume during pregnancy: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2017, 49, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Vargas, E.; Julian, C.G.; Armaza, J.F.; Rodriguez, A.; Tellez, W.; Niermeyer, S.; Wilson, M.; Parra, E.; Shriver, M.; et al. Determinants of blood oxygenation during pregnancy in Andean and European residents of high altitude. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1303–R1312. [Google Scholar] [CrossRef] [PubMed]
- Vricella, L.K. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), S1620–S1625. [Google Scholar] [CrossRef] [PubMed]
- Aguree, S.; Gernand, A.D. Plasma volume expansion across healthy pregnancy: A systematic review and meta-analysis of longitudinal studies. BMC Pregnancy Childbirth 2019, 19, 508. [Google Scholar] [CrossRef]
- Siauve, N.; Chalouhi, G.E.; Deloison, B.; Alison, M.; Clement, O.; Ville, Y.; Salomon, L.J. Functional imaging of the human placenta with magnetic resonance. Am. J. Obstet. Gynecol. 2015, 213 (Suppl. 4), S103–S114. [Google Scholar] [CrossRef]
- Vora, N.; Kalagiri, R.; Mallett, L.H.; Oh, J.H.; Wajid, U.; Munir, S.; Colon, N.; Raju, V.N.; Beeram, M.R.; Uddin, M.N.; et al. Proteomics and Metabolomics in Pregnancy-An Overview. Obstet. Gynecol. Surv. 2019, 74, 111–125. [Google Scholar] [CrossRef]
- McKeating, D.R.; Fisher, J.J.; Perkins, A.V. Elemental Metabolomics and Pregnancy Outcomes. Nutrients 2019, 11, 73. [Google Scholar] [CrossRef]
- Gyselaers, W. Hemodynamic pathways of gestational hypertension and preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S988–S1005. [Google Scholar] [CrossRef]
- Garrity, S.T.; Paques, M.; Gaudric, A.; Freund, K.B.; Sarraf, D. Considerations in the understanding of venous outflow in the retinal capillary plexus. Retina 2017, 37, 1809–1812. [Google Scholar] [CrossRef]
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2002, 99, 159–167. [Google Scholar]
- Chandran, J.R.; Narayanan, I.B.; Rajan, J. Ocular Manifestations: Are They Significant in Hypertensive Disorders of Pregnancy? J. Obstet. Gynecol. India 2021, 71, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Henriques, I.; Rocha-Sousa, A.; Barbosa-Breda, J. Optical coherence tomography angiography changes in cardiovascular systemic diseases and risk factors: A Review. Acta Ophthalmol. 2022, 100, e1–e15. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, R.; Ecker, J.L.; Kettyle, E.; Sandler, L.; Frigoletto, F.D. Pulse pressure and risk of preeclampsia: A prospective study. Obstet. Gynecol. 2001, 97, 515–520. [Google Scholar] [CrossRef] [PubMed]
- McCormick, P.A.; Higgins, M.; McCormick, C.A.; Nolan, N.; Docherty, J.R. Hepatic infarction, hematoma, and rupture in HELLP syndrome: Support for a vasospastic hypothesis. J. Matern. Fetal Neonatal Med. 2022, 35, 7942–7947. [Google Scholar] [CrossRef]
- Govindaraju, V.K.; Schimmel, O.C.; Trese, M.G.J.; Garretson, B.R.; Faia, L.J. Bilateral Exudative Retinal Detachments, Chemosis, and Impaired Ocular Motility in the Setting of Preeclampsia and HELLP Syndrome. J. Vitreoretin. Dis. 2022, 6, 255–258. [Google Scholar] [CrossRef]
- Velazquez-Villoria, D.; Marti Rodrigo, P.; DeNicola, M.L.; Zapata Vitori, M.A.; Segura García, A.; García-Arumí, J. Swept source optical coherence tomography evaluation of chorioretinal changes in hypertensive choroidopathy related to HELLP syndrome. Retin. Cases Brief Rep. 2019, 13, 30–33. [Google Scholar] [CrossRef]
- Cankaya, C.; Bozkurt, M.; Ulutas, O. Total macular volume and foveal retinal thickness alterations in healthy pregnant women. Semin. Ophthalmol. 2013, 28, 103–111. [Google Scholar] [CrossRef]
- Spaide, R.F. Retinal vascular cystoid macular edema: Review and new theory. Retina 2016, 36, 1823–1842. [Google Scholar] [CrossRef]
- Pournaras, C.J.; Rungger-Brändle, E.; Riva, C.E.; Hardarson, S.H.; Stefansson, E. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res. 2008, 27, 284–330. [Google Scholar] [CrossRef]
- Yu, D.-Y.; Cringle, S.J.; Yu, P.K.; Balaratnasingam, C.; Mehnert, A.; Sarunic, M.V.; An, D.; Su, E.-N. Retinal capillary perfusion: Spatial and temporal heterogeneity. Prog. Retin Eye Res. 2019, 70, 23–54. [Google Scholar] [CrossRef]
- Dadaci, Z.; Alptekin, H.; Acir, N.O.; Borazan, M. Changes in choroidal thickness during pregnancy detected by enhanced depth imaging optical coherence tomography. Br. J. Ophthalmol. 2015, 99, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Roskal-Wałek, J.; Laudańska-Olszewska, I.; Biskup, M.; Gierada, M.; Odrobina, D. Choroidal Thickness in Women with Uncomplicated Pregnancy: Literature Review. Biomed Res. Int. 2017, 2017, 5694235. [Google Scholar] [CrossRef] [PubMed]
- Kara, N.; Sayin, N.; Pirhan, D.; Vural, A.D.; Araz-Ersan, H.B.; Tekirdag, A.I.; Yildirim, G.Y.; Gulac, B.; Yilmaz, G. Evaluation of subfoveal choroidal thickness in pregnant women using enhanced depth imaging optical coherence tomography. Curr. Eye Res. 2014, 39, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Gil, G.; Fernández-Avellaneda, P.; Spaide, R.F. Swept-source optical coherence tomography angiography imaging of the choriocapillaris. Retina 2021, 41, 1373–1378. [Google Scholar] [CrossRef]
- Su, L.; Lin, B.R.; Lin, F.; Tsui, I.K.; Gaw, S.L.; Janzen, C.; Sadda, S.R.; Tsui, I. Maternal Optical Coherence Tomography Angiography Changes Related to Small for Gestational Age Pregnancies. Transl. Vis. Sci. Technol. 2020, 9, 4. [Google Scholar] [CrossRef]
- Christou, E.E.; Rallis, D.; Zafeiropoulos, P.; Asproudis, C.; Stefaniotou, M.; Giapros, V.; Asproudis, I. Assessment of Maternal Retinal Microvasculature in Preterm Pregnancy Using OCT-Angiography: A Cross-Sectional Study. Maedica 2023, 18, 623–630. [Google Scholar] [CrossRef]
- Tang, C.; Pan, S.; Zeng, X.; Fu, X.; Deng, J.; Shi, K. Diminished retinal microcirculation in mild preeclampsia without obvious retinopathy in high-altitude native Tibetans by OCTA. Photodiagn. Photodyn. Ther. 2024, 50, 104396. [Google Scholar] [CrossRef]
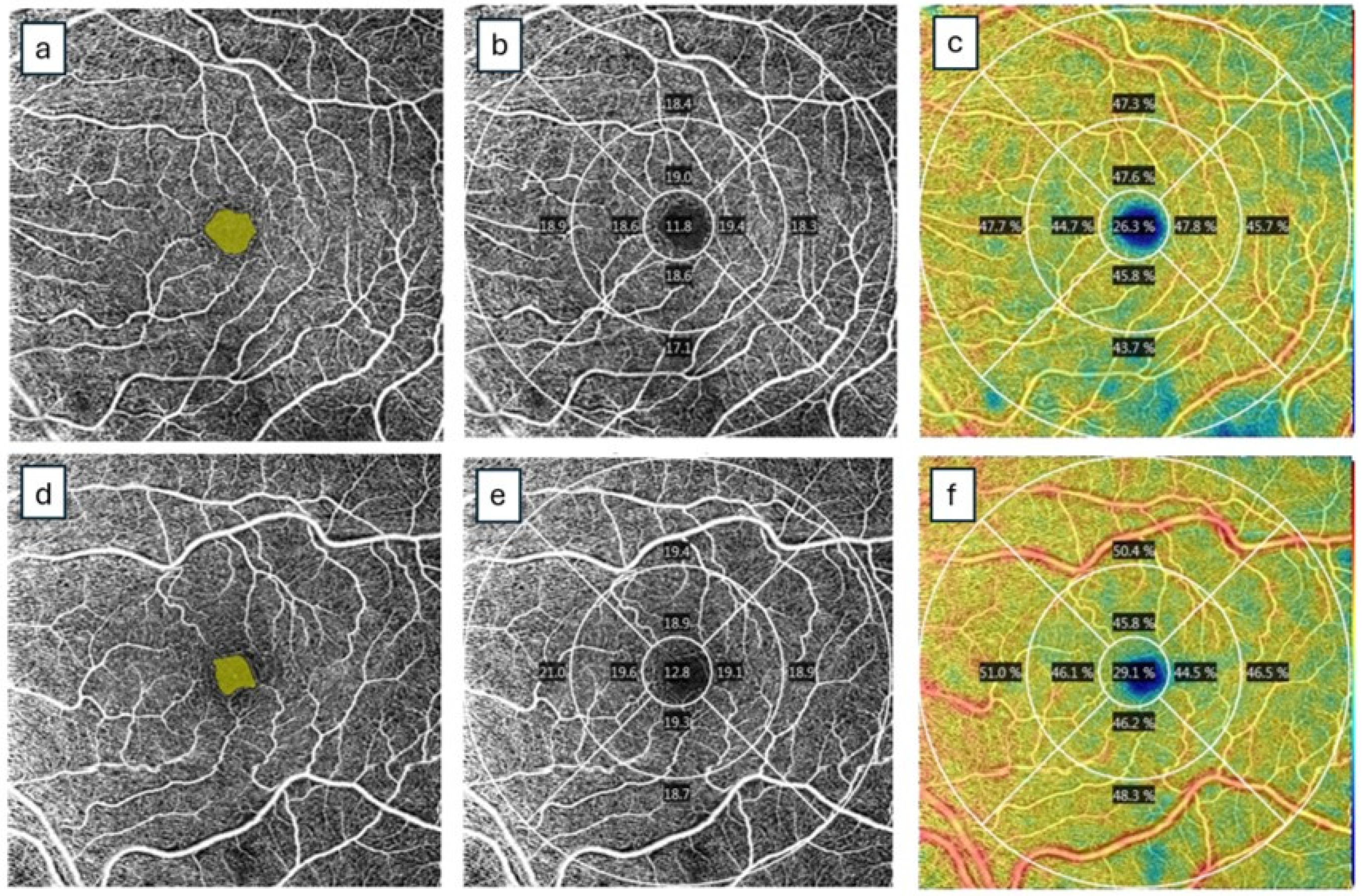
| Study | Design | Study Population | OCTA Device | Scan Area (mm²) | OCTA Characteristics | Study Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preeclampsia | Healthy Pregnancy | Non-Pregnancy | Macula | Optic Nerve | FAZ | VD Macula | VD Optic Nerve | |||||||
| SCP | DCP | SCP | DCP | CC | ||||||||||
| Urfalioglu et al., 2019 [11] | Prospective, cross-sectional | 27 | 26 | 25 | AngioVue, Optovue, RTVue-XR, Fremont, CA, USA | 6 × 6 | 6 × 6 | - | - | + | + | + | + | Retinal microcirculation maintained consistent flow; choriocapillaris and optic nerve head vasculature were attenuated in preeclampsia |
| Ciloglu et al., 2019 [12] | Prospective | 55 | 43 | 38 | AngioVue, OptoVue, RTVue-XR, Fremont, CA, USA | 3 × 3 | 4.5 × 4.5 | + | + | + | + | - | + | Retinal microvasculature structure changes in preeclampsia, even without confirmed retinal and optic disk pathology |
| Shim et al., 2021 [13] | Retrospective | 61 | - | - | DRI OCT, Triton, Topcon, Tokyo, Japan | 3 × 3 | - | + | + | + | + | - | - | Arterial pressure and protein–creatinine ratio indicated no association with retinal microvasculature |
| Ozcan et al., 2022 [14] | Prospective, cross-sectional | 50 | 50 | 50 | AngioVue, OptoVue, RTVue-XR, Fremont, CA, USA | 3 × 3 | 4.5 × 4.5 | + | + | + | + | + | + | There was a decreased macular DCP VD in preeclampsia, which showed improvement after delivery |
| Fayed et al., 2023 [15] | Prospective, observational, cross-sectional, comparative | 15 | 15 | - | AngioVue, OptoVue, RTVue-XR, Fremont, CA, USA | 3 × 3, 6 × 6 | - | - | - | - | - | + | - | There was low choroidal blood flow in preeclampsia compared to pregnancy with systemic hypertension and healthy pregnancy |
| Hoel et al., 2024 [16] | Pilot study | 27 | - | 23 | Zeiss PLEX Elite 9000, Carl Zeiss, Meditec Inc, Dublin, CA, USA | 6 × 6 | - | - | - | + | - | - | - | Microvasculature structural alterations were not biomarkers for cardiovascular disorders in healthy women 3 years postpartum with hypertensive disorders in pregnancy |
| Pota et al., 2024 [17] | Single center | 27 | 30 | 30 | DRI OCT, Triton, Topcon, Tokyo, Japan | 3 × 3 | - | + | + | + | + | + | - | In preeclampsia, a decreased central VD, increased FAZ with increased systolic blood pressure and decreased choroidalthickness could contribute to monitoring |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christou, E.E.; Ong, A.Y.; Frise, C.; Jalil, A.; Ivanova, T.; Georgalas, I.; de Silva, S.R. Evaluating the Chorioretinal Microcirculation in Preeclampsia with OCT-Angiography: A Narrative Literature Review. J. Clin. Med. 2025, 14, 3913. https://doi.org/10.3390/jcm14113913
Christou EE, Ong AY, Frise C, Jalil A, Ivanova T, Georgalas I, de Silva SR. Evaluating the Chorioretinal Microcirculation in Preeclampsia with OCT-Angiography: A Narrative Literature Review. Journal of Clinical Medicine. 2025; 14(11):3913. https://doi.org/10.3390/jcm14113913
Chicago/Turabian StyleChristou, Evita Evangelia, Ariel Yuhan Ong, Charlotte Frise, Assad Jalil, Tsveta Ivanova, Ilias Georgalas, and Samantha R. de Silva. 2025. "Evaluating the Chorioretinal Microcirculation in Preeclampsia with OCT-Angiography: A Narrative Literature Review" Journal of Clinical Medicine 14, no. 11: 3913. https://doi.org/10.3390/jcm14113913
APA StyleChristou, E. E., Ong, A. Y., Frise, C., Jalil, A., Ivanova, T., Georgalas, I., & de Silva, S. R. (2025). Evaluating the Chorioretinal Microcirculation in Preeclampsia with OCT-Angiography: A Narrative Literature Review. Journal of Clinical Medicine, 14(11), 3913. https://doi.org/10.3390/jcm14113913






