Ventilator-Induced Lung Injury: The Unseen Challenge in Acute Respiratory Distress Syndrome Management
Abstract
1. Introduction
2. Pathophysiological Mechanisms
2.1. Barotrauma and Volutrauma
2.2. Atelectrauma
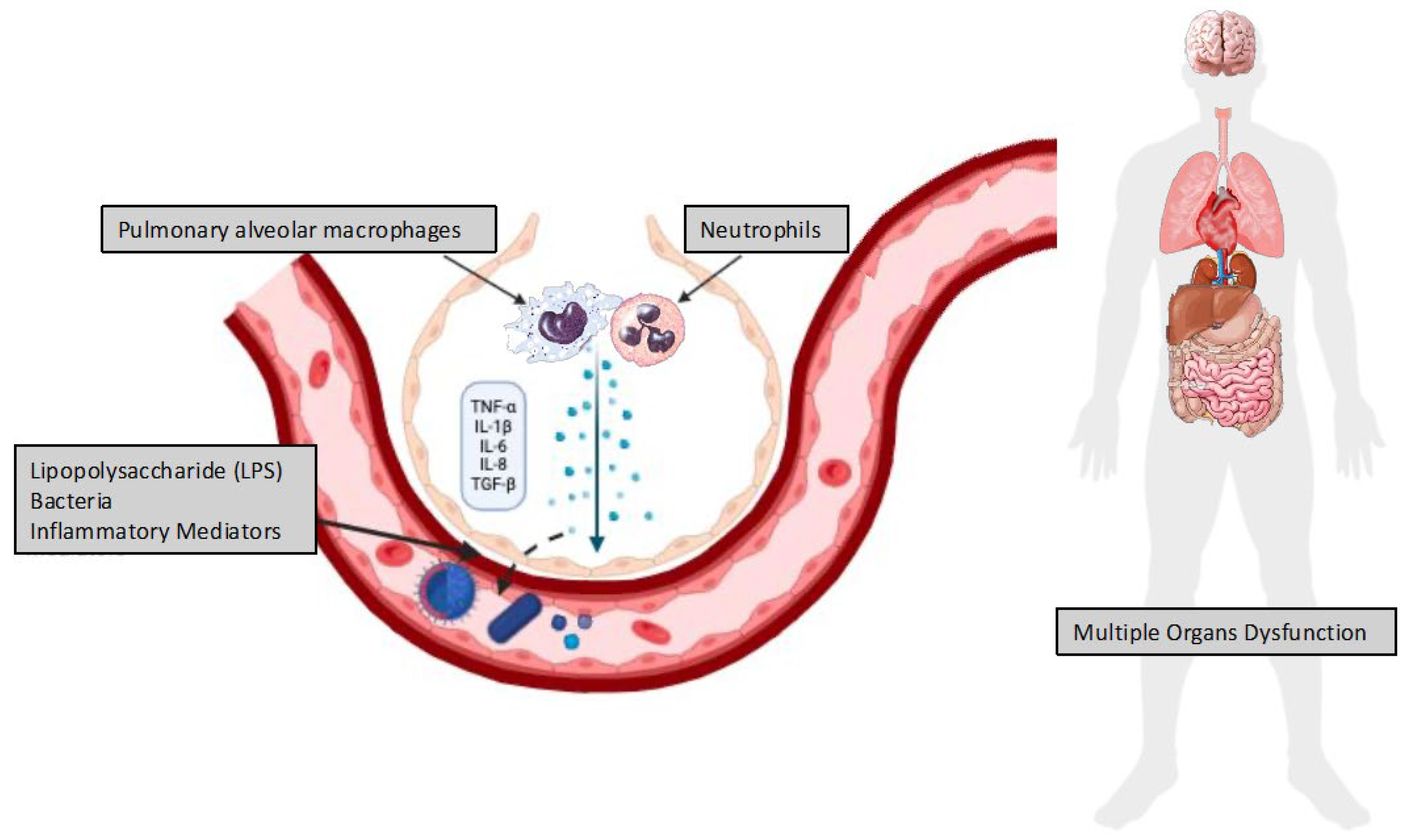
2.3. Biotrauma
2.4. Ergotrauma and Respiratory Mechanics
2.5. Concept of VILI in Clinical Practice
3. Clinical Management
3.1. Lung-Protective Ventilation
3.1.1. Positive End-Expiratory Pressure
3.1.2. Prone Position
3.1.3. Ultra–Lung-Protective Ventilation and Extracorporeal Life Support (ECLS)
3.1.4. Management of Sedation and Neuromuscular Blocking Agents and Early Transitioning from Controlled to Assisted Ventilation
3.1.5. ARDS Biological Subphenotypes and Pharmacologic Interventions
3.1.6. Emerging Insights in Biological Subphenotyping for ARDS Management
3.1.7. Critical Perspectives and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARDS | Acute Respiratory Distress Syndrome |
| CI | Confidence Interval |
| CT | Computed Tomography |
| ECLS | Extracorporeal Life Support |
| ECCO2R | Extracorporeal Carbon Dioxide Removal |
| ECMO | Extracorporeal Membrane Oxygenation |
| EELV | End-Expiratory Lung Volume |
| EIT | Electrical Impedance Tomography |
| FiO2 | fraction of inspired oxygen |
| HBO | Hyperbaric Oxygen |
| HCT | Hematocrit |
| ICU | Intensive Care Unit |
| IL | Interleukin |
| LPV | Lung-Protective Ventilation |
| MP | Mechanical Power |
| MV | Mechanical Ventilation |
| PaCO2 | Partial Pressure of Carbon Dioxide |
| PACO2 | Alveolar Partial Pressure of Carbon Dioxide |
| PEEP | Positive End-Expiratory Pressure |
| PET | Positron Emission Tomography |
| POCUS | Point-of-Care Ultrasound |
| PROSEVA | Proning Severe ARDS Patients |
| RCT | Randomized Controlled Trial |
| RM | Recruitment Maneuver |
| RR | Respiratory Rate |
| SUPERNOVA | Study on Ultra-Protective Ventilation and Extracorporeal Life Support |
| TGF | Transforming Growth Factor |
| TNF | tumor necrosis factor |
| Transp | Transpulmonary Pressure |
| V/Q | Ventilation/Perfusion Ratio |
| VILI | Ventilator-Induced Lung Injury |
| VT | Tidal Volume |
| ∆P | driving pressure |
References
- Lassen, H.C. A preliminary report on the 1952 epidemic of poliomyelitis in Copenhagen with special reference to the treatment of acute respiratory insufficiency. Lancet 1953, 1, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, B. The anaesthetist’s viewpoint on the treatment of respiratory complications in poliomyelitis during the epidemic in Copenhagen, 1952. Proc. R Soc. Med. 1954, 47, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, J. Observations of a case published in the last volume of the medi- cal essays of recovering a man dead in appearance, by distending the lungs with air. Philos. Trans. 1744, 43, 275–281. [Google Scholar]
- Slutsky, A.S. History of Mechanical Ventilation. From Vesalius to Ventilator-induced Lung Injury. Am. J. Respir. Crit. Care Med. 2015, 191, 1106–1115. [Google Scholar] [CrossRef]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar]
- Webb, H.H.; Tierney, D.F. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am. Rev. Respir. Dis. 1974, 110, 556–565. [Google Scholar]
- Dreyfuss, D.; Soler, P.; Basset, G.; Saumon, G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am. Rev. Respir. Dis. 1988, 137, 1159–1164. [Google Scholar] [CrossRef]
- Protti, A.; Cressoni, M.; Santini, A.; Langer, T.; Mietto, C.; Febres, D.; Chierichetti, M.; Coppola, S.; Conte, G.; Gatti, S.; et al. Lung stress and strain during mechanical ventilation: Any safe threshold? Am. J. Respir. Crit. Care Med. 2011, 183, 1354–1362. [Google Scholar] [CrossRef]
- Motta-Ribeiro, G.C.; Hashimoto, S.; Winkler, T.; Baron, R.M.; Grogg, K.; Paula, L.F.S.C.; Santos, A.; Zeng, C.; Hibbert, K.; Harris, R.S.; et al. Deterioration of Regional Lung Strain and Inflammation during Early Lung Injury. Am. J. Respir. Crit. Care Med. 2018, 198, 891–902. [Google Scholar] [CrossRef]
- Gattinoni, L.; Marini, J.J.; Pesenti, A.; Quintel, M.; Mancebo, J.; Brochard, L. The “baby lung” became an adult. Intensive Care Med. 2016, 42, 663–673. [Google Scholar] [CrossRef]
- Chiumello, D.; Marino, A.; Brioni, M.; Cigada, I.; Menga, F.; Colombo, A.; Crimella, F.; Algieri, I.; Cressoni, M.; Carlesso, E.; et al. Lung Recruitment Assessed by Respiratory Mechanics and Computed Tomography in Patients with Acute Respiratory Distress Syndrome. What Is the Relationship? Am. J. Respir. Crit. Care Med. 2016, 193, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.A.; Peevy, K.J.; Moise, A.A.; Parker, J.C. Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J. Appl. Physiol. (1985) 1989, 66, 2364–2368. [Google Scholar] [CrossRef] [PubMed]
- Carlton, D.P.; Cummings, J.J.; Scheerer, R.G.; Poulain, F.R.; Bland, R.D. Lung overexpansion increases pulmonary microvascular protein permeability in young lambs. J. Appl. Physiol. (1985) 1990, 69, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Adkins, W.K.; Hernandez, L.A.; Coker, P.J.; Buchanan, B.; Parker, J.C. Age effects susceptibility to pulmonary barotrauma in rabbits. Crit. Care Med. 1991, 19, 390–393. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Saumon, G. Ventilator-induced lung injury: Lessons from experimental studies. Am. J. Respir. Crit. Care Med. 1998, 157, 294–323. [Google Scholar] [CrossRef]
- Bouhuys, A. Physiology and musical instruments. Nature 1969, 221, 1199–1204. [Google Scholar] [CrossRef]
- Gattinoni, L.; Protti, A.; Caironi, P.; Carlesso, E. Ventilator-induced lung injury: The anatomical and physiological framework. Crit. Care Med. 2010, 38 (Suppl. S10), S539–S548. [Google Scholar] [CrossRef]
- Gattinoni, L.; Carlesso, E.; Cadringher, P.; Valenza, F.; Vagginelli, F.; Chiumello, D. Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur. Respir. J. Suppl. 2003, 47, 15s–25s. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Swenson, K.E.; Swenson, E.R. Pathophysiology of Acute Respiratory Distress Syndrome and COVID-19 Lung Injury. Crit. Care Clin. 2021, 37, 749–776. [Google Scholar] [CrossRef]
- Beitler, J.R.; Malhotra, A.; Thompson, B.T. Ventilator-induced Lung Injury. Clin. Chest Med. 2016, 37, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Song, Y.L.; Hu, Z.Y.; Zhang, S.; Chen, Y.Z. An estimation of mechanical stress on alveolar walls during repetitive alveolar reopening and closure. J. Appl. Physiol. (1985) 2015, 119, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Bilek, A.M.; Dee, K.C.; Gaver, D.P., 3rd. Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J. Appl. Physiol. (1985) 2003, 94, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Mead, J.; Takishima, T.; Leith, D. Stress distribution in lungs: A model of pulmonary elasticity. J. Appl. Physiol. 1970, 28, 596–608. [Google Scholar] [CrossRef]
- Albert, K.; Krischer, J.M.; Pfaffenroth, A.; Wilde, S.; Lopez-Rodriguez, E.; Braun, A.; Smith, B.J.; Knudsen, L. Hidden Microatelectases Increase Vulnerability to Ventilation-Induced Lung Injury. Front. Physiol. 2020, 11, 530485. [Google Scholar] [CrossRef]
- Cressoni, M.; Chiurazzi, C.; Gotti, M.; Amini, M.; Brioni, M.; Algieri, I.; Cammaroto, A.; Rovati, C.; Massari, D.; di Castiglione, C.B.; et al. Lung inhomogeneities and time course of ventilator-induced mechanical injuries. Anesthesiology 2015, 123, 618–627. [Google Scholar] [CrossRef]
- Retamal, J.; Hurtado, D.; Villarroel, N.; Bruhn, A.; Bugedo, G.; Amato, M.B.P.; Costa, E.L.V.; Hedenstierna, G.; Larsson, A.; Borges, J.B. Does Regional Lung Strain Correlate With Regional Inflammation in Acute Respiratory Distress Syndrome During Nonprotective Ventilation? An Experimental Porcine Study. Crit. Care Med. 2018, 46, e591–e599. [Google Scholar] [CrossRef]
- Retamal, J.; Bergamini, B.C.; Carvalho, A.R.; Bozza, F.A.; Borzone, G.; Borges, J.B.; Larsson, A.; Hedenstierna, G.; Bugedo, G.; Bruhn, A. Non-lobar atelectasis generates inflammation and structural alveolar injury in the surrounding healthy tissue during mechanical ventilation. Crit. Care 2014, 18, 505. [Google Scholar] [CrossRef]
- Seah, A.S.; Grant, K.A.; Aliyeva, M.; Allen, G.B.; Bates, J.H. Quantifying the roles of tidal volume and PEEP in the pathogenesis of ventilator-induced lung injury. Ann. Biomed. Eng. 2011, 39, 1505–1516. [Google Scholar] [CrossRef]
- Protti, A.; Andreis, D.T.; Iapichino, G.E.; Monti, M.; Comini, B.; Milesi, M.; Zani, L.; Gatti, S.; Lombardi, L.; Gattinoni, L. High positive end-expiratory pressure: Only a dam against oedema formation? Crit. Care 2013, 17, R131. [Google Scholar] [CrossRef]
- Malhotra, A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N. Engl. J. Med. 2007, 357, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Caironi, P.; Cressoni, M.; Chiumello, D.; Ranieri, M.; Quintel, M.; Russo, S.G.; Cornejo, R.; Bugedo, G.; Carlesso, E.; Russo, R.; et al. Lung opening and closing during ventilation of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2010, 181, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.B.; Barbas, C.S.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi-Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Munoz, C.; Oliveira, R.; et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Meade, M.O.; Cook, D.J.; Guyatt, G.H.; Slutsky, A.S.; Arabi, Y.M.; Cooper, D.J.; Davies, A.R.; Hand, L.E.; Zhou, Q.; Thabane, L.; et al. Ventilation strategy using low tidal volumes recruitment maneuvers high positive end-expiratory pressure for acute lung injury acute respiratory distress syndrome: A randomized controlled trial. JAMA 2008, 299, 637–645. [Google Scholar] [CrossRef]
- Terragni, P.P.; Rosboch, G.; Tealdi, A.; Corno, E.; Menaldo, E.; Davini, O.; Gandini, G.; Herrmann, P.; Mascia, L.; Quintel, M.; et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2007, 175, 160–166. [Google Scholar] [CrossRef]
- Grasso, S.; Stripoli, T.; De Michele, M.; Bruno, F.; Moschetta, M.; Angelelli, G.; Munno, I.; Ruggiero, V.; Anaclerio, R.; Cafarelli, A.; et al. ARDSnet ventilatory protocol and alveolar hyperinflation: Role of positive end-expiratory pressure. Am. J. Respir. Crit. Care Med. 2007, 176, 761–767. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Suter, P.M.; Tortorella, C.; De Tullio, R.; Dayer, J.M.; Brienza, A.; Bruno, F.; Slutsky, A.S. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: A randomized controlled trial. JAMA 1999, 282, 54–61. [Google Scholar] [CrossRef]
- Chiumello, D.; Pristine, G.; Slutsky, A.S. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1999, 160, 109–116. [Google Scholar] [CrossRef]
- Imai, Y.; Parodo, J.; Kajikawa, O.; de Perrot, M.; Fischer, S.; Edwards, V.; Cutz, E.; Liu, M.; Keshavjee, S.; Martin, T.R.; et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 2003, 289, 2104–2112. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Giunta, F.; Suter, P.M.; Slutsky, A.S. Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA 2000, 284, 43–44. [Google Scholar] [CrossRef]
- Dolinay, T.; Kim, Y.S.; Howrylak, J.; Hunninghake, G.M.; An, C.H.; Fredenburgh, L.; Massaro, A.F.; Rogers, A.; Gazourian, L.; Nakahira, K.; et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am. J. Respir. Crit. Care Med. 2012, 185, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Zhuo, H.; Brady, S.; Levitt, J.; Steingrub, J.; Siegel, M.D.; Soto, G.; Peterson, M.W.; Chesnutt, M.S.; Matthay, M.A.; et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: Results from two clinical trials. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L634–L639. [Google Scholar] [CrossRef] [PubMed]
- Parsons, P.E.; Eisner, M.D.; Thompson, B.T.; Matthay, M.A.; Ancukiewicz, M.; Bernard, G.R.; Wheeler, A.P.; NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit. Care Med. 2005, 33, 1–6; discussion 230–232. [Google Scholar] [CrossRef]
- Barbeta, E.; Ferrando, C.; López-Aladid, R.; Motos, A.; Bueno-Freire, L.; Fernández-Barat, L.; Soler-Comas, A.; Palomeque, A.; Gabarrús, A.; Artigas, A.; et al. Association between driving pressure, systemic inflammation and non-pulmonary organ dysfunction in patients with acute respiratory distress syndrome, a prospective pathophysiological study. Anaesth. Crit. Care Pain. Med. 2025, 44, 101458. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Tonetti, T.; Cressoni, M.; Cadringher, P.; Herrmann, P.; Moerer, O.; Protti, A.; Gotti, M.; Chiurazzi, C.; Carlesso, E.; et al. Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 2016, 42, 1567–1575. [Google Scholar] [CrossRef]
- Cressoni, M.; Gotti, M.; Chiurazzi, C.; Massari, D.; Algieri, I.; Amini, M.; Cammaroto, A.; Brioni, M.; Montaruli, C.; Nikolla, K.; et al. Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology 2016, 124, 1100–1108. [Google Scholar] [CrossRef]
- Tonetti, T.; Vasques, F.; Rapetti, F.; Maiolo, G.; Collino, F.; Romitti, F.; Camporota, L.; Cressoni, M.; Cadringher, P.; Quintel, M.; et al. Driving pressure and mechanical power: New targets for VILI prevention. Ann. Transl. Med. 2017, 5, 286. [Google Scholar] [CrossRef]
- Paudel, R.; Trinkle, C.A.; Waters, C.M.; Robinson, L.E.; Cassity, E.; Sturgill, J.L.; Broaddus, R.; Morris, P.E. Mechanical Power: A New Concept in Mechanical Ventilation. Am. J. Med. Sci. 2021, 362, 537–545. [Google Scholar] [CrossRef]
- Giosa, L.; Busana, M.; Pasticci, I.; Bonifazi, M.; Macrì, M.M.; Romitti, F.; Vassalli, F.; Chiumello, D.; Quintel, M.; Marini, J.J.; et al. Mechanical power at a glance: A simple surrogate for volume-controlled ventilation. Intensive Care Med. Exp. 2019, 7, 61. [Google Scholar] [CrossRef]
- Becher, T.; van der Staay, M.; Schädler, D.; Frerichs, I.; Weiler, N. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med. 2019, 45, 1321–1323. [Google Scholar] [CrossRef]
- Serpa Neto, A.; Deliberato, R.O.; Johnson, A.E.W.; Bos, L.D.; Amorim, P.; Pereira, S.M.; Cazati, D.C.; Cordioli, R.L.; Correa, T.D.; Pollard, T.J.; et al. Mechanical power of ventilation is associated with mortality in critically ill patients: An analysis of patients in two observational cohorts. Intensive Care Med. 2018, 44, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Parhar, K.K.S.; Zjadewicz, K.; Soo, A.; Sutton, A.; Zjadewicz, M.; Doig, L.; Lam, C.; Ferland, A.; Niven, D.J.; Fiest, K.M.; et al. Epidemiology, Mechanical Power, and 3-Year Outcomes in Acute Respiratory Distress Syndrome Patients Using Standardized Screening. An Observational Cohort Study. Ann. Am. Thorac. Soc. 2019, 16, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifah, A.S.; Rumindo, K.; Brincat, E.; Blanchard, F.; Helleberg, J.; Clarke, D.; Popoff, B.; Duranteau, O.; Mohamed, Z.U.; Senosy, A. Optimizing mechanical ventilation: Personalizing mechanical power to reduce ICU mortality—A retrospective cohort study. PLoS ONE 2025, 2, e0318018. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.C.; Lin, S.W.; Chuang, L.P.; Li, H.H.; Liu, P.H.; Tsai, F.C.; Chang, C.H.; Hung, C.Y.; Lee, C.S.; Leu, S.W.; et al. Mechanical power during extracorporeal membrane oxygenation and hospital mortality in patients with acute respiratory distress syndrome. Crit. Care 2021, 25, 13. [Google Scholar] [CrossRef]
- Battaglini, D.; Fazzini, B.; Silva, P.L.; Cruz, F.F.; Ball, L.; Robba, C.; Rocco, P.R.M.; Pelosi, P. Challenges in ARDS Definition, Management, and Identification of Effective Personalized Therapies. J. Clin. Med. 2023, 12, 1381. [Google Scholar] [CrossRef]
- Costa, E.L.V.; Slutsky, A.S.; Brochard, L.J.; Brower, R.; Serpa-Neto, A.; Cavalcanti, A.B.; Mercat, A.; Meade, M.; Morais, C.C.A.; Goligher, E.; et al. Ventilatory Variables and Mechanical Power in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2021, 204, 303–311. [Google Scholar] [CrossRef]
- Robba, C.; Badenes, R.; Battaglini, D.; Ball, L.; Brunetti, I.; Jakobsen, J.C.; Lilja, G.; Friberg, H.; Wendel-Garcia, P.D.; Young, P.J.; et al. Ventilatory settings in the initial 72 h and their association with outcome in out-of-hospital cardiac arrest patients: A preplanned secondary analysis of the targeted hypothermia versus targeted normothermia after out-of-hospital cardiac arrest (TTM2) trial. Intensive Care Med. 2022, 48, 1024–1038. [Google Scholar]
- Maeda, Y.; Fujino, Y.; Uchiyama, A.; Matsuura, N.; Mashimo, T.; Nishimura, M. Effects of peak inspiratory flow on development of ventilator-induced lung injury in rabbits. Anesthesiology 2004, 101, 722–728. [Google Scholar] [CrossRef]
- Felix, N.S.; Samary, C.S.; Cruz, F.F.; Rocha, N.N.; Fernandes, M.V.S.; Machado, J.A.; Bose-Madureira, R.L.; Capelozzi, V.L.; Pelosi, P.; Silva, P.L.; et al. Gradually Increasing Tidal Volume May Mitigate Experimental Lung Injury in Rats. Anesthesiology 2019, 130, 767–777. [Google Scholar] [CrossRef]
- Borsellino, B.; Schultz, M.J.; Gama de Abreu, M.; Robba, C.; Bilotta, F. Mechanical ventilation in neurocritical care patients: A systematic literature review. Expert. Rev. Respir. Med. 2016, 10, 1123–1132. [Google Scholar] [CrossRef]
- Nucci, G.; Suki, B.; Lutchen, K. Modeling airflow-related shear stress during heterogeneous constriction and mechanical ventilation. J. Appl. Physiol. (1985) 2003, 95, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Wittenstein, J.; Huhle, R.; Scharffenberg, M.; Kiss, T.; Herold, J.; Vivona, L.; Bergamaschi, A.; Schultz, M.J.; Pelosi, P.; Gama de Abreu, M.; et al. Effects of two stepwise lung recruitment strategies on respiratory function and haemodynamics in anaesthetised pigs: A randomised crossover study. Eur. J. Anaesthesiol. 2021, 38, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Serpa Neto, A.; Trifiletti, V.; Mandelli, M.; Firpo, I.; Robba, C.; Gama de Abreu, M.; Schultz, M.J.; Patroniti, N.; Rocco, P.R.M.; et al. Effects of higher PEEP and recruitment manoeuvres on mortality in patients with ARDS: A systematic review, meta-analysis, meta-regression and trial sequential analysis of randomized controlled trials. Intensive Care Med. Exp. 2020, 8 (Suppl. S1), 39. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators; Cavalcanti, A.B.; Suzumura, É.A.; Laranjeira, L.N.; Paisani, D.M.; Damiani, L.P.; Guimarães, H.P.; Romano, E.R.; Regenga, M.M.; Taniguchi, L.N.T.; et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017, 318, 1335–1345. [Google Scholar]
- Hodgson, C.L.; Cooper, D.J.; Arabi, Y.; King, V.; Bersten, A.; Bihari, S.; Brickell, K.; Davies, A.; Fahey, C.; Fraser, J.; et al. Maximal Recruitment Open Lung Ventilation in Acute Respiratory Distress Syndrome (PHARLAP). A Phase II, Multicenter Randomized Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 1363–1372. [Google Scholar] [CrossRef]
- Katira, B.H.; Engelberts, D.; Otulakowski, G.; Giesinger, R.E.; Yoshida, T.; Post, M.; Kuebler, W.M.; Connelly, K.A.; Kavanagh, B.P. Abrupt Deflation after Sustained Inflation Causes Lung Injury. Am. J. Respir. Crit. Care Med. 2018, 198, 1165–1176. [Google Scholar] [CrossRef]
- Rocha, N.N.; Samary, C.S.; Antunes, M.A.; Oliveira, M.V.; Hemerly, M.R.; Santos, P.S.; Capelozzi, V.L.; Cruz, F.F.; Marini, J.J.; Silva, P.L.; et al. The impact of fluid status and decremental PEEP strategy on cardiac function and lung and kidney damage in mild-moderate experimental acute respiratory distress syndrome. Respir. Res. 2021, 22, 214. [Google Scholar] [CrossRef]
- Xavier, P.H.; Fonseca, A.C.F.; Gonçalves, L.A.; de Sousa, G.C.; Silva, M.C.D.; Sacramento, R.F.M.; Samary, C.D.S.; Medeiros, M.; Cruz, F.F.; Capelozzi, V.L.; et al. Lung Injury Is Induced by Abrupt Increase in Respiratory Rate but Prevented by Recruitment Maneuver in Mild Acute Respiratory Distress Syndrome in Rats. Anesthesiology 2023, 138, 420–435. [Google Scholar] [CrossRef]
- Pelosi, P.; D’Andrea, L.; Vitale, G.; Pesenti, A.; Gattinoni, L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1994, 149, 8–13. [Google Scholar] [CrossRef]
- Albert, R.K. The role of ventilation-induced surfactant dysfunction and atelectasis in causing acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2012, 185, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; D’Andrea, L.; Pelosi, P.; Vitale, G.; Pesenti, A.; Fumagalli, R. Regional Effects and Mechanism of Positive End-Expiratory Pressure in Early Adult Respiratory Distress Syndrome. JAMA 1993, 269, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A. The concept of “baby lung”. Intensive Care Med. 2005, 31, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A.; Carlesso, E. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure: Impact and clinical fallout through the following 20 years. Intensive Care Med. 2013, 39, 1909–1915. [Google Scholar] [CrossRef]
- Gattinoni, L.; Pelosi, P.; Vitale, G.; Pesenti, A.; D’Andrea, L.; Mascheroni, D. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure. Anesthesiology 1991, 74, 15–23. [Google Scholar] [CrossRef]
- Cressoni, M.; Chiumello, D.; Chiurazzi, C.; Brioni, M.; Algieri, I.; Gotti, M.; Nikolla, K.; Massari, D.; Cammaroto, A.; Colombo, A.; et al. Lung inhomogeneities, inflation and [18F]2-fluoro-2-deoxy-D-glucose uptake rate in acute respiratory distress syndrome. Eur. Respir. J. 2016, 47, 233–242. [Google Scholar] [CrossRef]
- Bates, J.H.T.; Smith, B.J. Ventilator-induced lung injury and lung mechanics. Ann. Transl. Med. 2018, 6, 378. [Google Scholar] [CrossRef]
- Perlman, C.E.; Lederer, D.J.; Bhattacharya, J. Micromechanics of alveolar edema. Am. J. Respir. Cell Mol. Biol. 2011, 44, 34–39. [Google Scholar] [CrossRef]
- Wellman, T.J.; Winkler, T.; Costa, E.L.; Musch, G.; Harris, R.S.; Zheng, H.; Venegas, J.G.; Vidal Melo, M.F. Effect of local tidal lung strain on inflammation in normal and lipopolysaccharide-exposed sheep*. Crit. Care Med. 2014, 42, e491–e500. [Google Scholar] [CrossRef]
- Bellani, G.; Messa, C.; Guerra, L.; Spagnolli, E.; Foti, G.; Patroniti, N.; Fumagalli, R.; Musch, G.; Fazio, F.; Pesenti, A. Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: A [18F]-fluoro-2-deoxy-D-glucose PET/CT study. Crit. Care Med. 2009, 37, 2216–2222. [Google Scholar] [CrossRef]
- Cressoni, M.; Cadringher, P.; Chiurazzi, C.; Amini, M.; Gallazzi, E.; Marino, A.; Brioni, M.; Carlesso, E.; Chiumello, D.; Quintel, M.; et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2014, 189, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zhong, M.; Dong, M.H.; Song, J.Q.; Zheng, Y.J.; Wu, W.; Tao, J.L.; Zhu, L.; Zheng, X. Prone positioning improves ventilation-perfusion matching assessed by electrical impedance tomography in patients with ARDS: A prospective physiological study. Crit. Care 2022, 26, 154. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Taccone, P.; Carlesso, E.; Marini, J.J. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am. J. Respir. Crit. Care Med. 2013, 188, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pelosi, P.; Crotti, S.; Valenza, F. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1995, 151, 1807–1814. [Google Scholar] [CrossRef]
- Beitler, J.R.; Guérin, C.; Ayzac, L.; Mancebo, J.; Bates, D.M.; Malhotra, A.; Talmor, D. PEEP titration during prone positioning for acute respiratory distress syndrome. Crit. Care. 2015, 19, 436. [Google Scholar] [CrossRef][Green Version]
- Protti, A.; Andreis, D.T.; Monti, M.; Santini, A.; Sparacino, C.C.; Langer, T.; Votta, E.; Gatti, S.; Lombardi, L.; Leopardi, O.; et al. Lung stress and strain during mechanical ventilation: Any difference between statics and dynamics? Crit. Care Med. 2013, 41, 1046–1055. [Google Scholar] [CrossRef]
- Sahetya, S.K.; Goligher, E.C.; Brower, R.G. Fifty Years of Research in ARDS. Setting Positive End-Expiratory Pressure in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1429–1438. [Google Scholar] [CrossRef]
- Malo, J.; Ali, J.; Wood, L.D. How does positive end-expiratory pressure reduce intrapulmonary shunt in canine pulmonary edema? J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1002–1010. [Google Scholar] [CrossRef]
- Paré, P.D.; Warriner, B.; Baile, E.M.; Hogg, J.C. Redistribution of pulmonary extravascular water with positive end-expiratory pressure in canine pulmonary edema. Am. Rev. Respir. Dis. 1983, 127, 590–593. [Google Scholar] [CrossRef]
- Suter, P.M.; Fairley, B.; Isenberg, M.D. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N. Engl. J. Med. 1975, 292, 284–289. [Google Scholar] [CrossRef]
- Cournand, A.; Motley, H.L. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am. J. Physiol. 1948, 152, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Dhainaut, J.F.; Devaux, J.Y.; Monsallier, J.F.; Brunet, F.; Villemant, D.; Huyghebaert, M.F. Mechanisms of decreased left ventricular preload during continuous positive pressure ventilation in ARDS. Chest 1986, 90, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Puybasset, L.; Gusman, P.; Muller, J.C.; Cluzel, P.; Coriat, P.; Rouby, J.J. Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure. CT Scan ARDS Study Group. Adult Respiratory Distress Syndrome. Intensive Care Med. 2000, 26, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Caironi, P.; Cressoni, M.; Chiumello, D.; Ranieri, V.M.; Quintel, M.; Russo, S.; Patroniti, N.; Cornejo, R.; Bugedo, G. Lung recruitment in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2006, 354, 1775–1786. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology Patterns of Care Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Pelosi, P.; Brazzi, L.; Gattinoni, L. Prone position in acute respiratory distress syndrome. Eur. Respir. J. 2002, 20, 1017–1028. [Google Scholar] [CrossRef]
- Guérin, C.; Albert, R.K.; Beitler, J.; Gattinoni, L.; Jaber, S.; Marini, J.J.; Munshi, L.; Papazian, L.; Pesenti, A.; Vieillard-Baron, A.; et al. Prone position in ARDS patients: Why, when, how and for whom. Intensive Care Med. 2020, 46, 2385–2396. [Google Scholar] [CrossRef]
- Raimondi Cominesi, D.; Forcione, M.; Pozzi, M.; Giani, M.; Foti, G.; Rezoagli, E.; Cipulli, F. Pulmonary shunt in critical care: A practical approach with clinical scenarios. J. Anesth. Analg. Crit. Care 2024, 4, 18. [Google Scholar] [CrossRef]
- Scholten, E.L.; Beitler, J.R.; Prisk, G.K.; Malhotra, A. Treatment of ARDS With Prone Positioning. Chest 2017, 151, 215–224. [Google Scholar] [CrossRef]
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Munshi, L.; Del Sorbo, L.; Adhikari, N.K.J.; Hodgson, C.L.; Wunsch, H.; Meade, M.O.; Uleryk, E.; Mancebo, J.; Pesenti, A.; Ranieri, V.M.; et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S4), S280–S288. [Google Scholar] [CrossRef] [PubMed]
- Estrella-Alonso, A.; Silva-Obregón, J.A.; Fernández-Tobar, R.; Marián-Crespo, C.; Ruiz de Santaquiteria-Torres, V.; Jiménez-Puente, G.; Arroyo-Espliguero, R.; Viana-Llamas, M.C.; Ramírez-Cervantes, K.L.; Quintana-Díaz, M. Extended Prone Position and 90-Day Mortality in Mechanically Ventilated Patients With COVID-19. Respir. Care 2024, 69, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Sella, N.; Bellani, G.; Foti, G.; Cortegiani, A.; Lorenzoni, G.; Gregori, D.; Boscolo, A.; Cattin, L.; Elhadi, M.; et al. Oxygenation improvement and duration of prone positioning are associated with ICU mortality in mechanically ventilated COVID-19 patients. Ann. Intensive Care. 2025, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Yang, T.; Dinglas, V.D.; Mendez-Tellez, P.A.; Shanholtz, C.; Sevransky, J.E.; Brower, R.G.; Pronovost, P.J.; Colantuoni, E. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am. J. Respir. Crit. Care Med. 2015, 191, 177–185. [Google Scholar] [CrossRef]
- Fuller, B.M.; Ferguson, I.T.; Mohr, N.M.; Drewry, A.M.; Palmer, C.; Wessman, B.T.; Ablordeppey, E.; Keeperman, J.; Stephens, R.J.; Briscoe, C.C.; et al. Lung-Protective Ventilation Initiated in the Emergency Department (LOV-ED): A Quasi-Experimental, Before-After Trial. Ann. Emerg. Med. 2017, 70, 406–418.e4. [Google Scholar] [CrossRef]
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023, 49, 727–759. [Google Scholar] [CrossRef]
- Hager, D.N.; Krishnan, J.A.; Hayden, D.L.; Brower, R.G.; ARDS Clinical Trials Network. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am. J. Respir. Crit. Care Med. 2005, 172, 1241–1245. [Google Scholar] [CrossRef]
- Deans, K.J.; Minneci, P.C.; Cui, X.; Banks, S.M.; Natanson, C.; Eichacker, P.Q. Mechanical ventilation in ARDS: One size does not fit all. Crit. Care Med. 2005, 33, 1141–1143. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Cook, D.J.; Guyatt, G.H.; Mehta, S.; Hand, L.; Austin, P.; Zhou, Q.; Matte, A.; Walter, S.D.; Lamontagne, F.; et al. High-frequency oscillation in early acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 795–805. [Google Scholar] [CrossRef]
- Young, D.; Lamb, S.E.; Shah, S.; MacKenzie, I.; Tunnicliffe, W.; Lall, R.; Rowan, K.; Cuthbertson, B.H.; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 806–813. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, Q.; Yang, H.; Dong, W.; Wang, B.; Zhang, Z.; Liang, G. Airway pressure release ventilation versus low tidal volume ventilation for patients with acute respiratory distress syndrome/acute lung injury: A meta-analysis of randomized clinical trials. Ann. Transl. Med. 2020, 8, 1641. [Google Scholar] [CrossRef] [PubMed]
- Brower, R.G.; Lanken, P.N.; MacIntyre, N.; Matthay, M.A.; Morris, A.; Ancukiewicz, M.; Schoenfeld, D.; Thompson, B.T.; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2004, 351, 327–336. [Google Scholar] [PubMed]
- Mercat, A.; Richard, J.C.; Vielle, B.; Jaber, S.; Osman, D.; Diehl, J.L.; Lefrant, J.Y.; Prat, G.; Richecoeur, J.; Nieszkowska, A.; et al. Positive end-expiratory pressure setting in adults with acute lung injury acute respiratory distress syndrome: A randomized controlled trial. JAMA 2008, 299, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Briel, M.; Meade, M.; Mercat, A.; Brower, R.G.; Talmor, D.; Walter, S.D.; Slutsky, A.S.; Pullenayegum, E.; Zhou, Q.; Cook, D.; et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA 2010, 303, 865–873. [Google Scholar] [CrossRef]
- Fougères, E.; Teboul, J.L.; Richard, C.; Osman, D.; Chemla, D.; Monnet, X. Hemodynamic impact of a positive end-expiratory pressure setting in acute respiratory distress syndrome: Importance of the volume status. Crit. Care Med. 2010, 38, 802–807. [Google Scholar] [CrossRef]
- Mekontso Dessap, A.; Boissier, F.; Charron, C.; Bégot, E.; Repessé, X.; Legras, A.; Brun-Buisson, C.; Vignon, P.; Vieillard-Baron, A. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: Prevalence, predictors, and clinical impact. Intensive Care Med. 2016, 42, 862–870. [Google Scholar] [CrossRef]
- Ball, L.; Talmor, D.; Pelosi, P. Transpulmonary pressure monitoring in critically ill patients: Pros and cons. Crit Care. 2024, 28, 177. [Google Scholar] [CrossRef]
- Cressoni, M.; Chiumello, D.; Carlesso, E.; Chiurazzi, C.; Amini, M.; Brioni, M.; Cadringher, P.; Quintel, M.; Gattinoni, L. Compressive forces and computed tomography-derived positive end-expiratory pressure in acute respiratory distress syndrome. Anesthesiology 2014, 121, 572–581. [Google Scholar] [CrossRef]
- Radwan, W.A.; Khaled, M.M.; Salman, A.G.; Fakher, M.A.; Khatab, S. Use of lung ultrasound for assessment of lung recruitment maneuvers in patients with ARDS. J. Med. Sci. 2021, 9, 952–963. [Google Scholar] [CrossRef]
- Bouhemad, B.; Brisson, H.; Le-Guen, M.; Arbelot, C.; Lu, Q.; Rouby, J.J. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am. J. Respir. Crit. Care Med. 2011, 183, 341–347. [Google Scholar] [CrossRef]
- Tusman, G.; Acosta, C.M.; Costantini, M. Ultrasonography for the assessment of lung recruitment maneuvers. Crit. Ultrasound J. 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, P.; Marra, A.; Iacovazzo, C.; Merola, R.; De Siena, A.U.; Servillo, G.; Vargas, M. Electric impedance tomography and protective mechanical ventilation in elective robotic-assisted laparoscopy surgery with steep Trendelenburg position: A randomized controlled study. Sci. Rep. 2023, 13, 2753. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.V.; Weirauch, A.J.; Culter, C.A.; Choi, P.J.; Hyzy, R.C. Electrical Impedance Tomography in Acute Respiratory Distress Syndrome Management. Crit. Care Med. 2022, 50, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.L.; Borges, J.B.; Melo, A.; Suarez-Sipmann, F.; Toufen CJr Bohm, S.H.; Amato, M.B. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009, 35, 1132–1137. [Google Scholar] [CrossRef]
- Frerichs, I.; Amato, M.B.; van Kaam, A.H.; Tingay, D.G.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Böhm, S.H.; Teschner, E.; et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2017, 72, 83–93. [Google Scholar] [CrossRef]
- He, H.; Chi, Y.; Yang, Y.; Yuan, S.; Long, Y.; Zhao, P.; Frerichs, I.; Fu, F.; Möller, K.; Zhao, Z. Early individualized positive end-expiratory pressure guided by electrical impedance tomography in acute respiratory distress syndrome: A randomized controlled clinical trial. Crit. Care 2021, 25, 230. [Google Scholar] [CrossRef]
- Hsu, H.J.; Chang, H.T.; Zhao, Z.; Wang, P.H.; Zhang, J.H.; Chen, Y.S.; Frerichs, I.; Möller, K.; Fu, F.; Hsu, H.S.; et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve: A randomized trial in moderate to severe ARDS. Physiol. Meas. 2021, 42, 014002. [Google Scholar] [CrossRef]
- Jimenez, J.V.; Munroe, E.; Weirauch, A.J.; Fiorino, K.; Culter, C.A.; Nelson, K.; Labaki, W.W.; Choi, P.J.; Co, I.; Standiford, T.J.; et al. Electric impedance tomography-guided PEEP titration reduces mechanical power in ARDS: A randomized crossover pilot trial. Crit. Care 2023, 27, 21. [Google Scholar] [CrossRef]
- Constantin, J.M.; Jabaudon, M.; Lefrant, J.Y.; Jaber, S.; Quenot, J.P.; Langeron, O.; Ferrandière, M.; Grelon, F.; Seguin, P.; Ichai, C.; et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): A multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 2019, 7, 870–880. [Google Scholar] [CrossRef]
- Bellani, G.; Guerra, L.; Musch, G.; Zanella, A.; Patroniti, N.; Mauri, T.; Messa, C.; Pesenti, A. Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. Am. J. Respir. Crit. Care Med. 2011, 183, 1193–1199. [Google Scholar] [CrossRef]
- Abrams, D.; Schmidt, M.; Pham, T.; Beitler, J.R.; Fan, E.; Goligher, E.C.; McNamee, J.J.; Patroniti, N.; Wilcox, M.E.; Combes, A.; et al. Mechanical Ventilation for Acute Respiratory Distress Syndrome during Extracorporeal Life Support. Research and Practice. Am. J. Respir. Crit. Care Med. 2020, 201, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Umbrello, M.; Marino, A.; Chiumello, D. Tidal volume in acute respiratory distress syndrome: How best to select it. Ann. Transl. Med. 2017, 5, 287. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Agerstrand, C.; Beitler, J.R.; Karagiannidis, C.; Madahar, P.; Yip, N.H.; Pesenti, A.; Slutsky, A.S.; Brochard, L.; Brodie, D. Risks and Benefits of Ultra-Lung-Protective Invasive Mechanical Ventilation Strategies with a Focus on Extracorporeal Support. Am. J. Respir. Crit. Care Med. 2022, 205, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Brodie, D.; Bacchetta, M. Extracorporeal membrane oxygenation for ARDS in adults. N. Engl. J. Med. 2011, 365, 1905–1914. [Google Scholar] [CrossRef]
- Brodie, D.; Slutsky, A.S.; Combes, A. Extracorporeal Life Support for Adults With Respiratory Failure and Related Indications: A Review. JAMA 2019, 322, 557–568. [Google Scholar] [CrossRef]
- Combes, A.; Fanelli, V.; Pham, T.; Ranieri, V.M.; European Society of Intensive Care Medicine Trials Group and the “Strategy of Ultra-Protective lung ventilation with Extracorporeal CO2 Removal for New-Onset moderate to severe ARDS” (SUPERNOVA) investigators. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: The SUPERNOVA study. Intensive Care Med. 2019, 45, 592–600. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Goligher, E.C.; Tomlinson, G.; Hajage, D.; Wijeysundera, D.N.; Fan, E.; Jüni, P.; Brodie, D.; Slutsky, A.S.; Combes, A. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome and Posterior Probability of Mortality Benefit in a Post Hoc Bayesian Analysis of a Randomized Clinical Trial. JAMA 2018, 320, 2251–2259. [Google Scholar] [CrossRef]
- Papazian, L.; Forel, J.M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.M.; Perez, D.; Seghboyan, J.M.; et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef]
- Merola, R.; Vargas, M.; Sanfilippo, F.; Vergano, M.; Mistraletti, G.; Vetrugno, L.; De Pascale, G.; Bignami, E.G.; Servillo, G.; Battaglini, D. Tracheostomy Practice in the Italian Intensive Care Units: A Point-Prevalence Survey. Medicina 2025, 61, 87. [Google Scholar] [CrossRef]
- Forel, J.M.; Roch, A.; Marin, V.; Michelet, P.; Demory, D.; Blache, J.L.; Perrin, G.; Gainnier, M.; Bongrand, P.; Papazian, L. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit. Care Med. 2006, 34, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.T.N.; Patolia, S.; Guervilly, C. Neuromuscular blockade in acute respiratory distress syndrome: A systematic review and meta-analysis of randomized controlled trials. J. Intensive Care. 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Laghi, F.; Cattapan, S.E.; Jubran, A.; Parthasarathy, S.; Warshawsky, P.; Choi, Y.S.; Tobin, M.J. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am. J. Respir. Crit. Care Med. 2003, 167, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Vassilakopoulos, T.; Petrof, B.J. Ventilator-induced diaphragmatic dysfunction. Am. J. Respir. Crit. Care Med. 2004, 169, 336–341. [Google Scholar] [CrossRef]
- Battaglini, D.; Robba, C.; Ball, L.; Silva, P.L.; Cruz, F.F.; Pelosi, P.; Rocco, P.R.M. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: A narrative review. Br. J. Anaesth. 2021, 127, 353–364. [Google Scholar] [CrossRef]
- Esteban, A.; Ferguson, N.D.; Meade, M.O.; Frutos-Vivar, F.; Apezteguia, C.; Brochard, L.; Raymondos, K.; Nin, N.; Hurtado, J.; Tomicic, V.; et al. Evolution of mechanical ventilation in response to clinical research. Am. J. Respir. Crit. Care Med. 2008, 177, 170–177. [Google Scholar] [CrossRef]
- Gama de Abreu, M.; Cuevas, M.; Spieth, P.M.; Carvalho, A.R.; Hietschold, V.; Stroszczynski, C.; Wiedemann, B.; Koch, T.; Pelosi, P.; Koch, E. Regional lung aeration and ventilation during pressure support and biphasic positive airway pressure ventilation in experimental lung injury. Crit. Care 2010, 14, R34. [Google Scholar] [CrossRef]
- Yoshida, T.; Uchiyama, A.; Matsuura, N.; Mashimo, T.; Fujino, Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: High transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit. Care Med. 2012, 40, 1578–1585. [Google Scholar] [CrossRef]
- Yoshida, T.; Grieco, D.L.; Brochard, L.; Fujino, Y. Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr. Opin. Crit. Care 2020, 26, 59–65. [Google Scholar] [CrossRef]
- Chanques, G.; Constantin, J.M.; Devlin, J.W.; Ely, E.W.; Fraser, G.L.; Gélinas, C.; Girard, T.D.; Guérin, C.; Jabaudon, M.; Jaber, S.; et al. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020, 46, 2342–2356. [Google Scholar] [CrossRef]
- Rasulo, F.A.; Badenes, R.; Longhitano, Y.; Racca, F.; Zanza, C.; Marchesi, M.; Piva, S.; Beretta, S.; Nocivelli, G.P.; Matta, B.; et al. Excessive Sedation as a Risk Factor for Delirium: A Comparison between Two Cohorts of ARDS Critically Ill Patients with and without COVID-19. Life 2022, 12, 2031. [Google Scholar] [CrossRef] [PubMed]
- Fusina, F.; Albani, F.; de Vries, H.J.; Pisani, L.; Natalini, G.; Tuinman, P.R.; Heunks, L. Flow Index as a Noninvasive Method to Evaluate Inspiratory Effort in Patients on Pressure Support Ventilation. Respir. Care 2025. [Google Scholar] [CrossRef] [PubMed]
- Rolland-Debord, C.; Poitou, T.; Bureau, C.; Rivals, I.; Similowski, T.; Demoule, A. Decreased breathing variability is associated with poorer outcome in mechanically ventilated patients. ERJ Open Res. 2023, 9, 00544–02022. [Google Scholar] [CrossRef] [PubMed]
- Boles, J.M.; Bion, J.; Connors, A.; Herridge, M.; Marsh, B.; Melot, C.; Pearl, R.; Silverman, H.; Stanchina, M.; Vieillard-Baron, A.; et al. Weaning from mechanical ventilation. Eur. Respir. J. 2007, 29, 1033–1056. [Google Scholar] [CrossRef]
- Saddy, F.; Oliveira, G.P.; Garcia, C.S.N.B.; Nardelli, L.M.; Rzezinski, A.F.; Ornellas, D.S.; Morales, M.M.; Capelozzi, V.L.; Pelosi, P.; Rocco, P.R.M. Assisted Ventilation Modes Reduce the Expression of Lung Inflammatory and Fibrogenic Mediators in a Model of Mild Acute Lung Injury. Intensive Care Med. 2010, 36, 1417–1426. [Google Scholar] [CrossRef]
- Saddy, F.; Moraes, L.; Santos, C.L.; Oliveira, G.P.; Cruz, F.F.; Morales, M.M.; Capelozzi, V.L.; de Abreu, M.G.; Garcia, C.S.; Pelosi, P.; et al. Biphasic positive airway pressure minimizes biological impact on lung tissue in mild acute lung injury independent of etiology. Crit. Care 2013, 17, R228. [Google Scholar] [CrossRef]
- da Cruz, D.G.; de Magalhães, R.F.; Padilha, G.A.; da Silva, M.C.; Braga, C.L.; Silva, A.R.; Gonçalves de Albuquerque, C.F.; Capelozzi, V.L.; Samary, C.S.; Pelosi, P.; et al. Impact of positive biphasic pressure during low and high inspiratory efforts in Pseudomonas aeruginosa-induced pneumonia. PLoS ONE 2021, 16, e0246891. [Google Scholar] [CrossRef]
- Thompson, A.F.; Moraes, L.; Rocha, N.N.; Fernandes, M.V.S.; Antunes, M.A.; Abreu, S.C.; Santos, C.L.; Capelozzi, V.L.; Samary, C.S.; de Abreu, M.G.; et al. Impact of different frequencies of controlled breath and pressure-support levels during biphasic positive airway pressure ventilation on the lung and diaphragm in experimental mild acute respiratory distress syndrome. PLoS ONE 2021, 16, e0256021. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A.; NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]
- Famous, K.R.; Delucchi, K.; Ware, L.B.; Kangelaris, K.N.; Liu, K.D.; Thompson, B.T.; Calfee, C.S.; ARDS Network. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am. J. Respir. Crit. Care Med. 2017, 195, 331–338. [Google Scholar] [CrossRef]
- Sinha, P.; Delucchi, K.L.; Thompson, B.T.; McAuley, D.F.; Matthay, M.A.; Calfee, C.S.; NHLBI ARDS Network. Latent class analysis of ARDS subphenotypes: A secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018, 44, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Delucchi, K.; Famous, K.R.; Ware, L.B.; Parsons, P.E.; Thompson, B.T.; Calfee, C.S.; ARDS Network. Stability of ARDS subphenotypes over time in two randomised controlled trials. Thorax 2018, 73, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F.; et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.; Schouten, L.R.; van Vught, L.A.; Wiewel, M.A.; Ong, D.S.Y.; Cremer, O.; Artigas, A.; Martin-Loeches, I.; Hoogendijk, A.J.; van der Poll, T.; et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax 2017, 72, 876–883. [Google Scholar] [CrossRef]
- Craig, T.R.; Duffy, M.J.; Shyamsundar, M.; McDowell, C.; O’Kane, C.M.; Elborn, J.S.; McAuley, D.F. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am. J. Respir. Crit. Care Med. 2011, 183, 620–626. [Google Scholar] [CrossRef]
- Uhlig, S.; Uhlig, U. Pharmacological interventions in ventilator-induced lung injury. Trends Pharmacol. Sci. 2004, 25, 592–600. [Google Scholar] [CrossRef]
- Curley, G.F.; Hayes, M.; Ansari, B.; Shaw, G.; Ryan, A.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax 2012, 67, 496–501. [Google Scholar] [CrossRef]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef]
- Nasa, P.; Bos, L.D.; Estenssoro, E.; van Haren, F.M.P.; Neto, A.S.; Rocco, P.R.M.; Slutsky, A.S.; Schultz, M.J.; DIOS investigators. Defining and subphenotyping ARDS: Insights from an international Delphi expert panel. Lancet Respir Med. 2025, S2213-2600(25)00115-8. [Google Scholar] [CrossRef]
- Xiong, T.; Bai, X.; Wei, X.; Wang, L.; Li, F.; Shi, H.; Shi, Y. Exercise Rehabilitation and Chronic Respiratory Diseases: Effects, Mechanisms, and Therapeutic Benefits. Int. J. Chron. Obstruct Pulmon Dis. 2023, 18, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Yan, J.; Wang, Y. Aerobic exercise alleviates ventilator-induced lung injury by inhibiting NLRP3 inflammasome activation. BMC Anesthesiol. 2022, 22, 369. [Google Scholar] [CrossRef] [PubMed]
- Parzibut, G.; Henket, M.; Moermans, C.; Struman, I.; Louis, E.; Malaise, M.; Louis, R.; Misset, B.; Njock, M.S.; Guiot, J. A Blood Exosomal miRNA Signature in Acute Respiratory Distress Syndrome. Front. Mol. Biosci. 2021, 8, 640042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, M.; Zhao, Y.; Kabir, M.A.; Peng, X. Exosomal microRNA/miRNA Dysregulation in Respiratory Diseases: From Mycoplasma-Induced Respiratory Disease to COVID-19 and Beyond. Cells 2023, 12, 2421. [Google Scholar] [CrossRef]
- Rahmel, T.; Rump, K.; Adamzik, M.; Peters, J.; Frey, U.H. Increased circulating microRNA-122 is associated with mortality and acute liver injury in the acute respiratory distress syndrome. BMC Anesthesiol. 2018, 18, 75. [Google Scholar] [CrossRef]
- Xu, F.; Yuan, J.; Tian, S.; Chen, Y.; Zhou, F. MicroRNA-92a serves as a risk factor in sepsis-induced ARDS and regulates apoptosis and cell migration in lipopolysaccharide-induced HPMEC and A549 cell injury. Life Sci. 2020, 256, 117957. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, L.; Zhang, R.; Wei, Y.; Su, L.; Tejera, P.; Guo, Y.; Wang, Z.; Lu, Q.; Baccarelli, A.A.; et al. Whole blood microRNA markers are associated with acute respiratory distress syndrome. Intensive Care Med. Exp. 2017, 5, 38. [Google Scholar] [CrossRef]
- Neto, A.S.; Tomlinson, G.; Sahetya, S.K.; Ball, L.; Nichol, A.D.; Hodgson, C.; Cavalcanti, A.B.; Briel, M.; de Abreu, M.G.; Pelosi, P.; et al. Higher PEEP for acute respiratory distress syndrome: A Bayesian meta-analysis of randomised clinical trials. Crit. Care Resusc. 2023, 23, 171–182. [Google Scholar] [CrossRef]
- Hickling, K.G.; Henderson, S.J.; Jackson, R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990, 16, 372–377. [Google Scholar] [CrossRef]
- Hickling, K.G.; Walsh, J.; Henderson, S.; Jackson, R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: A prospective study. Crit. Care Med. 1994, 22, 1568–1578. [Google Scholar] [CrossRef]
- Botta, M.; Tsonas, A.M.; Sinnige, J.S.; De Bie, A.J.R.; Bindels, A.J.G.H.; Ball, L.; Battaglini, D.; Brunetti, I.; Buiteman-Kruizinga, L.A.; van der Heiden, P.L.J.; et al. Effect of Automated Closed-loop ventilation versus convenTional VEntilation on duration and quality of ventilation in critically ill patients (ACTiVE)—Study protocol of a randomized clinical trial. Trials 2022, 23, 348. [Google Scholar] [CrossRef] [PubMed]
- Jansson, M.; Rubio, J.; Gavaldà, R.; Rello, J. Artificial Intelligence for clinical decision support in Critical Care, required and accelerated by COVID-19. Anaesth. Crit. Care Pain Med. 2020, 39, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Merola, R.; Marra, A.; Simone, S.; Vargas, M. Telemedicine in Intensive Care Unit: Current Practice and Future Prospect. J. Intensive Care Med. 2025, 40, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, S.J.; Cecconi, M. Setting the ventilator with AI support: Challenges and perspectives. Intensive Care Med. 2025, 51, 593–595. [Google Scholar] [CrossRef]
- Viderman, D.; Ayazbay, A.; Kalzhan, B.; Bayakhmetova, S.; Tungushpayev, M.; Abdildin, Y. Artificial Intelligence in the Management of Patients with Respiratory Failure Requiring Mechanical Ventilation: A Scoping Review. J. Clin. Med. 2024, 13, 7535. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merola, R.; Vargas, M.; Battaglini, D. Ventilator-Induced Lung Injury: The Unseen Challenge in Acute Respiratory Distress Syndrome Management. J. Clin. Med. 2025, 14, 3910. https://doi.org/10.3390/jcm14113910
Merola R, Vargas M, Battaglini D. Ventilator-Induced Lung Injury: The Unseen Challenge in Acute Respiratory Distress Syndrome Management. Journal of Clinical Medicine. 2025; 14(11):3910. https://doi.org/10.3390/jcm14113910
Chicago/Turabian StyleMerola, Raffaele, Maria Vargas, and Denise Battaglini. 2025. "Ventilator-Induced Lung Injury: The Unseen Challenge in Acute Respiratory Distress Syndrome Management" Journal of Clinical Medicine 14, no. 11: 3910. https://doi.org/10.3390/jcm14113910
APA StyleMerola, R., Vargas, M., & Battaglini, D. (2025). Ventilator-Induced Lung Injury: The Unseen Challenge in Acute Respiratory Distress Syndrome Management. Journal of Clinical Medicine, 14(11), 3910. https://doi.org/10.3390/jcm14113910







