Anti-Inflammatory and Immunomodulatory Effects of Intravenous Lidocaine in Surgery: A Narrative Review
Abstract
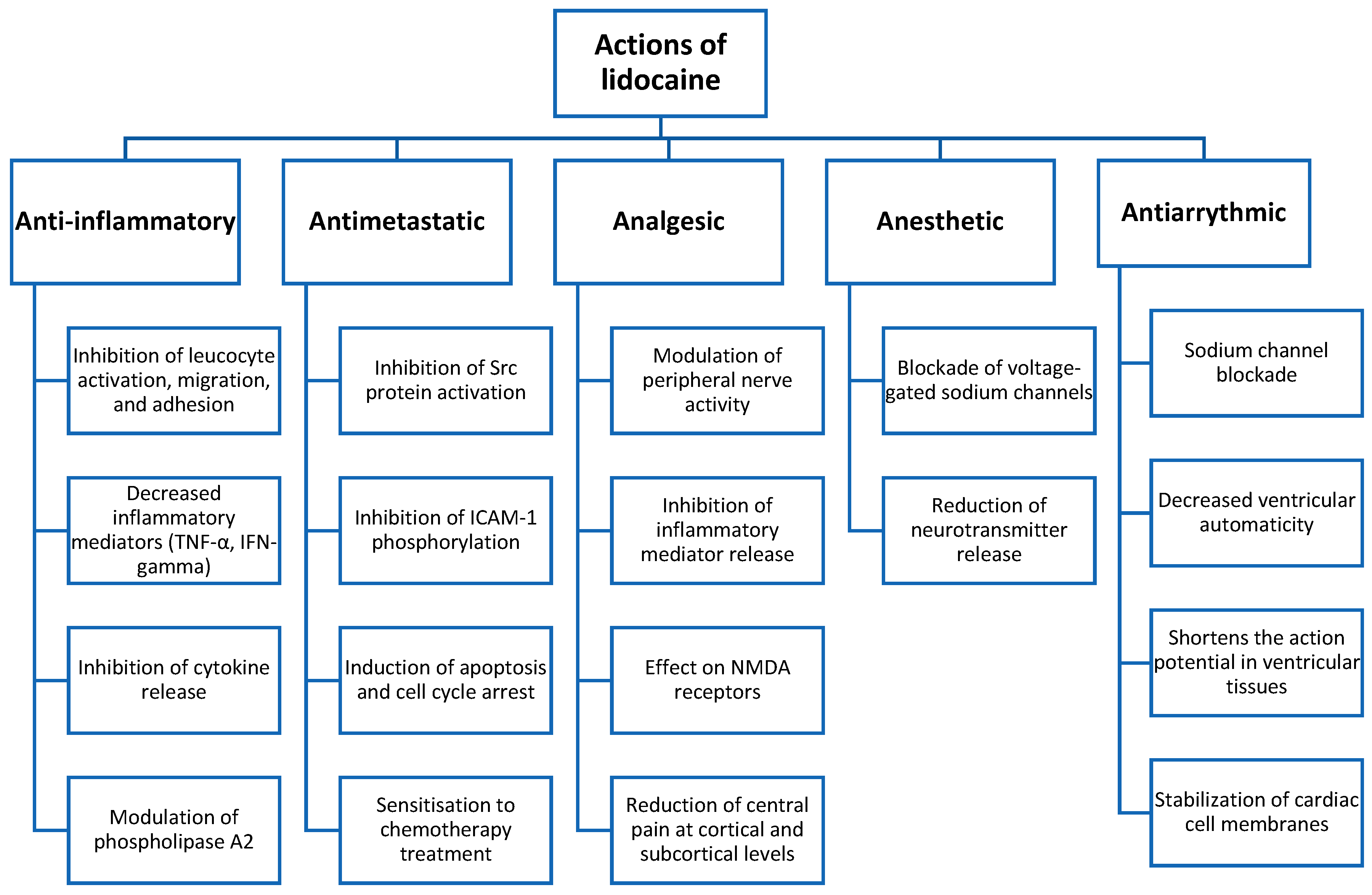
1. Introduction
2. Methodology
3. Review of the Evidence
3.1. Inflammation and Surgery
3.2. Immunomodulatory and Antimetastatic Effects of Lidocaine
3.3. Anti-Inflammatory Effect of Lidocaine
3.4. Pharmacokinetics, Dosing, and Toxicity
3.5. Abdominal Surgery
3.6. Genitourinary Surgery
3.7. Gynaecological and Obstetric Surgery
3.8. Breast Surgery
3.9. Cardiac Surgery
3.10. Thoracic Surgery
3.11. Hip and Spine Surgery
4. Limitations
5. Conclusions
Funding
Conflicts of Interest
References
- Alazawi, W.; Pirmadjid, N.; Lahiri, R.; Bhattacharya, S. Inflammatory and Immune Responses to Surgery and Their Clinical Impact. Ann. Surg. 2016, 264, 73. [Google Scholar] [CrossRef] [PubMed]
- Karnina, R.; Arif, S.K.; Hatta, M.; Bukhari, A. Molecular mechanisms of lidocaine. Ann. Med. Surg. 2021, 69, 102733. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bian, Q.; Chen, X.; Feng, Y.; Zhang, L.; Chen, P. The mechanism of perioperative intravenous lidocaine in regulating the inflammatory response: A review. Medicine 2024, 103, e39574. [Google Scholar] [CrossRef] [PubMed]
- Margraf, A.; Ludwig, N.; Zarbock, A.; Rossaint, J. Systemic Inflammatory Response Syndrome After Surgery: Mechanisms and Protection. Anesth. Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef]
- Castro, I.; Carvalho, P.; Vale, N.; Monjardino, T.; Mourão, J. Systemic Anti-Inflammatory Effects of Intravenous Lidocaine in Surgical Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3772. [Google Scholar] [CrossRef]
- Perioperative Cytokine Release During Coronary Artery Bypass Grafting in Patients of Different Ages|Clinical and Experimental Immunology|Oxford Academic. Available online: https://academic.oup.com/cei/article-abstract/114/1/26/6480346?redirectedFrom=fulltext (accessed on 1 November 2024).
- Pinheiro de Oliveira, R.; Hetzel, M.P.; dos Anjos Silva, M.; Dallegrave, D.; Friedman, G. Mechanical ventilation with high tidal volume induces inflammation in patients without lung disease. Crit. Care 2010, 14, R39. [Google Scholar] [CrossRef]
- Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China—The Lancet. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30183-5/fulltext (accessed on 1 November 2024).
- Volmering, S.; Block, H.; Boras, M.; Lowell, C.A.; Zarbock, A. The Neutrophil Btk Signalosome Regulates Integrin Activation during Sterile Inflammation. Immunity 2016, 44, 73–87. [Google Scholar] [CrossRef]
- Retsky, M.; Demicheli, R.; Hrushesky, W.; Baum, M.; Gukas, I. Surgery triggers outgrowth of latent distant disease in breast cancer: An inconvenient truth? Cancers 2010, 2, 305–337. [Google Scholar] [CrossRef]
- Chamaraux-Tran, T.-N.; Piegeler, T. The Amide Local Anesthetic Lidocaine in Cancer Surgery—Potential Antimetastatic Effects and Preservation of Immune Cell Function? A Narrative Review. Front. Med. 2017, 4, 235. [Google Scholar] [CrossRef]
- Piegeler, T.; Votta-Velis, E.G.; Liu, G.; Place, A.T.; Schwartz, D.E.; Beck-Schimmer, B.; Minshall, R.D.; Borgeat, A. Antimetastatic potential of amide-linked local anesthetics: Inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology 2012, 117, 548–559. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Liu, C.-L.; Chen, M.-J.; Hsu, Y.-W.; Chen, S.-N.; Lin, C.-H.; Chen, C.M.; Yang, F.M.; Hu, M.C. Local anesthetics induce apoptosis in human breast tumor cells. Anesth. Analg. 2014, 118, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Piegeler, T.; Schläpfer, M.; Dull, R.O.; Schwartz, D.E.; Borgeat, A.; Minshall, R.D.; Beck-Schimmer, B. Clinically relevant concentrations of lidocaine and ropivacaine inhibit TNFα-induced invasion of lung adenocarcinoma cells in vitro by blocking the activation of Akt and focal adhesion kinase. Br. J. Anaesth. 2015, 115, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Hsu, Y.-C.; Liu, C.-L.; Huang, S.-Y.; Hu, M.-C.; Cheng, S.-P. Local Anesthetics Induce Apoptosis in Human Thyroid Cancer Cells through the Mitogen-Activated Protein Kinase Pathway. PLoS ONE 2014, 9, e89563. [Google Scholar] [CrossRef] [PubMed]
- Le Gac, G.; Angenard, G.; Clément, B.; Laviolle, B.; Coulouarn, C.; Beloeil, H. Local Anesthetics Inhibit the Growth of Human Hepatocellular Carcinoma Cells. Anesth. Analg. 2017, 125, 1600–1609. [Google Scholar] [CrossRef]
- Xing, W.; Chen, D.-T.; Pan, J.-H.; Chen, Y.-H.; Yan, Y.; Li, Q.; Xue, R.F.; Yuan, Y.F.; Zeng, W.A. Lidocaine Induces Apoptosis and Suppresses Tumor Growth in Human Hepatocellular Carcinoma Cells In Vitro and in a Xenograft Model In Vivo. Anesthesiology 2017, 126, 868–881. [Google Scholar] [CrossRef]
- Jaura, A.I.; Flood, G.; Gallagher, H.C.; Buggy, D.J. Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: A pilot study. Br. J. Anaesth. 2014, 113 (Suppl. S1), i63–i67. [Google Scholar] [CrossRef]
- Schmidt, W.; Schmidt, H.; Bauer, H.; Gebhard, M.M.; Martin, E. Influence of lidocaine on endotoxin-induced leukocyte-endothelial cell adhesion and macromolecular leakage in vivo. Anesthesiology 1997, 87, 617–624. [Google Scholar] [CrossRef]
- MacGregor, R.R.; Thorner, R.E.; Wright, D.M. Lidocaine inhibits granulocyte adherence and prevents granulocyte delivery to inflammatory sites. Blood 1980, 56, 203–209. [Google Scholar] [CrossRef]
- Rancan, L.; Simón, C.; Marchal-Duval, E.; Casanova, J.; Paredes, S.D.; Calvo, A.; García, C.; Rincón, D.; Turrero, A.; Garutti, I.; et al. Lidocaine Administration Controls MicroRNAs Alterations Observed After Lung Ischemia-Reperfusion Injury. Anesth. Analg. 2016, 123, 1437–1447. [Google Scholar] [CrossRef]
- Jönsson, A.; Cassuto, J.; Tarnow, P.; Sinclair, R.; Bennett, A.; Tavares, I.A. Effects of amide local anaesthetics on eicosanoid formation in burned skin. Acta Anaesthesiol. Scand. 1999, 43, 618–622. [Google Scholar] [CrossRef]
- Flynn, J.T. Effect of lidocaine on hepatic prostanoid production in vitro following 2,4-dinitrophenol administration. Adv. Shock Res. 1983, 10, 149–159. [Google Scholar] [PubMed]
- Modig, J. Influence of regional anesthesia, local anesthetics, and sympathicomimetics on the pathophysiology of deep vein thrombosis. Acta Chir. Scand. Suppl. 1989, 550, 119–124; discussion 124–127. [Google Scholar] [PubMed]
- Lo, B.; Hönemann, C.W.; Kohrs, R.; Hollmann, M.W.; Polanowska-Grabowska, R.K.; Gear, A.R.; Durieux, M.E. Local anesthetic actions on thromboxane-induced platelet aggregation. Anesth. Analg. 2001, 93, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Cassuto, J.; Tarnow, P. Potent inhibition of burn pain without use of opiates. Burns. J. Int. Soc. Burn. Inj. 2003, 29, 163–166. [Google Scholar] [CrossRef]
- Jönsson, A.; Cassuto, J.; Hanson, B. Inhibition of burn pain by intravenous lignocaine infusion. Lancet Lond. Engl. 1991, 338, 151–152. [Google Scholar]
- Hendrickson, H.S.; van Dam-Mieras, M.C. Local anesthetic inhibition of pancreatic phospholipase A2 action on lecithin monolayers. J. Lipid Res. 1976, 17, 399–405. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; He, N. Lidocaine attenuates hypoxia/reoxygenation-induced inflammation, apoptosis and ferroptosis in lung epithelial cells by regulating the p38 MAPK pathway. Mol. Med. Rep. 2022, 25, 150. [Google Scholar] [CrossRef]
- Cusati, G.; Garutti Martínez, I.; Vara Ameigeiras, E. Universidad Complutense de Madrid Facultad de Medicina Departamento de Cirugía. Efecto de la Lidocaína en la Modulación del daño Pulmonar en un Modelo Experimental de Cirugía de Resección pulmonar en Cerdos. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2015. [Google Scholar]
- Lin, S.; Jin, P.; Shao, C.; Lu, W.; Xiang, Q.; Jiang, Z.; Zhang, Y.; Bian, J. Lidocaine attenuates lipopolysaccharide-induced inflammatory responses and protects against endotoxemia in mice by suppressing HIF1α-induced glycolysis. Int. Immunopharmacol. 2020, 80, 106150. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, X.; Yang, S. Lidocaine Potentiates SOCS3 to Attenuate Inflammation in Microglia and Suppress Neuropathic Pain. Cell. Mol. Neurobiol. 2019, 39, 1081–1092. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, G.-J.; Hu, L.; Qu, J.; Han, Y.; Zhang, G.; Qian, Y.; Jiang, C.-Y.; Liu, W.-T. Lidocaine alleviates morphine tolerance via AMPK-SOCS3-dependent neuroinflammation suppression in the spinal cord. J. Neuroinflammation 2017, 14, 211. [Google Scholar] [CrossRef]
- Chiu, K.M.; Lu, C.W.; Lee, M.Y.; Wang, M.J.; Lin, T.Y.; Wang, S.J. Neuroprotective and anti-inflammatory effects of lidocaine in kainic acid-injected rats. Neuroreport 2016, 27, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Dai, J.; Wang, Q.; Deng, H.-W.; Liu, Y.; He, G.-F.; Guo, H.-J.; Li, Y.-L. Intravenous lidocaine improves postoperative cognition in patients undergoing laparoscopic colorectal surgery: A randomized, double-blind, controlled study. BMC Anesthesiol. 2023, 23, 243. [Google Scholar] [CrossRef] [PubMed]
- Beaussier, M.; Delbos, A.; Maurice-Szamburski, A.; Ecoffey, C.; Mercadal, L. Perioperative Use of Intravenous Lidocaine. Drugs 2018, 78, 1229–1246. [Google Scholar] [CrossRef] [PubMed]
- Eipe, N.; Gupta, S.; Penning, J. Intravenous lidocaine for acute pain: An evidence-based clinical update. BJA Educ. 2016, 16, 292–298. [Google Scholar] [CrossRef]
- Lauretti, G.R. Mechanisms of analgesia of intravenous lidocaine. Rev. Bras. Anestesiol. 2008, 58, 280–286. [Google Scholar] [CrossRef]
- DeToledo, J.C. Lidocaine and Seizures. Ther. Drug Monit. 2000, 22, 320. [Google Scholar] [CrossRef]
- Yang, S.Y.; Kang, H.; Choi, G.J.; Shin, H.Y.; Baek, C.W.; Jung, Y.H.; Choi, Y.S. Efficacy of intraperitoneal and intravenous lidocaine on pain relief after laparoscopic cholecystectomy. J. Int. Med. Res. 2014, 42, 307–319. [Google Scholar] [CrossRef]
- Shakir, F.T.Z.; Sultan, R.; Siddiqui, R.; Shah, M.Z.; Javed, A.; Jamal, A. Perioperative Intravenous Lidocaine Infusion for Postlaparoscopic Cholecystectomy Pain. J. Coll. Physicians Surg. Pak. JCPSP 2023, 33, 5–9. [Google Scholar]
- Bakan, M.; Umutoglu, T.; Topuz, U.; Uysal, H.; Bayram, M.; Kadioglu, H.; Salihoglu, Z. Opioid-free total intravenous anesthesia with propofol, dexmedetomidine and lidocaine infusions for laparoscopic cholecystectomy: A prospective, randomized, double-blinded study. Braz. J. Anesthesiol. 2015, 65, 191–199. [Google Scholar] [CrossRef]
- Ortiz, M.P.; de Mello Godoy, M.C.; Schlosser, R.S.; Ortiz, R.P.; Godoy, J.P.M.; Santiago, E.S.; Rigo, F.K.; Beck, V.; Duarte, T.; Duarte, M.M.M.F.; et al. Effect of endovenous lidocaine on analgesia and serum cytokines: Double-blinded and randomized trial. J. Clin. Anesth. 2016, 35, 70–77. [Google Scholar] [CrossRef]
- Casas-Arroyave, F.D.; Osorno-Upegui, S.C.; Zamudio-Burbano, M.A. Therapeutic efficacy of intravenous lidocaine infusion compared with thoracic epidural analgesia in major abdominal surgery: A noninferiority randomised clinical trial. Br. J. Anaesth. 2023, 131, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Swenson, B.R.; Gottschalk, A.; Wells, L.T.; Rowlingson, J.C.; Thompson, P.W.; Barclay, M.; Sawyer, R.G.; Friel, C.M.; Foley, E.; Durieux, M.E. Intravenous lidocaine is as effective as epidural bupivacaine in reducing ileus duration, hospital stay, and pain after open colon resection: A randomized clinical trial. Reg. Anesth. Pain Med. 2010, 35, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, A.S.; Tsang, S.; Kazemi, A.; Morton, S.; Luo, R.; Sanders, D.T.; Regali, L.A.; Columbano, H.; Kurtzeborn, N.Y.; Durieux, M.E. A Clinical Comparison of Intravenous and Epidural Local Anesthetic for Major Abdominal Surgery. Reg. Anesth. Pain Med. 2016, 41, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, S.; Wang, J.; Gao, X.; Wang, G. Effect of Intravenous Infusion of Lidocaine Compared with Ultrasound-Guided Transverse Abdominal Plane Block on the Quality of Postoperative Recovery in Patients Undergoing Laparoscopic Bariatric Surgery. Drug Des. Devel. Ther. 2022, 16, 739. [Google Scholar] [CrossRef]
- Plass, F.; Nicolle, C.; Zamparini, M.; Al Issa, G.; Fiant, A.L.; Le Roux, Y.; Gérard, J.L.; Fischer, M.O.; Alvès, A.; Hanouz, J.L. Effect of intra-operative intravenous lidocaine on opioid consumption after bariatric surgery: A prospective, randomised, blinded, placebo-controlled study. Anaesthesia 2021, 76, 189–198. [Google Scholar] [CrossRef]
- Vigneault, L.; Turgeon, A.F.; Côté, D.; Lauzier, F.; Zarychanski, R.; Moore, L.; McIntyre, L.A.; Nicole, P.C.; Fergusson, D.A. Perioperative intravenous lidocaine infusion for postoperative pain control: A meta-analysis of randomized controlled trials. Can. J. Anaesth. J. Can. Anesth. 2011, 58, 22–37. [Google Scholar] [CrossRef]
- De Oliveira, G.S.; Duncan, K.; Fitzgerald, P.; Nader, A.; Gould, R.W.; McCarthy, R.J. Systemic lidocaine to improve quality of recovery after laparoscopic bariatric surgery: A randomized double-blinded placebo-controlled trial. Obes. Surg. 2014, 24, 212–218. [Google Scholar] [CrossRef]
- Marret, E.; Rolin, M.; Beaussier, M.; Bonnet, F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br. J. Surg. 2008, 95, 1331–1338. [Google Scholar] [CrossRef]
- Groudine, S.B.; Fisher, H.A.; Kaufman, R.P.; Patel, M.K.; Wilkins, L.J.; Mehta, S.A.; Lumb, P.D. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth. Analg. 1998, 86, 235–239. [Google Scholar] [CrossRef]
- Nakhli, M.S.; Kahloul, M.; Guizani, T.; Zedini, C.; Chaouch, A.; Naija, W. Intravenous lidocaine as adjuvant to general anesthesia in renal surgery. Libyan J. Med. 2018, 13, 1433418. [Google Scholar] [CrossRef]
- Wuethrich, P.Y.; Romero, J.; Burkhard, F.C.; Curatolo, M. No benefit from perioperative intravenous lidocaine in laparoscopic renal surgery: A randomised, placebo-controlled study. Eur. J. Anaesthesiol. 2012, 29, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Grady, M.V.; Mascha, E.; Sessler, D.I.; Kurz, A. The effect of perioperative intravenous lidocaine and ketamine on recovery after abdominal hysterectomy. Anesth. Analg. 2012, 115, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Bryson, G.L.; Charapov, I.; Krolczyk, G.; Taljaard, M.; Reid, D. Intravenous lidocaine does not reduce length of hospital stay following abdominal hysterectomy. Can. J. Anaesth. J. Can. Anesth. 2010, 57, 759–766. [Google Scholar] [CrossRef] [PubMed]
- El-Tahan, M.R.; Warda, O.M.; Diab, D.G.; Ramzy, E.A.; Matter, M.K. A randomized study of the effects of perioperative i.v. lidocaine on hemodynamic and hormonal responses for cesarean section. J. Anesth. 2009, 23, 215–221. [Google Scholar] [CrossRef]
- Terkawi, A.S.; Durieux, M.E.; Gottschalk, A.; Brenin, D.; Tiouririne, M. Effect of intravenous lidocaine on postoperative recovery of patients undergoing mastectomy: A double-blind, placebo-controlled randomized trial. Reg. Anesth. Pain Med. 2014, 39, 472–477. [Google Scholar] [CrossRef]
- Grigoras, A.; Lee, P.; Sattar, F.; Shorten, G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin. J. Pain. 2012, 28, 567–572. [Google Scholar] [CrossRef]
- Terkawi, A.S.; Sharma, S.; Durieux, M.E.; Thammishetti, S.; Brenin, D.; Tiouririne, M. Perioperative lidocaine infusion reduces the incidence of post-mastectomy chronic pain: A double-blind, placebo-controlled randomized trial. Pain Physician 2015, 18, E139–E146. [Google Scholar]
- Klinger, R.Y.; Cooter, M.; Berger, M.; Podgoreanu, M.V.; Stafford-Smith, M.; Ortel, T.L.; Welsby, I.J.; Levy, J.H.; Rinder, H.M.; Newman, M.F.; et al. Effect of intravenous lidocaine on the transcerebral inflammatory response during cardiac surgery: A randomized-controlled trial. Can. J. Anaesth. J. Can. Anesth. 2016, 63, 1223–1232. [Google Scholar] [CrossRef]
- Cui, W.; Li, Y.; Li, S.; Wang, R.; Li, J. Systemic administration of lidocaine reduces morphine requirements and postoperative pain of patients undergoing thoracic surgery after propofol-remifentanil-based anaesthesia. Eur. J. Anaesthesiol. 2010, 27, 41–46. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Zhang, X.; Wang, G. The Effect of Lidocaine on Postoperative Quality of Recovery and Lung Protection of Patients Undergoing Thoracoscopic Radical Resection of Lung Cancer. Drug Des. Devel. Ther. 2021, 15, 1485. [Google Scholar] [CrossRef]
- Yao, Y.; Jiang, J.; Lin, W.; Yu, Y.; Guo, Y.; Zheng, X. Efficacy of systemic lidocaine on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: A randomized controlled trial. J. Clin. Anesth. 2021, 71, 110223. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Cherif, K.; Gentili, M.E.; Enel, D.; Abe, E.; Alvarez, J.C.; Mazoit, J.X.; Chauvin, M.; Bouhassira, D.; Fletcher, D. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology 2008, 109, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Farag, E.; Ghobrial, M.; Sessler, D.I.; Dalton, J.E.; Liu, J.; Lee, J.H.; Zaky, S.; Benzel, E.; Bingaman, W.; Kurz, A. Effect of perioperative intravenous lidocaine administration on pain, opioid consumption, and quality of life after complex spine surgery. Anesthesiology 2013, 119, 932–940. [Google Scholar] [CrossRef] [PubMed]
| Background | Surgical procedures and associated invasive techniques trigger an inflammatory response, which, although necessary to some extent, can lead to serious postoperative complications if excessively activated. These complications may include sepsis, anastomotic dehiscence, and cardiopulmonary events, among others. Lidocaine has been shown in multiple in vitro and in vivo studies to modulate the immune system and attenuate the inflammatory response associated with surgical trauma. |
| Methodology | A literature search was conducted using PubMed databases. The search strategy included the following terms: (anti-inflammatory effect) AND (lidocaine) and (systemic inflammation) AND (lidocaine). The following filters were applied: reviews, systematic reviews, clinical trials, and meta-analyses published within the last 10 years. Relevant studies cited in the bibliographies of key selected articles were also included. |
| Results | Perioperative intravenous administration of lidocaine has been shown in multiple studies to reduce postoperative pain, opioid consumption, paralytic ileus, and length of hospital stay, particularly in abdominal surgery, where it has been most extensively studied. In spinal and breast surgery, lidocaine has demonstrated efficacy in reducing chronic postoperative pain for up to three months. Furthermore, it appears to reduce the intraoperative dissemination of tumour cells during oncological procedures and modulates the immune system by promoting tumour cell apoptosis and cell cycle arrest. |
| Conclusions | Intravenous lidocaine shows promising potential as an immunomodulatory agent in surgical settings. However, further research is needed to establish optimal dosing regimens, determine the appropriate duration of administration, and assess its clinical impact in both the short and long term. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Martínez, A.; García, J.G.; López-Picado, A. Anti-Inflammatory and Immunomodulatory Effects of Intravenous Lidocaine in Surgery: A Narrative Review. J. Clin. Med. 2025, 14, 3883. https://doi.org/10.3390/jcm14113883
Fernández-Martínez A, García JG, López-Picado A. Anti-Inflammatory and Immunomodulatory Effects of Intravenous Lidocaine in Surgery: A Narrative Review. Journal of Clinical Medicine. 2025; 14(11):3883. https://doi.org/10.3390/jcm14113883
Chicago/Turabian StyleFernández-Martínez, Ana, Joseba González García, and Amanda López-Picado. 2025. "Anti-Inflammatory and Immunomodulatory Effects of Intravenous Lidocaine in Surgery: A Narrative Review" Journal of Clinical Medicine 14, no. 11: 3883. https://doi.org/10.3390/jcm14113883
APA StyleFernández-Martínez, A., García, J. G., & López-Picado, A. (2025). Anti-Inflammatory and Immunomodulatory Effects of Intravenous Lidocaine in Surgery: A Narrative Review. Journal of Clinical Medicine, 14(11), 3883. https://doi.org/10.3390/jcm14113883






