Retinal Ischemic Perivascular Lesions: An Exploratory Study of Their Potential as Biomarkers for Cardiovascular Disease
Abstract
1. Introduction
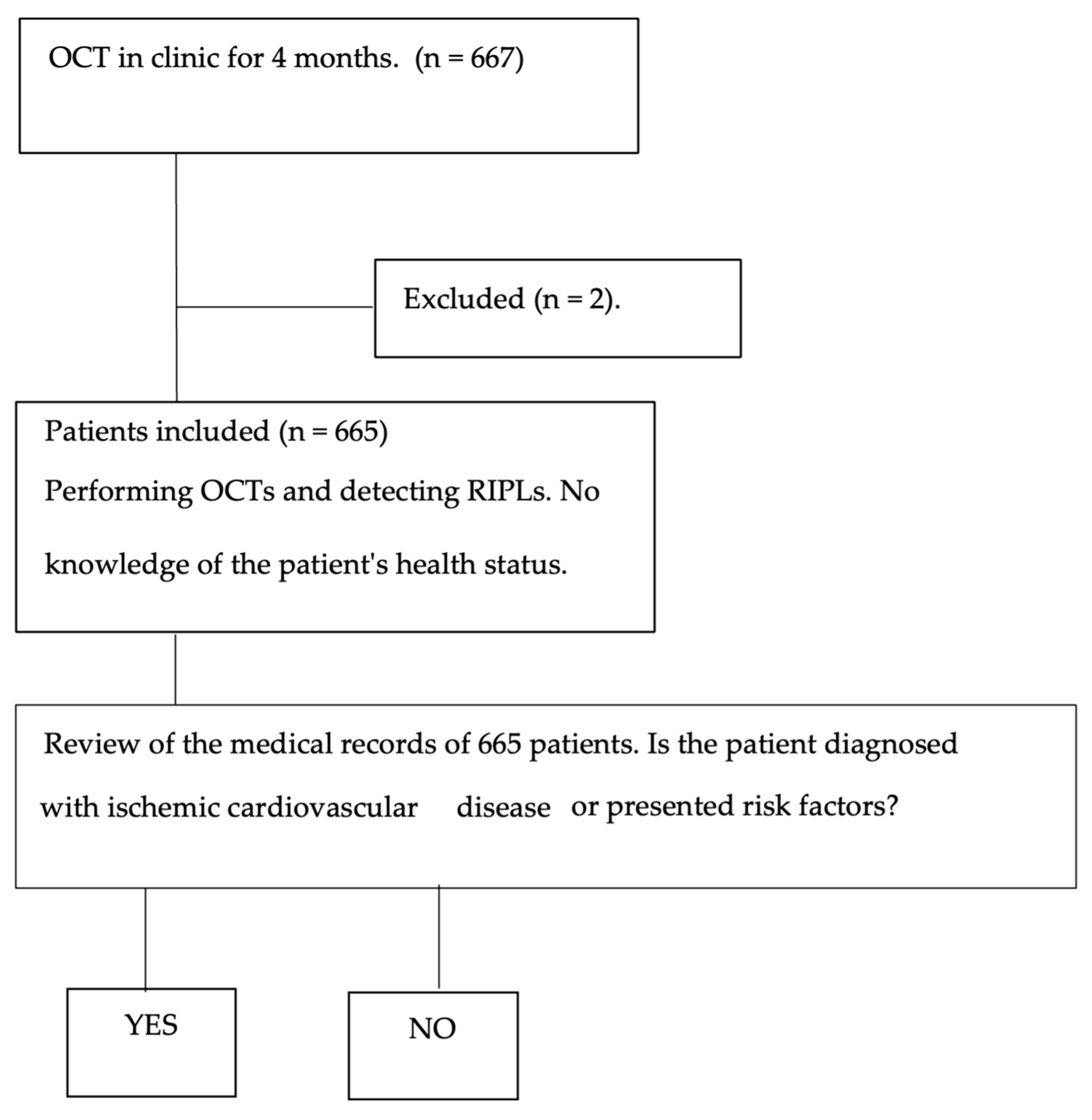
2. Materials and Methods
2.1. Clinical Procedure
2.2. Statistical Analysis
3. Results
Association Between RIPL Demographic and Clinical Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics: 2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Banegas, J.R.; Villar, F.; Graciani, A.; Rodríguez-Artalejo, F. Cardiovascular disease epidemiology in Spain. Gac. Sanit. 2006, 6, 3G–12G. [Google Scholar] [CrossRef]
- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE), 1990–2016: A systematic analysis. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef]
- Michniewicz, E.; Mlodawska, E.; Lopatowska, P.; Tomaszuk-Kazberuk, A.; Malyszko, J. Patients with atrial fibrillation and coronary artery disease: Double problem. Adv. Med. Sci. 2018, 63, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Saposnik, G.; Biessels, G.J.; Doubal, F.N.; Fornage, M.; Gorelick, P.B.; Greenberg, S.M.; Higashida, R.T.; Kasner, S.E.; Seshadri, S. Stroke prevention in patients with silent cerebrovascular disease. Stroke 2017, 48, e44–e71. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, C.A. Atrial fibrillation and risks of cardiovascular disease, kidney disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef]
- Chan, A.X.; Bakhoum, C.Y.; Bangen, K.J.; Bakhoum, M.F. Relationship between retinal vascular occlusions and cognitive dementia. Am. J. Ophthalmol. 2021, 226, 201–205. [Google Scholar] [CrossRef]
- Lavin, P.; Patrylo, M.; Hollar, M.; Espaillat, K.B.; Kirshner, H.; Schrag, M. Stroke risk in patients with central retinal artery occlusion. Am. J. Ophthalmol. 2018, 196, 96–100. [Google Scholar] [CrossRef]
- Moura-Coelho, N.; Gaspar, T.; Ferreira, J.T.; Dutra-Medeiros, M.; Cunha, J.P. Acute paracentral middle maculopathy: A literature review. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2583–2596. [Google Scholar] [CrossRef]
- Burnasheva, M.A.; Maltsev, D.S.; Kulikov, A.N.; Sherbakova, K.A.; Barsukov, A.V. Chronic paracentral acute middle maculopathy and hypertension. Ophthalmol. Retin. 2020, 4, 504–509. [Google Scholar] [CrossRef]
- Leisser, C.; Findl, O. Stroke rate 1 year after retinal artery occlusion with risk group analysis. Eur. J. Ophthalmol. 2020, 30, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Patton, N.; Aslam, T.; Macgillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, C.Y.; Madala, S.; Lando, L.; Yarmohammadi, A.; Long, C.P.; Miguez, S.; Chan, A.X.; Singer, M.; Jin, A.; Steren, B.J.; et al. Retinal ischemic perivascular lesions in individuals with atrial fibrillation. J. Am. Heart Assoc. 2023, 12, e028853. [Google Scholar] [CrossRef] [PubMed]
- Long, C.P.; Chan, A.X.; Bakhoum, C.Y.; Toomey, C.B.; Madala, S.; Garg, A.K.; Freeman, W.R.; Goldbaum, M.H.; DeMaria, A.N.; Bakhoum, M.F. Prevalence of subclinical retinal ischemia in cardiovascular disease. EClinicalMedicine 2021, 33, 100775. [Google Scholar] [CrossRef]
- Chen, X.; Rahimy, E.; Sergott, R.C.; Nunes, R.P.; Souza, E.C.; Choudhry, N.; Cutler, N.E.; Houston, S.K.; Munk, M.R.; Fawzi, A.A.; et al. Retinal vascular diseases associated with paracentral acute middle maculopathy. Am. J. Ophthalmol. 2015, 160, 26–34.e1. [Google Scholar] [CrossRef]
- Abtahi, S.H.; Nourinia, R.; Mazloumi, M.; Nouri, H.; Arevalo, J.F.; Ahmadieh, H. Ischemic cascade of the retina: New insights. Surv. Ophthalmol. 2023, 68, 380–387. [Google Scholar] [CrossRef]
- Rahimy, E.; Sarraf, D. Acute paracentral middle maculopathy and deep capillary ischemia. Curr. Opin. Ophthalmol. 2014, 25, 207–212. [Google Scholar] [CrossRef]
- Drakopoulos, M.; Zhang, D.L.; Cheng, B.T.; Sadiq, S.A.; Nadel, A.; Marchese, A.; Eskandari, M.; Mirza, R.G. SS-OCT angiography of perivascular lesions in carotid stenosis. BMJ Open Ophthalmol. 2023, 8, e001226. [Google Scholar] [CrossRef]
- Madala, S.; Adabifirouzjaei, F.; Lando, L.; Yarmohammadi, A.; Long, C.P.; Bakhoum, C.Y.; Goldbaum, M.H.; Sarraf, D.; DeMaria, A.N.; Bakhoum, M.F. Retinal ischemic perivascular lesions as CVD biomarkers. Ophthalmol. Retin. 2022, 6, 865–867. [Google Scholar] [CrossRef]
- Topcon Corporation. Maestro2 OCT/Fundus Camera: Product Specifications; Topcon Corporation: Tokyo, Japan, 2018; Available online: https://www.topconhealthcare.com/products/maestro2 (accessed on 22 May 2025).
- Bousquet, E.; Santina, A.; Au, A.; Somisetty, S.; Abraham, N.; Voichanski, S.; Estawro, R.; Fouad, Y.A.; Romero-Morales, V.; Bakhoum, M.F.; et al. Retinal Ischemic Perivascular Lesions Are Associated with Myocardial Infarction in Patients with Coronary Artery Disease. Am. J. Ophthalmol. 2024, 264, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in OCT angiography. Retina 2018, 38, 2073–2080. [Google Scholar] [CrossRef]
- Yatsuya, H.; Folsom, A.R.; Wong, T.Y.; Klein, R.; Klein, B.E.K.; Sharrett, A.R. Retinal microvascular abnormalities and lacunar stroke. Lancet Neurol. 2010, 9, 689–697. [Google Scholar]
- Torjesen, I. Care not health should be centre of more integrated system, former health ministers agree. BMJ 2012, 345, e7846. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Adults aged 40 years or older | Poor-quality OCT images (signal strength < 6 or artifacts) |
| Underwent macular OCT scan with Topcon Maestro 2 | Media opacities affecting image quality |
| Provided written informed consent | Fixation instability or segmentation errors |
| History of ocular disease affecting retinal structure (e.g., diabetic retinopathy, retinal vein occlusion) | |
| Previous ocular surgeries (except uncomplicated cataract surgery) | |
| Refusal to participate |
| Variable | Total (n = 665) | Diseased Group (n = 297) | Healthy Group (n = 368) |
|---|---|---|---|
| Mean age (SD) | 61.64 ± 11.63 | 57.53 ± 11.05 | 66.67 ± 10.29 |
| Female (%) | 68.57% | 71.04% | 65.32% |
| Male (%) | 31.43% | 28.96% | 34.68% |
| RIPLs (%) | 0.75% (five cases) | 1.68% (five cases) | 0% (zero cases) |
| Covariates | Odds Ratio (CI 95%) | p-Value |
|---|---|---|
| Sex | 0.9 (0.64–1.18) | 0.51 |
| RIPLs | 13.3 (0.73–239.53) | 0.08 |
| Hypertension | 49.4 (28.58–82.48) | <0.001 |
| Ischemic heart disease | 26.8 (10.69–66.75) | <0.001 |
| Dyslipidemia | 44.3 (19.16–101.60) | <0.001 |
| Thrombosis | 32.2 (9.97–103.13) | <0.001 |
| Myocardial infarction | 8.8 (0.45–167.6) | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carretero, M.M.; Parejo, A.d.l.R.S.; Biarnés, M.; Amores, R.R.; Gómez, Á.P.; Martinez-Perez, C. Retinal Ischemic Perivascular Lesions: An Exploratory Study of Their Potential as Biomarkers for Cardiovascular Disease. J. Clin. Med. 2025, 14, 3837. https://doi.org/10.3390/jcm14113837
Carretero MM, Parejo AdlRS, Biarnés M, Amores RR, Gómez ÁP, Martinez-Perez C. Retinal Ischemic Perivascular Lesions: An Exploratory Study of Their Potential as Biomarkers for Cardiovascular Disease. Journal of Clinical Medicine. 2025; 14(11):3837. https://doi.org/10.3390/jcm14113837
Chicago/Turabian StyleCarretero, Manuel Moriche, Ana de los Reyes Sánchez Parejo, Marc Biarnés, Remedios Revilla Amores, Ángel Pérez Gómez, and Clara Martinez-Perez. 2025. "Retinal Ischemic Perivascular Lesions: An Exploratory Study of Their Potential as Biomarkers for Cardiovascular Disease" Journal of Clinical Medicine 14, no. 11: 3837. https://doi.org/10.3390/jcm14113837
APA StyleCarretero, M. M., Parejo, A. d. l. R. S., Biarnés, M., Amores, R. R., Gómez, Á. P., & Martinez-Perez, C. (2025). Retinal Ischemic Perivascular Lesions: An Exploratory Study of Their Potential as Biomarkers for Cardiovascular Disease. Journal of Clinical Medicine, 14(11), 3837. https://doi.org/10.3390/jcm14113837





