Efficacy and Safety of Uro-Vaxom in Urinary Tract Infection Prevention: A Systematic Literature Review
Abstract
1. Introduction
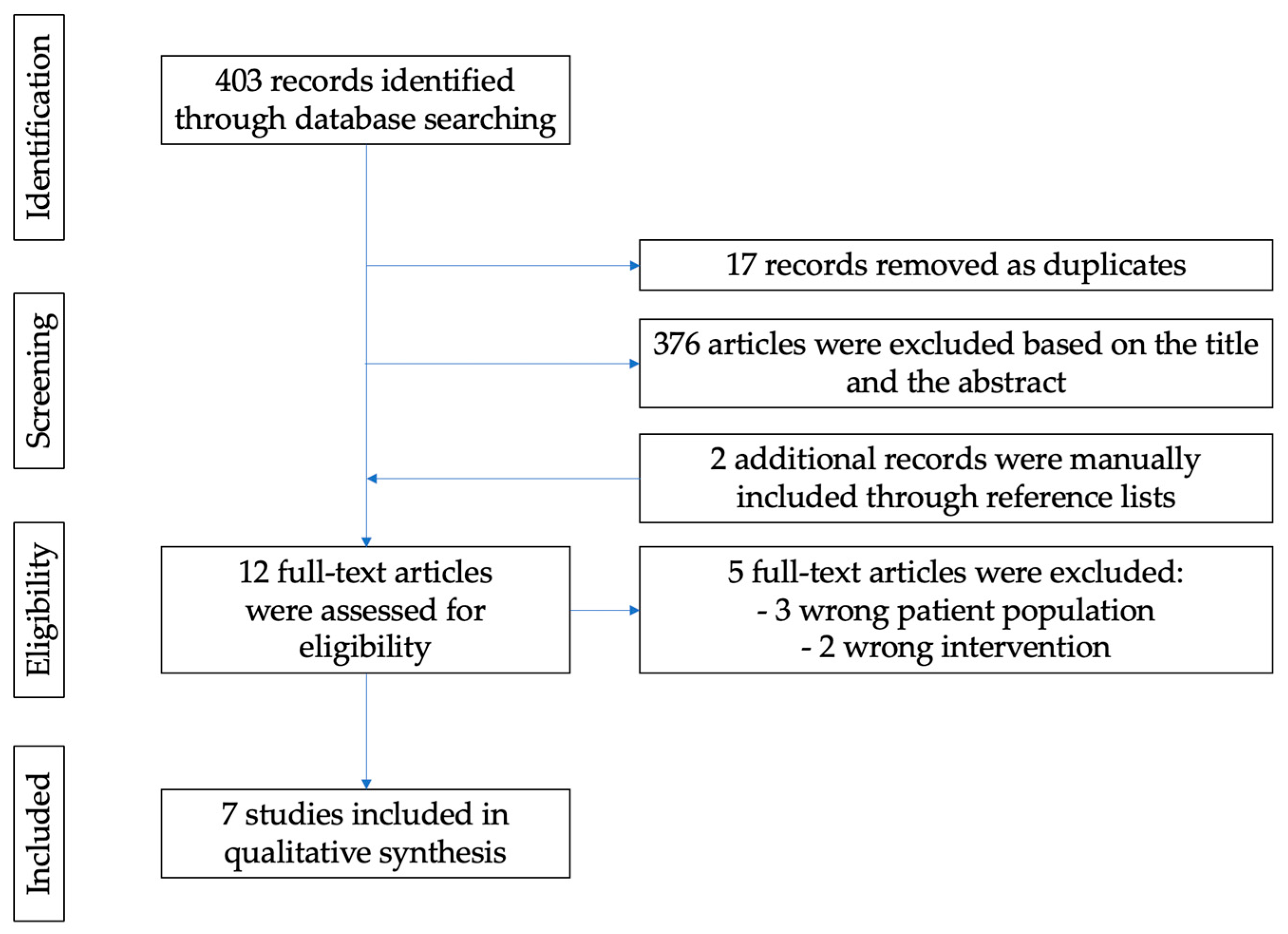
2. Materials and Methods
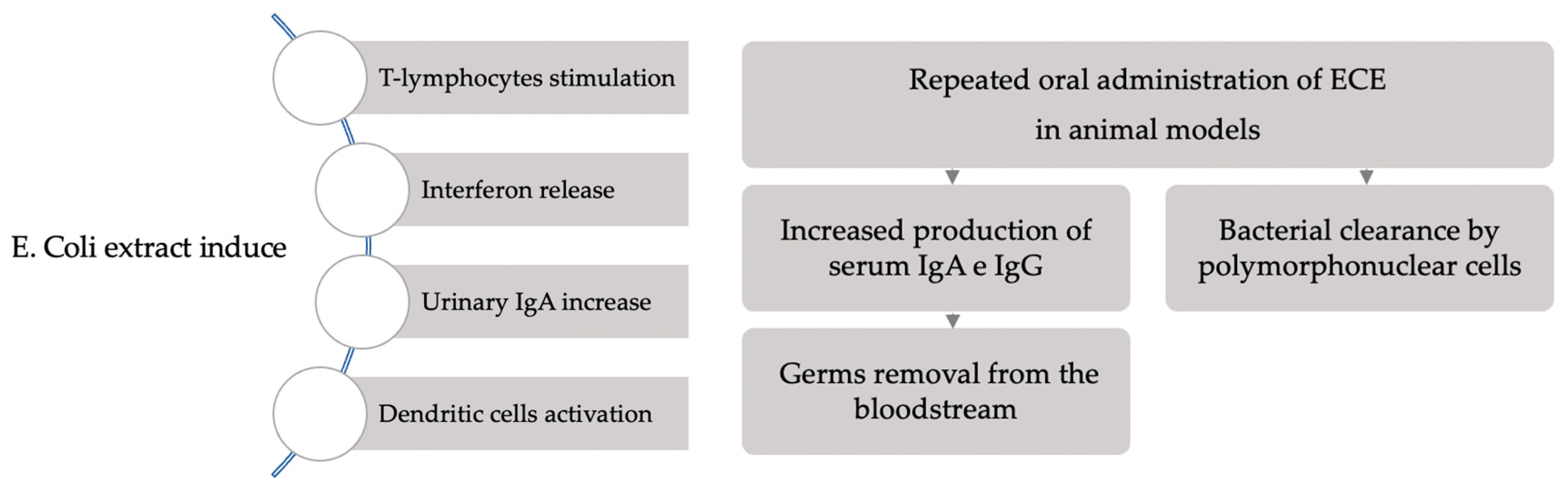
3. Results
4. Main Findings
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taha Neto, K.A.; Nogueira Castilho, L.; Reis, L.O. Oral vaccine (OM-89) in the recurrent urinary tract infection prophylaxis: A realistic systematic review with meta-analysis. Actas Urol. Esp. 2016, 40, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.W.; Alloussi, S.; Egger, G.; Blümlein, H.M.; Cozma, G.; Schulman, C.C.; Multicenter UTI Study Group. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur. Urol. 2005, 47, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Grabe, M.; Bartoletti, R.; Bjerklund-Johansen, T.E.; Guidelines on Urological Infections. European Association of Urology Website. Available online: http://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf (accessed on 2 April 2025).
- Cruz, F.; Dambros, M.; Naber, K.G.; Bauer, H.W.; Cozma, G. Recurrent Urinary Tract Infections: Uro-Vaxom, a New Alternative. Eur. Urol. Suppl. 2009, 8, 762–768. [Google Scholar] [CrossRef]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Dis. Mon. 2003, 49, 53–70. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef]
- Jung, C.; Brubaker, L. The etiology and management of recurrent urinary tract infections in postmenopausal women. Climacteric 2019, 22, 242–249. [Google Scholar] [CrossRef]
- Schneeberger, C.; Geerlings, S.E.; Middleton, P.; Crowther, C.A. Interventions for preventing recurrent urinary tract infection during pregnancy. Cochrane Database Syst. Rev. 2015, 2015, Cd009279. [Google Scholar] [CrossRef]
- Matthews, S.J.; Lancaster, J.W. Urinary tract infections in the elderly population. Am. J. Geriatr. Pharmacother. 2011, 9, 286–309. [Google Scholar] [CrossRef]
- Becknell, B.; Schober, M.; Korbel, L.; Spencer, J.D. The diagnosis, evaluation and treatment of acute and recurrent pediatric urinary tract infections. Expert Rev. Anti-Infect. Ther. 2015, 13, 81–90. [Google Scholar] [CrossRef]
- Simoões, E.; Silva, A.C.; Oliveira, E.A.; Mak, R.H. Urinary tract infection in pediatrics: An overview. J. Pediatr. (Rio. J.) 2020, 96, 65–79. [Google Scholar] [CrossRef]
- Frey, C.H.; Obolensky, W.; Wyss, H. Treatment of recurrent urinary tract infections: Efficacy of an orally administered biological response modifier. Urol. Int. 1986, 41, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Piatek, B.M. Urinary tract infection of Escherichia coli strains of chaperone-usher system. Pol. J. Microbiol. 2011, 60, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, H.L., 4th; Conover, M.S.; Chou, W.C.; Hibbing, M.E.; Manson, A.L.; Dodson, K.W.; Hannan, T.J.; Roberts, P.L.; Stapleton, A.E.; Hooton, T.M.; et al. Bacterial virulence phenotypes of Escherichia coli and host susceptibility determine risk for urinary tract infections. Sci. Transl. Med. 2017, 9, eaaf1283. [Google Scholar] [CrossRef] [PubMed]
- Ejrnæs, K. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan. Med. Bull. 2011, 58, B4187. [Google Scholar]
- Luo, Y.; Ma, Y.; Zhao, Q.; Wang, L.; Guo, L.; Ye, L.; Zhang, Y.; Yang, J. Similarity and divergence of phylogenies, antimicrobial susceptibilities, and virulence factor profiles of Escherichia coli isolates causing recurrent urinary tract infections that persist or result from reinfection. J. Clin. Microbiol. 2012, 50, 4002–4007. [Google Scholar] [CrossRef]
- Blango, M.G.; Mulvey, M.A. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 2010, 54, 1855–1863. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 2 April 2025).
- Erman, A.; Hergouth, V.K.; Blango, M.G.; Kos, M.K.; Mulvey, M.A.; Veranic, P. Repeated Treatments with Chitosan in Combination with Antibiotics Completely Eradicate Uropathogenic Escherichia coli From Infected Mouse Urinary Bladders. J. Infect. Dis. 2017, 216, 375–381. [Google Scholar] [CrossRef]
- Lichtenberger, P.; Hooton, T.M. Antimicrobial prophylaxis in women with recurrent urinary tract infections. Int. J. Antimicrob. Agents. 2011, 38, 36–41. [Google Scholar] [CrossRef]
- Lenger, S.M.; Bradley, M.S.; Thomas, D.A.; Bertolet, M.H.; Lowder, J.L.; Sutcliffe, S. D-mannose vs other agents for recurrent urinary tract infection prevention in adult women: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020, 223, 265.e1–265.e13. [Google Scholar] [CrossRef]
- Lianos, E.; Mountrakis, S.; Zampiozis, E. Effectiveness of Uro-vaxom and vitamin E in delaying recurrences of E. coli LUTS in geriatric patients. Eur. Urol. Suppl. 2005, 4, 134. [Google Scholar] [CrossRef]
- Schmidhammer, S.; Ramoner, R.; Holtl, L.; Bartsch, G.; Thurnher, M.; Zelle-Rieser, C. An Escherichia coli-based oral vaccine against urinary tract infections potently activates human dendritic cells. Urology 2002, 60, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Baier, W.; Sedelmeier, E.A.; Bessler, W.G. Studies on the immunogenicity of an Escherichia coli extract after oral application in mice. Arzneimittelforschung 1997, 47, 980–985. [Google Scholar] [PubMed]
- Nauck, M.; Matthys, H.; Emmons, L.R.; Perruchoud, A.; Reichel, H.; Pfleger, S.; Breyer, S.; Paupe, J. The immunomodulators Broncho-Vaxom and Uro-Vaxom stimulate the bacterial killing and oxidative metabolism of polymorphonuclear leukocytes by the activation of phosphatidyli-nositol turnover. Int. J. Exp. Clin. Chemoter. 1991, 4, 1–11. [Google Scholar]
- Magasi, P.; Pánovics, J.; Illés, A.; Nagy, M. Uro-Vaxom and the management of recurrent urinary tract infection in adults: A randomized multicenter double-blind trial. Eur. Urol. 1994, 26, 137–140. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.Y.; Jeong, I.G.; Paick, J.S.; Son, H.; Lim, D.J.; Shim, H.B.; Park, W.H.; Jung, H.C.; Choo, M.S. A prospective multi-center trial of Escherichia coli extract for the prophylactic treatment of patients with chronically recurrent cystitis. J. Korean Med. Sci. 2010, 25, 435–439. [Google Scholar] [CrossRef]
- Tammen, H. Immunobiotherapy with Uro-Vaxom in recurrent urinary tract infection. The German Urinary Tract Infection Study Group. Br. J. Urol. 1990, 65, 6–9. [Google Scholar] [CrossRef]
- Schulman, C.C.; Corbusier, A.; Michiels, H.; Taenzer, H.J. Oral immunotherapy of recurrent urinary tract infections: A double-blind placebo-controlled multicenter study. J. Urol. 1993, 150, 917–921. [Google Scholar] [CrossRef]
- Brodie, A.; El-Taji, O.; Jour, I.; Foley, C.; Hanbury, D. A Retrospective Study of Immunotherapy Treatment with Uro-Vaxom (OM-89®) for Prophylaxis of Recurrent Urinary Tract Infections. Curr. Urol. 2020, 14, 130–134. [Google Scholar] [CrossRef]
- Kranz, J.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F.M.E.; et al. European Association of Urology Guidelines on Urological Infections: Summary of the 2024 Guidelines. Eur. Urol. 2024, 86, 27–41. [Google Scholar] [CrossRef]
| First Author Year, Country | Study Design | Population | Mean Age | Gender Representation | Follow-Up | Dose |
|---|---|---|---|---|---|---|
| Bauer, HW 2005, Switzerland [2] | Multicenter, randomized double-blind controlled study | 454 (232 intervention group; 222 placebo group) | 41.7 ±15.3 (intervention), 39.8 ± 15.1 (placebo) | 100% female | 12 months | 600 mg daily for 90 days or placebo then 600 mg daily for the first 10 days of months 7–9 or placebo |
| Kim, KS 2010, South Korea [27] | Prospective multicenter trial | 34 | 56.4 (range 34–75) | 100% female | 8 months | 600 mg daily for 90 days or placebo then 600 mg daily for the first 10 days of months 7–9 or placebo |
| Brodie, A 2020, USA [30] | Retrospective trial | 79 | 56 (range 19–90) | 95% female | 12 months | 600 mg daily for 90 days |
| Tammen, H 1990, UK [28] | Randomized controlled trial | 120 | 51.2 ± 2.4 (intervention), 50.4 ± 2.3 (placebo) | 52 women/9 men in control group vs. 51 women/8 men in placebo group | 8 months | 600 mg daily for 90 days or placebo |
| Schulman, CC 1993, USA [29] | Multicenter, randomized double-blind controlled study | 142 (74 under Uro-Vaxom, 68 under placebo) | Not reported | Not specified | 3 months | 600 mg daily for 90 days or placebo |
| Frey, C 1986, Switzerland [12] | Multicenter, randomized double-blind controlled study | 64 (32 under Uro-Vaxom, 32 under placebo) | Not reported (age range 22–84) | 64 patients, “mostly women” | 3 months | 600 mg daily for 90 days or placebo |
| Magasi, P 1994, Switzerland [26] | Multicenter, randomized double-blind controlled study | 112 (58 under Uro-Vaxom, 54 under placebo) | Not reported (age range 16–82) | 48 women/10 men in control group vs. 47 women/7 men in placebo group | 6 months | 600 mg daily for 90 days or placebo |
| Study | Bias Due to Confounding | Bias in Selection of Participants | Bias in Classification of Interventions | Bias Due to Deviation from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result |
|---|---|---|---|---|---|---|---|
| Bauer | Low | Low | Low | Low | Low | Low | Low |
| Kim | Moderate | Moderate | Low | Moderate | Low | Low | Moderate |
| Brodie | Moderate | Moderate | Low | Moderate | Moderate | Moderate | Moderate |
| Tammen | Low | Low | Low | Low | Moderate | Low | Low |
| Schulman | Low | Low | Low | Low | Low | Low | Low |
| Frey | Low | Low | Low | Low | Low | Low | Low |
| Magasi | Low | Low | Low | Low | Low | Low | Low |
| First Author | UTI Recurrence Reduction | Side Effects Reported | Patient Satisfaction |
|---|---|---|---|
| Bauer, HW | 1.28 → 0.84 (34% reduction, p < 0.003) | Good tolerability; no serious adverse effects | Not reported |
| Kim, KS | Mean number of UTI: 4.26 → 0.35 (p < 0.001) | 2 patients excluded for gastrointestinal symptoms | Not reported |
| Brodie, A | Mean number of UTI: 3.14 → 1.53 (p < 0.05) | 9.4% (rash, gastrointestinal) | 60.4% positive satisfaction |
| Tammen, H | Mean number of UTI: 3.5 → 0.82 in control group vs. 3.5 → 1.8 in placebo group | 4.5% (pruritus, diarrhea, headache with flushing) | Not reported |
| Schulman, CC | Mean number of UTI: 1.5 placebo vs. 0.7 Uro-Vaxom (p < 0.0001) | 2% Uro-Vaxom vs. 6% placebo (gastrointestinal, rash, vertigo, sleep disorders) | Not reported |
| Frey, C | Significative reduction in control group (qualitative data) | 1 case of allergic exanthema | Not reported |
| Magasi, P | 67.2% no recurrence vs. 22.2% placebo (p < 0.0005) | None reported | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volontè, S.; De Vicari, D.; Cola, A.; Barba, M.; Frigerio, M. Efficacy and Safety of Uro-Vaxom in Urinary Tract Infection Prevention: A Systematic Literature Review. J. Clin. Med. 2025, 14, 3836. https://doi.org/10.3390/jcm14113836
Volontè S, De Vicari D, Cola A, Barba M, Frigerio M. Efficacy and Safety of Uro-Vaxom in Urinary Tract Infection Prevention: A Systematic Literature Review. Journal of Clinical Medicine. 2025; 14(11):3836. https://doi.org/10.3390/jcm14113836
Chicago/Turabian StyleVolontè, Silvia, Desireè De Vicari, Alice Cola, Marta Barba, and Matteo Frigerio. 2025. "Efficacy and Safety of Uro-Vaxom in Urinary Tract Infection Prevention: A Systematic Literature Review" Journal of Clinical Medicine 14, no. 11: 3836. https://doi.org/10.3390/jcm14113836
APA StyleVolontè, S., De Vicari, D., Cola, A., Barba, M., & Frigerio, M. (2025). Efficacy and Safety of Uro-Vaxom in Urinary Tract Infection Prevention: A Systematic Literature Review. Journal of Clinical Medicine, 14(11), 3836. https://doi.org/10.3390/jcm14113836







