Adherence to Exercise Training Within a Multimodal Prehabilitation Program: An Exploratory Study of Influencing Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Study Variables
2.5. Outcomes
2.6. Data Analysis
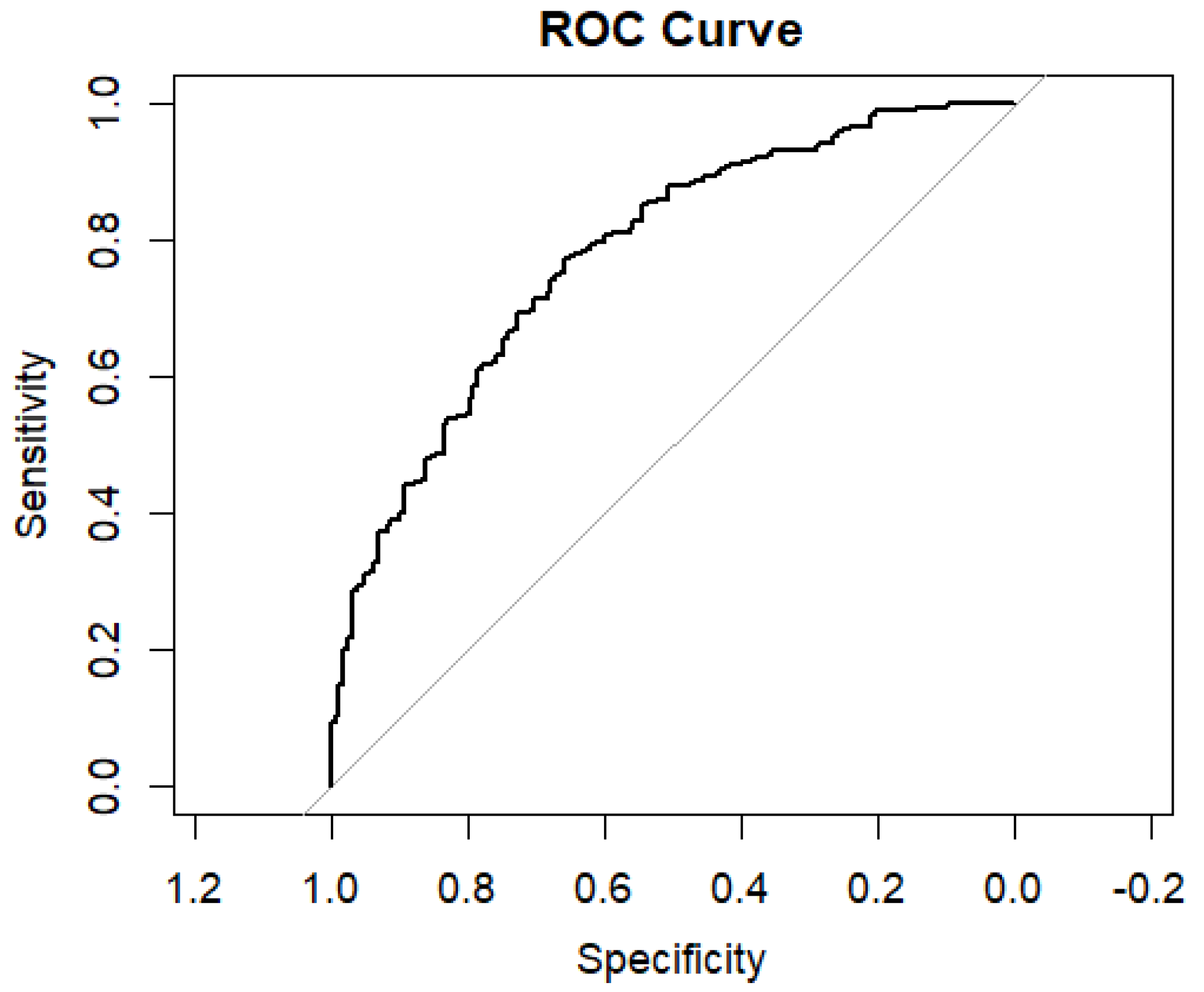
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.M.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised Prehabilitation in High-risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann. Surg. 2018, 267, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, C.J.L.; Minnella, E.M.; Coca-Martinez, M.; Cate, D.W.G.T.; Regis, M.; Awasthi, R.; Martínez-Palli, G.; López-Baamonde, M.; Sebio-Garcia, R.; Feo, C.V.; et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery: The PREHAB Randomized Clinical Trial. JAMA Surg. 2023, 158, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Berkel, A.E.M.; Bongers, B.C.; Kotte, H.; Weltevreden, P.; de Jongh, F.H.C.; Eijsvogel, M.M.M.; Wymenga, M.; Bigirwamungu-Bargeman, M.; van der Palen, J.; van Det, M.J.; et al. Effects of Community-based Exercise Prehabilitation for Patients Scheduled for Colorectal Surgery With High Risk for Postoperative Complications: Results of a Randomized Clinical Trial. Ann. Surg. 2022, 275, e299–e306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Y.; Ding, L.; Miao, X.; Jiang, X.; Xu, T.; Xu, X.; Zhu, S.; Xu, Q.; Hu, J. Effects of prehabilitation on postoperative outcomes in frail cancer patients undergoing elective surgery: A systematic review and meta-analysis. Support. Care Cancer 2022, 31, 57. [Google Scholar] [CrossRef] [PubMed]
- Nordon, C.; Karcher, H.; Groenwold, R.H.; Ankarfeldt, M.Z.; Pichler, F.; Chevrou-Severac, H.; Rossignol, M.; Abbe, A.; Abenhaim, L.; GetReal consortium. The “Efficacy-Effectiveness Gap”: Historical Background and Current Conceptualization. Value Health 2016, 19, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Risco, R.; González-Colom, R.; Montané-Muntané, M.; Cano, I.; Vela, E.; Sebio, R.; Dana, F.; Faner, J.; Coca, M.; Laxe, S.; et al. Actionable Factors Fostering Health Value Generation and Scalability of Prehabilitation: A Prospective Cohort Study. Ann. Surg. 2023, 278, e217–e225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tweed, T.T.T.; Sier, M.A.T.; Van Bodegraven, A.A.; Van Nie, N.C.; Sipers, W.M.W.H.; Boerma, E.G.; Stoot, J.H.M.B. Feasibility and Efficiency of the BEFORE (Better Exercise and Food, Better Recovery) Prehabilitation Program. Nutrients 2021, 13, 3493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voorn, M.J.J.; Driessen, E.J.M.; Reinders, R.J.E.F.; van Kampen-van den Boogaart, V.E.M.; Bongers, B.C.; Janssen-Heijnen, M.L.G. Evidence base for exercise prehabilitation suggests favourable outcomes for patients undergoing surgery for non-small cell lung cancer despite being of low therapeutic quality: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2023, 49, 879–894. [Google Scholar] [CrossRef]
- Watts, T.; Courtier, N.; Fry, S.; Gale, N.; Gillen, E.; McCutchan, G.; Patil, M.; Rees, T.; Roche, D.; Wheelwright, S.; et al. Access, acceptance and adherence to cancer prehabilitation: A mixed-methods systematic review. J. Cancer Surviv. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Agnihotram, R.V.; Bergdahl, A.; van Rooijen, S.J.; Awasthi, R.; Carli, F.; Scheede-Bergdahl, C. Maximizing patient adherence to prehabilitation: What do the patients say? Support. Care Cancer 2018, 26, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Talen, A.D.; van Meeteren, N.L.U.; Barten, J.A.; Pereboom, I.; Krijnen, W.P.; Jager-Wittenaar, H.; Bongers, B.C.; van der Sluis, G. The challenges of evidence-based prehabilitation in a real-life context for patients preparing for colorectal surgery—A cohort study and multiple case analysis. Perioper. Med. 2025, 14, 7. [Google Scholar] [CrossRef]
- Manocchio, N.; Ljoka, C.; Ferdinandi, V.; Cicchi, L.; Foti, C. Commentary on “The learning rehabilitation system: Strengthening an intersectoral strategy to improve functioning of an ageing population” by Bickenbach et al. Health Policy 2025, 155, 105303. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Hasil, L.; Keane, C.; Brassard, D.; Kiernan, F.; Bellafronte, N.T.; Culos-Reed, S.N.; Gramlich, L.; Ljungqvist, O.; Fenton, T.R. A multimodal prehabilitation class for Enhanced Recovery After Surgery: A pragmatic randomised type 1 hybrid effectiveness-implementation trial. Br. J. Anaesth. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A review of ASA physical status—Historical perspectives and modern developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Donati, A.; Ruzzi, M.; Adrario, E.; Pelaia, P.; Coluzzi, F.; Gabbanelli, V.; Pietropaoli, P. A new and feasible model for predicting operative risk. Br. J. Anaesth. 2004, 93, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Hlatky, M.A.; Boineau, R.E.; Higginbotham, M.B.; Lee, K.L.; Mark, D.B.; Califf, R.M.; Cobb, F.R.; Pryor, D.B. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am. J. Cardiol. 1989, 64, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, S.A.; Wershof Schwartz, A.; Karunananthan, S.; Bergman, H.; Mark Clarfield, A. The identification of frailty: A systematic literature review. J. Am. Geriatr. Soc. 2011, 59, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Strassmann, A.; Steurer-Stey, C.; Lana, K.D.; Zoller, M.; Turk, A.J.; Suter, P.; Puhan, M.A. Population-based reference values for the 1-min sit-to-stand test. Int. J. Public Health 2013, 58, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Donaire-Gonzalez, D.; Gimeno-Santos, E.; Serra, I.; Roca, J.; Balcells, E.; Rodríguez, E.; Farrero, E.; Antó, J.M.; Garcia-Aymerich, J. Validation of the Yale Physical Activity Survey in chronic obstructive pulmonary disease patients. Arch. Bronconeumol. 2011, 47, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Craig, C.L.; Aoyagi, Y.; Bell, R.C.; Croteau, K.A.; De Bourdeaudhuij, I.; Ewald, B.; Gardner, A.W.; Hatano, Y.; Lutes, L.D.; et al. How many steps/day are enough? For older adults and special populations. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.E.; ten Hacken, N.H.; Boezen, H.M.; de Greef, M.H. Self-efficacy for physical activity and insight into its benefits are modifiable factors associated with physical activity in people with COPD: A mixed-methods study. J. Physiother. 2013, 59, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, O.; Johnston, K.; Kumar, S. Barriers and enablers to physical activity participation in patients with COPD: A systematic review. J. Cardiopulm. Rehabil. Prev. 2012, 32, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, O.; Kumar, S.; Johnston, K. Barriers to and enablers of physical activity in patients with COPD following a hospital admission: A qualitative study. Int. J. Chronic Obs. Pulmon Dis. 2014, 9, 115–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oates, G.R.; Niranjan, S.J.P.; Ott, C.; Scarinci, I.C.; Schumann, C.; Parekh, T.D.; Dransfield, M.T. Adherence to Pulmonary Rehabilitation in copd: A qualitative exploration of patient perspectives on barriers and facilitators. J. Cardiopulm. Rehabil. Prev. 2019, 39, 344–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogerson, M.C.; Murphy, B.M.; Bird, S.; Morris, T. “I don’t have the heart”: A qualitative study of barriers to and facilitators of physical activity for people with coronary heart disease and depressive symptoms. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finch, A.; Benham, A. Patient attitudes and experiences towards exercise during oncological treatment. A qualitative systematic review. Support. Care Cancer 2024, 32, 509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Potiaumpai, M.; Schleicher, E.A.; Wang, M.; Campbell, K.L.; Sturgeon, K.; Schmitz, K.H. Exercise during chemotherapy: Friend or foe? Cancer Med. 2023, 12, 10715–10724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maurer, T.; Belau, M.H.; von Grundherr, J.; Schlemmer, Z.; Patra, S.; Becher, H.; Schulz, K.-H.; Zyriax, B.-C.; Schmalfeldt, B.; Chang-Claude, J. Randomised controlled trial testing the feasibility of an exercise and nutrition intervention for patients with ovarian cancer during and after first-line chemotherapy (BENITA-study). BMJ Open 2022, 12, e054091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bullard, T.; Ji, M.; An, R.; Trinh, L.; Mackenzie, M.; Mullen, S.P. A systematic review and meta-analysis of adherence to physical activity interventions among three chronic conditions: Cancer, cardiovascular disease, and diabetes. BMC Public Health 2019, 19, 636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaw, J.F.; Hladkowicz, E.; McCartney, C.J.L.; Bryson, G.L.; McIsaac, D.I. A model to predict level of adherence to prehabilitation in older adults with frailty having cancer surgery. Can. J. Anaesth. 2023, 70, 1950–1956. (In English) [Google Scholar] [CrossRef] [PubMed]
- Halliday, L.J.; Doganay, E.; Wynter-Blyth, V.; Osborn, H.; Buckley, J.; Moorthy, K. Adherence to Pre-operative Exercise and the Response to Prehabilitation in Oesophageal Cancer Patients. J. Gastrointest. Surg. 2021, 25, 890–899. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costello, E.; Kafchinski, M.; Vrazel, J.; Sullivan, P. Motivators, barriers, and beliefs regarding physical activity in an older adult population. J. Geriatr. Phys. Ther. 2011, 34, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Picorelli, A.M.; Pereira, L.S.; Pereira, D.S.; Felício, D.; Sherrington, C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: A systematic review. J. Physiother. 2014, 60, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Waterland, J.L.; Ismail, H.; Amin, B.; Granger, C.L.; Denehy, L.; Riedel, B. Patient acceptance of prehabilitation for major surgery: An exploratory survey. Support. Care Cancer 2021, 29, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.O.; Lange, P.; Hilberg, O.; Farver-Vestergaard, I.; Ibsen, R.; Løkke, A. COPD and Smoking Status—It Does Matter: Characteristics and Prognosis of COPD According to Smoking Status. Chronic Obs. Pulmon Dis. 2024, 11, 56–67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strine, T.W.; Okoro, C.A.; Chapman, D.P.; Balluz, L.S.; Ford, E.S.; Ajani, U.A.; Mokdad, A.H. Health-related quality of life and health risk behaviors among smokers. Am. J. Prev. Med. 2005, 28, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Nyrop, K.A.; Deal, A.M.; Choi, S.K.; Wagoner, C.W.; Lee, J.T.; Wood, W.A.; Anders, C.; Carey, L.A.; Dees, E.C.; Jolly, T.A.; et al. Measuring and understanding adherence in a home-based exercise intervention during chemotherapy for early breast cancer. Breast Cancer Res. Treat. 2018, 168, 43–55, Erratum in Breast Cancer Res. Treat. 2019, 173, 245. [Google Scholar] [CrossRef] [PubMed]
- Casanovas-Álvarez, A.; Sebio-Garcia, R.; Masià, J.; Mateo-Aguilar, E. Experiences of Patients with Breast Cancer Participating in a Prehabilitation Program: A Qualitative Study. J. Clin. Med. 2024, 13, 3732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooper, M.; Chmelo, J.; Sinclair, R.C.F.; Charman, S.; Hallsworth, K.; Welford, J.; Phillips, A.W.; Greystoke, A.; Avery, L. Exploring factors influencing uptake and adherence to a home-based prehabilitation physical activity and exercise intervention for patients undergoing chemotherapy before major surgery (ChemoFit): A qualitative study. BMJ Open 2022, 12, e062526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Sebio-García, R.; Iglesias-Garcia, E.; Reguart, N.; Martinez-Palli, G.; Bello, I. Prehabilitation for patients undergoing neoadjuvant therapy prior to cancer resection: A systematic review and meta-analysis. Support. Care Cancer 2024, 32, 749. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value 1 |
|---|---|
| Sociodemographics | |
| Age | 69.8 (11.4) |
| Gender | |
| Male | 342 (61.1%) |
| Female | 215 (38.4%) |
| Living area | |
| AISBE 2 | 312 (55.7%) |
| Barcelona | 71 (12.7%) |
| Metropolitan area | 63 (11.3%) |
| Rest of Catalonia | 98 (17.5%) |
| Living status | |
| Living alone | 146 (26.1%) |
| Co-living | 398 (71.1%) |
| Missing | 16 (2.9%) |
| Level of studies | |
| No studies | 12 (2.3%) |
| Primary incomplete | 45 (8%) |
| Primary complete | 172 (30.7%) |
| Secondary | 194 (34.6%) |
| University degree | 98 (17.5%) |
| Postgraduate/Doctorate | 20 (3.6%) |
| Missing | 19 (3.4%) |
| Civil status | |
| Single | 53 (9.5%) |
| Separated/divorced | 33 (5.9%) |
| Widowed | 102 (18.2%) |
| Married/in couple | 349 (62.3%) |
| Missing | 23 (4.1%) |
| Working status | |
| Actively working | 53 (9.5%) |
| Household labor | 23 (5.9%) |
| Retired | 382 (68.2%) |
| Unemployed | 15 (2.7%) |
| Sick leave | 72 (12.9%) |
| Student/others | 5 (0.9%) |
| Missing | 10 (1.8%) |
| Means of transportation to hospital | |
| Walking | 82 (14.6%) |
| Public transportation | 262 (46.8%) |
| Own vehicle | 186 (33.2%) |
| Ambulance | 17 (3%) |
| Missing | 13 (2.3%) |
| Needs accompaniment for hospital visit | |
| Yes | 146 (26.1%) |
| No | 398 (71.1%) |
| Missing | 16 (2.9%) |
| Clinical features | |
| Type of surgery | |
| Colorectal surgery | 137 (24.5%) |
| Valve replacement surgery | 65 (11.6%) |
| Esophagectomy | 62 (11.1%) |
| Lung resection surgery | 52 (9.3%) |
| Cystectomy | 47 (8.4%) |
| Gastrectomy | 37 (6.6%) |
| CRS + HIPEC 3 | 29 (5.2%) |
| CAGB 4 | 21 (3.8%) |
| Pancreatic Surgery | 19 (3.4%) |
| Other cardiac surgeries | 18 (3.2%) |
| Liver transplantation | 13 (2.3%) |
| Other digestive surgeries | 12 (2.1%) |
| Bariatric surgery | 12 (2.1%) |
| CAGB + valve replacement | 8 (1.4%) |
| Hepatic resection | 7 (1.3%) |
| Heart transplant | 7 (1.3%) |
| Other surgeries | 11 (2%) |
| ASA | |
| II | 162 (28.9%) |
| III | 335 (59.8%) |
| IV | 62 (11.1%) |
| Frailty (CSF) | |
| 1 | 16 (2.9%) |
| 2 | 69 (12.3%) |
| 3 | 185 (33%) |
| 4 | 223 (39.8%) |
| 5 | 14 (2.5%) |
| 6 | 46 (8.2%) |
| 7 | 3 (0.5%) |
| Oncological surgery | |
| Yes | 379 (67.7%) |
| No | 165 (31.4%) |
| Missing | 10 (1.8%) |
| Neoadjuvant therapy | |
| Radiotherapy | 4 (0.7%) |
| Chemotherapy | 73 (13%) |
| Chemoradiotherapy | 37 (6.6%) |
| None | 431 (77%) |
| Missing | 14 (2.5%) |
| Polypharmacy (>5 drugs) | |
| Yes | 296 (52.9%) |
| No | 237 (42.3%) |
| Missing | 27 (4.8%) |
| Body Mass Index (kg/m2) | 27.9 (7.5) |
| Hemoglobin (mg/dL) | 12.9 (2) |
| Albumin (mg/dL) | 42.4 (4.6) |
| Charlson Comorbidity Index | 5.7 (2.4) |
| Smoking | |
| Former smoker | 291 (52%) |
| Current smoker | 62 (11.1%) |
| Non-smoker | 195 (34.8%) |
| Missing | 12 (2.1%) |
| Nutritional Status 5 | |
| No malnutrition | 373 (66.6%) |
| Moderate malnutrition | 127 (22.7%) |
| Severe malnutrition | 59 (10.5%) |
| DASI (points) | 27.6 (13.6) |
| 6MWT | |
| ≤400 m | 167 (29.8%) |
| >400 m | 285 (50.9%) |
| Missing | 108 (19.3%) |
| Handgrip strength (kg) | |
| Women | 20.2 (5.8) |
| Men | 33.5 (9.1) |
| Sit-to-Stand (reps) | |
| Women | 10.6 (4.4) |
| Men | 11.4 (4.8) |
| Physical Activity Levels (YPAS) | |
| Not physically active (≤38 points) | 368 (65.7%) |
| Physically active (>38 points) | 185 (33%) |
| Missing | 7 (1.3%) |
| Mood (HADS) | |
| Anxiety | 5 (3.7) |
| Depression | 4.1 (3.3) |
| Total | 9.1 (6.3) |
| Variable | Adherent * | Non-Adherent * | p Value |
|---|---|---|---|
| Age | 71 (10.8) | 67.9 (12.2) | 0.002 |
| Gender (% male) | 227 (63.9%) | 114 (56.7%) | 0.056 |
| Living Area | 0.552 | ||
| AISBE 1 | 198 (57.6%) | 113 (56.8%) | |
| Barcelona | 46 (13.4%) | 25 (12.6%) | |
| Metropolitan area | 35 (10.2%) | 28 (14.1%) | |
| Rest of Catalonia | 65 (18.9%) | 33 (16.6%) | |
| Working Status | 0.014 | ||
| Actively working | 26 (7.5%) | 27 (13.4%) | |
| Household work | 11 (3.2%) | 12 (6%) | |
| Retired | 258 (74.1%) | 124 (61.7%) | |
| Unemployed | 8 (2.3%) | 7 (3.5%) | |
| Sick leave | 44 (12.6%) | 27 (13.4%) | |
| Student/other | 0 (0%) | 3 (1.5%) | |
| Neoadjuvant therapy | 0.079 | ||
| Yes | 66 (19.1%) | 49 (24.6%) | |
| No | 280 (80.9%) | 150 (75.4%) | |
| Charlson Comorbidity Score | 5.9 (2.3) | 5.5 (2.5) | 0.025 |
| Type of exercise program | 0.001 | ||
| Home-based | 51 (14.3%) | 47 (23.3%) | |
| Supervised | 286 (80.3%) | 134 (66.3%) | |
| Smoking | 0.023 | ||
| Non-smoker | 118 (34%) | 77 (38.5%) | |
| Former smoker | 198 (57.1%) | 93 (46.5%) | |
| Current smoker | 31 (8.9%) | 30 (15%) | |
| YPAS | 35.7 (18.3) | 32.1 (16) | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Risco, R.; Sebio-García, R.; González-Colom, R.; Ubré, M.; Dana, F.; Iglesias-García, E.; Martínez-Pallí, G.; on behalf of the Hospital Clínic de Barcelona Prehabilitation Group. Adherence to Exercise Training Within a Multimodal Prehabilitation Program: An Exploratory Study of Influencing Factors. J. Clin. Med. 2025, 14, 3813. https://doi.org/10.3390/jcm14113813
Risco R, Sebio-García R, González-Colom R, Ubré M, Dana F, Iglesias-García E, Martínez-Pallí G, on behalf of the Hospital Clínic de Barcelona Prehabilitation Group. Adherence to Exercise Training Within a Multimodal Prehabilitation Program: An Exploratory Study of Influencing Factors. Journal of Clinical Medicine. 2025; 14(11):3813. https://doi.org/10.3390/jcm14113813
Chicago/Turabian StyleRisco, Raquel, Raquel Sebio-García, Rubèn González-Colom, Marta Ubré, Fernando Dana, Edgar Iglesias-García, Graciela Martínez-Pallí, and on behalf of the Hospital Clínic de Barcelona Prehabilitation Group. 2025. "Adherence to Exercise Training Within a Multimodal Prehabilitation Program: An Exploratory Study of Influencing Factors" Journal of Clinical Medicine 14, no. 11: 3813. https://doi.org/10.3390/jcm14113813
APA StyleRisco, R., Sebio-García, R., González-Colom, R., Ubré, M., Dana, F., Iglesias-García, E., Martínez-Pallí, G., & on behalf of the Hospital Clínic de Barcelona Prehabilitation Group. (2025). Adherence to Exercise Training Within a Multimodal Prehabilitation Program: An Exploratory Study of Influencing Factors. Journal of Clinical Medicine, 14(11), 3813. https://doi.org/10.3390/jcm14113813






