Selective Angiography of Stimulant-Exposed Cardiac Donors Following Circulatory Death Does Not Impact Post-Transplant Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Data Source, and Study Population
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection and Variable Selection
2.4. Statistical Analyses
3. Results
3.1. Donor and Recipient Demographics and Clinical Characteristics
3.2. Post-Transplant Outcomes
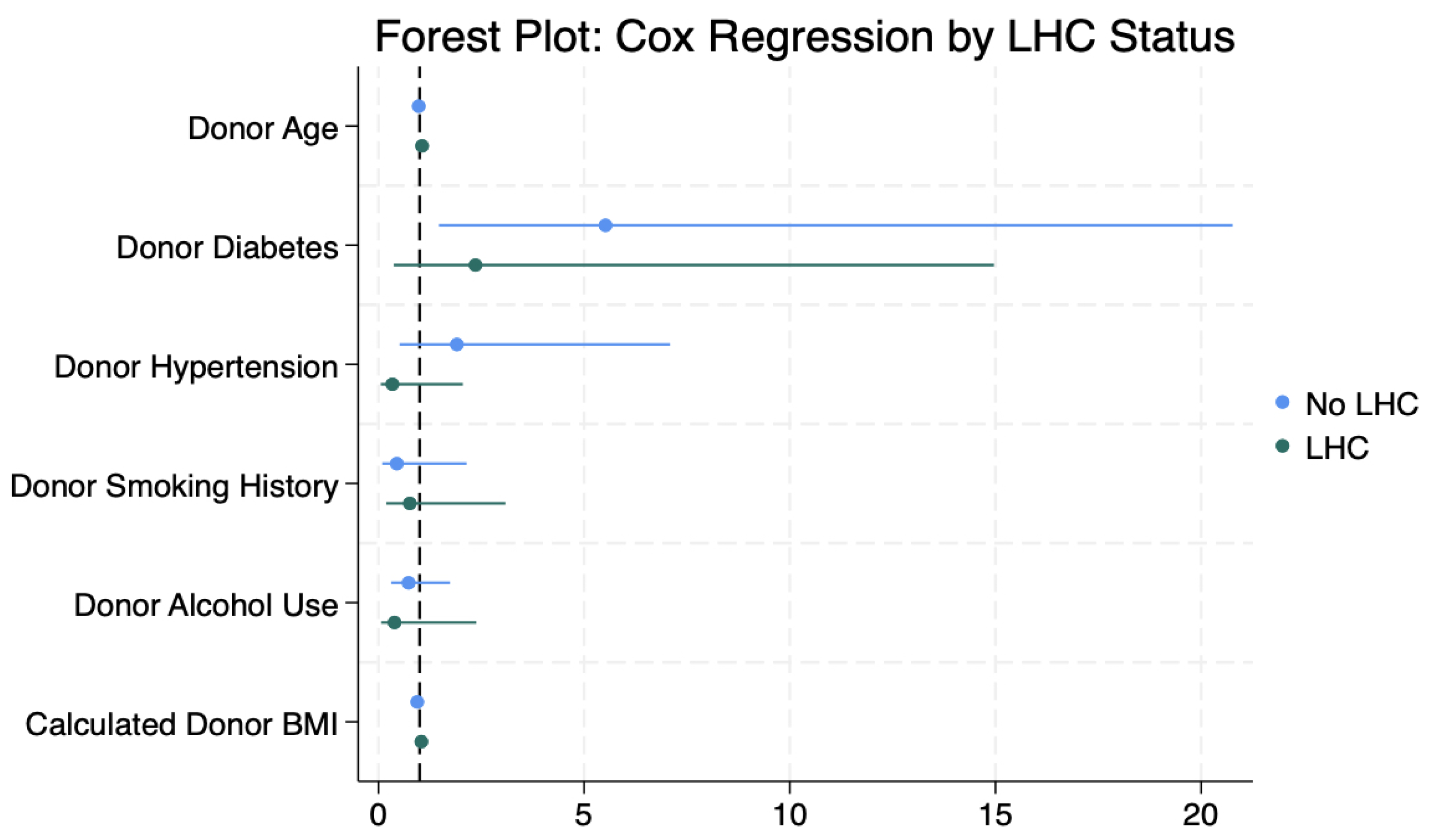
3.3. Cox Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD | Coronary Artery Disease |
| CVA | Cerebrovascular Accident |
| DBD | Donation after Brain Death |
| DCD | Donation after Circulatory Death |
| ECMO | Extracorporeal Membrane Oxygenation |
| HR | Hazard Ratio |
| IABP | Intra-Aortic Balloon Pump |
| IQR | Interquartile Range |
| ISHLT | International Society for Heart and Lung Transplantation |
| LHC | Left Heart Catheterization |
| MI | Myocardial Infarction |
| OPTN | Organ Procurement and Transplantation Network |
| UNOS | United Network for Organ Sharing |
References
- Copeland, H.; Hayanga, J.W.A.; Neyrinck, A.; MacDonald, P.; Dellgren, G.; Bertolotti, A.; Khuu, T.; Burrows, F.; Copeland, J.G.; Gooch, D.; et al. Donor Heart and Lung Procurement: A Consensus Statement. J. Heart Lung Transplant. 2020, 39, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Khush, K.K.; Potena, L.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Sadavarte, A.; Singh, T.P.; Zuckermann, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th Adult Heart Transplantation Report—2020; Focus on Deceased Donor Characteristics. J. Heart Lung Transplant. 2020, 39, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Velleca, A.; Shullo, M.A.; Dhital, K.; Azeka, E.; Colvin, M.; DePasquale, E.; Farrero, M.; García-Guereta, L.; Jamero, G.; Khush, K.; et al. The International Society for Heart and Lung Transplantation (ISHLT) Guidelines for the Care of Heart Transplant Recipients. J. Heart Lung Transplant. 2023, 42, e1–e141. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.; Scheuer, S.; Chew, H.; Ru Qiu, M.; Soto, C.; Villanueva, J.; Gao, L.; Doyle, A.; Takahara, S.; Jenkinson, C.; et al. Heart Transplantation From DCD Donors in Australia: Lessons Learned From the First 74 Cases. Transplantation 2023, 107, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.N.; Patel, C.B.; DeVore, A.D.; Bryner, B.S.; Casalinova, S.; Shah, A.; Smith, J.W.; Fiedler, A.G.; Daneshmand, M.; Silvestry, S.; et al. Transplantation Outcomes with Donor Hearts after Circulatory Death. N. Engl. J. Med. 2023, 388, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, A.; Miller, P.N.; Smith, J.W. Expanding the Donation After Circulatory Death Transplant Pool in the United States. Ann. Thorac. Surg. 2023, 116, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.N.; Scheuer, S.; Catarino, P.; Caplan, A.; Silvestry, S.C.; Jeevanandam, V.; Large, S.; Shah, A.; MacDonald, P.; Slaughter, M.S.; et al. The American Association for Thoracic Surgery 2023 Expert Consensus Document: Adult Cardiac Transplantation Utilizing Donors after Circulatory Death. J. Thorac. Cardiovasc. Surg. 2023, 166, 856–869.e5. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.; Wang, K.; MacLean, C.; Villanueva, J.; Gao, L.; Watson, A.; Iyer, A.; Connellan, M.; Granger, E.; Jansz, P.; et al. The Rapidly Evolving Landscape of DCD Heart Transplantation. Curr. Cardiol. Rep. 2024, 26, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.; Nzemenoh, B.; Jackson, S.; Chaikijurajai, T.; Halmosi, R.; Toth, K.; Khan, W.J.; Alexy, T. Substance Use-Associated Mortality among Heart Donors after the COVID-19 National Emergency Increased but Did Not Affect Peri-Transplant Outcomes. J. Cardiovasc. Dev. Dis. 2023, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.G.; Rezkalla, S.; Kloner, R.A. Cardiovascular Effects of Cocaine. Circulation 2010, 122, 2558–2569. [Google Scholar] [CrossRef] [PubMed]
- Kevil, C.G.; Goeders, N.E.; Woolard, M.D.; Bhuiyan, M.S.; Dominic, P.; Kolluru, G.K.; Arnold, C.L.; Traylor, J.G.; Orr, A.W. Methamphetamine Use and Cardiovascular Disease: In Search of Answers. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.; Kittleson, M.; Patel, J.; Stimpson, E.; Kao, T.; Liou, F.; Aintablian, T.; Siddiqui, S.; Chang, D.H.; Czer, L.; et al. Is There a Risk of Cocaine and Methamphetamine Use in Heart Donors? J. Heart Lung Transplant. 2015, 34, S276–S277. [Google Scholar] [CrossRef]
- Johnstad, C.M.; Fiedler, A.G. Coronary Angiography Utilization in the Evaluation of a Potential Heart Donor by Age: A Narrative Review. J. Thorac. Dis. 2021, 13, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Meredith, T.; Scheuer, S.; Hoffman, M.; Joshi, Y.; Kathir, K.; Gunalingam, B.; Roy, D.; Wilson, S.; Jansz, P.; Macdonald, P.; et al. Coronary Angiography of the Ex-situ Beating Donor Heart in a Portable Organ Care System. Catheter. Cardiovasc. Interv. 2022, 100, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Ghodsizad, A.; Bordel, V.; Ungerer, M.; Karck, M.; Bekeredjian, R.; Ruhparwar, A. Ex Vivo Coronary Angiography of a Donor Heart in the Organ Care System. Heart Surg. Forum 2012, 15, E161–E163. [Google Scholar] [CrossRef] [PubMed]
- Nadel, J.; Scheuer, S.; Kathir, K.; Muller, D.; Jansz, P.; Macdonald, P. Successful Transplantation of High-Risk Cardiac Allografts from DCD Donors Following Ex Vivo Coronary Angiography. J. Heart Lung Transplant. 2020, 39, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Tatum, R.; Briasoulis, A.; Tchantchaleishvili, V.; Massey, H.T. Evaluation of Donor Heart for Transplantation. Heart Fail. Rev. 2022, 27, 1819–1827. [Google Scholar] [CrossRef]
- Hussain, Z.; Yu, M.; Wozniak, A.; Kim, D.; Krepostman, N.; Liebo, M.; Raichlin, E.; Heroux, A.; Joyce, C.; Ilias-Basha, H. Impact of Donor Smoking History on Post Heart Transplant Outcomes: A Propensity-matched Analysis of ISHLT Registry. Clin. Transplant. 2021, 35, e14127. [Google Scholar] [CrossRef]
- Gavin, J.L.; Ganapathi, A.M.; Mokadam, N.A.; Whitson, B.A.; Henn, M.C.; Haas, G.J.; Hasan, A.K.; Franco, V.; Vallakati, A.; Emani, S.; et al. Heart Transplantation Characteristics and Outcomes in Recipients of Diabetic Donor Hearts. J. Heart Lung Transplant. 2023, 42, S328. [Google Scholar] [CrossRef]
- Joseph, J.T.; Mulvihill, M.S.; Yerokun, B.A.; Bell, S.M.; Milano, C.A.; Hartwig, M.G. Elevated Donor Hemoglobin A1c Does Not Impair Early Survival in Cardiac Transplant Recipients. Clin. Transplant. 2017, 31, e12995. [Google Scholar] [CrossRef] [PubMed]

| Variable Stratified Variable | LHC (n = 135) | Non-LHC (n = 350) | Total (n = 485) | p-Value |
|---|---|---|---|---|
| Recipient | ||||
| Age (years) | 54 ± 11 | 54 ± 12 | 54 ± 12 | 0.70 |
| Sex | ||||
| Male | 106 (79%) | 294 (84%) | 400 (83%) | 0.20 |
| Female | 29 (21%) | 56 (16%) | 85 (17%) | - |
| Race/Ethnicity | ||||
| White | 93 (69%) | 227 (65%) | 320 (66%) | 0.47 |
| Black | 19 (14%) | 70 (20%) | 89 (18%) | - |
| Hispanic | 20 (15%) | 42 (12%) | 62 (13%) | - |
| Insurance Type | ||||
| Private | 70 (52%) | 162 (46%) | 204 (45%) | 0.46 |
| Medicare/Medicaid | 62 (46%) | 183 (52%) | 245 (50%) | - |
| Self-Pay | - | - | - | - |
| Education Level | ||||
| High school or less | 48 (36%) | 133 (38%) | 181 (37%) | 0.29 |
| College or beyond | 77 (57%) | 203 (58%) | 280 (58%) | - |
| Employed | ||||
| Yes | 22 (16%) | 76 (22%) | 98 (20%) | 0.30 |
| Body Mass Index (kg/m2) | 28 ± 5 | 28 ± 5 | 28 ± 5 | 0.50 |
| Diabetes | 41 (30%) | 113 (32%) | 154 (32%) | 0.64 |
| Cerebrovascular Disease | 6 (5%) | 34 (10%) | 40 (9%) | 0.07 |
| Dialysis | 2 (1%) | 7 (2%) | 9 (2%) | 0.77 |
| Cancer | 12 (9%) | 33 (9%) | 45 (9%) | 0.89 |
| Smoking History | 56 (41%) | 166 (47%) | 222 (46%) | 0.32 |
| Life Support | ||||
| ECMO | 3 (2%) | 8 (2%) | 11 (2%) | 0.83 |
| IABP | 18 (13%) | 54 (15%) | 72 (15%) | 0.57 |
| Mechanical Ventilator | 2 (1%) | 4 (1%) | 6 (1%) | 0.83 |
| Variable Stratified Variable | LHC (n = 135) | Non-LHC (n = 350) | Total (n = 485) | p-Value |
|---|---|---|---|---|
| Donor | ||||
| Age (years) | 39 ± 6 | 31 ± 7 | 33 ± 8 | <0.001 |
| Sex | ||||
| Male | 112 (83%) | 302 (86%) | 414 (85%) | 0.43 |
| Female | 23 (17%) | 48 (14%) | 71 (15%) | - |
| Race/Ethnicity | ||||
| White | 107 (79%) | 265 (76%) | 372 (77%) | 0.89 |
| Black | 8 (6%) | 27 (8%) | 35 (7%) | - |
| Hispanic | 16 (12%) | 49 (14%) | 65 (13%) | - |
| Body Mass Index (kg/m2) | 29 ± 7 | 28 ± 6 | 28 ± 6 | 0.05 |
| Diabetes | 11 (8%) | 11 (3%) | 22 (5%) | 0.02 |
| Hypertension | 28 (21%) | 43 (12%) | 71 (14%) | 0.02 |
| Myocardial Infarction | 4 (3%) | 3 (1%) | 7 (1%) | 0.08 |
| Smoking History | 49 (37%) | 57 (17%) | 106 (22%) | <0.001 |
| Heavy Alcohol Use | 61 (47%) | 124 (37%) | 185 (40%) | 0.06 |
| Cocaine Use History | 55 (41%) | 200 (57%) | 255 (52%) | 0.001 |
| Amphetamine Use History | 54 (40%) | 95 (27%) | 149 (31%) | 0.001 |
| Variable Stratified Variable | LHC (n = 135) | Non-LHC (n = 350) | Total (n = 485) | p-Value |
|---|---|---|---|---|
| Match Run | ||||
| Waitlist Time (Days) | 22 [9, 167] | 26 [8, 135] | 25 [8, 147] | 0.44 |
| cPRA | 21 ± 31 | 16 ± 24 | 18 ± 27 | 0.32 |
| HLA Mismatch | 5 [4, 5] | 5 [4, 5] | 5 [4, 5] | 0.29 |
| Sex Mismatch | 20 (15%) | 54 (15%) | 74 (15%) | 0.88 |
| Ischemic Time (minutes) | 309 [183, 381] | 306 [217, 382] | 307 [214, 382] | 0.79 |
| Outcomes | ||||
| Renal Replacement | 27 (20%) | 54 (15%) | 81 (17%) | 0.22 |
| Cerebrovascular Accident | 6 (4%) | 13 (4%) | 19 (4%) | 0.71 |
| ECMO | 2 (1%) | 11 (3%) | 13 (3%) | 0.31 |
| Acute Cellular Rejection | 14 (10%) | 40 (11%) | 54 (11%) | 0.75 |
| Treated for Rejection | 3 (2%) | 25 (7%) | 28 (6%) | 0.04 |
| Length of Stay | 16 [13, 27] | 16 [12, 23] | 16 [12, 24] | 0.93 |
| Variable Stratified Variable | LHC (n = 135) | Non-LHC (n = 350) | Total (n = 485) | p-Value |
|---|---|---|---|---|
| Graft Failure | ||||
| 30-day Graft Failure | 9 (7%) | 11 (3%) | 20 (4%) | 0.08 |
| 90-day Graft Failure | 9 (7%) | 13 (4%) | 22 (5%) | 0.16 |
| 1-year Graft Failure | 10 (7%) | 21 (6%) | 31 (6%) | 0.57 |
| Recipient Mortality | ||||
| 30-day Mortality | 8 (6%) | 10 (3%) | 18 (4%) | 0.11 |
| 90-day Mortality | 8 (6%) | 12 (3%) | 20 (4%) | 0.21 |
| 1-year Mortality | 10 (7%) | 20 (6%) | 30 (6%) | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rust, C.J.; Reul, R.M., Jr.; Abadiotakis, H.; Kodimerla, R.; Preston, J.D.; Randhawa, S.S.; Halkos, M.E.; Bishawi, M.M.; Daneshmand, M.A.; Chan, J.L. Selective Angiography of Stimulant-Exposed Cardiac Donors Following Circulatory Death Does Not Impact Post-Transplant Outcomes. J. Clin. Med. 2025, 14, 3809. https://doi.org/10.3390/jcm14113809
Rust CJ, Reul RM Jr., Abadiotakis H, Kodimerla R, Preston JD, Randhawa SS, Halkos ME, Bishawi MM, Daneshmand MA, Chan JL. Selective Angiography of Stimulant-Exposed Cardiac Donors Following Circulatory Death Does Not Impact Post-Transplant Outcomes. Journal of Clinical Medicine. 2025; 14(11):3809. https://doi.org/10.3390/jcm14113809
Chicago/Turabian StyleRust, Clayton J., Ross Michael Reul, Jr., Helen Abadiotakis, Reshma Kodimerla, Joshua D. Preston, Supreet S. Randhawa, Michael E. Halkos, Muath M. Bishawi, Mani A. Daneshmand, and Joshua L. Chan. 2025. "Selective Angiography of Stimulant-Exposed Cardiac Donors Following Circulatory Death Does Not Impact Post-Transplant Outcomes" Journal of Clinical Medicine 14, no. 11: 3809. https://doi.org/10.3390/jcm14113809
APA StyleRust, C. J., Reul, R. M., Jr., Abadiotakis, H., Kodimerla, R., Preston, J. D., Randhawa, S. S., Halkos, M. E., Bishawi, M. M., Daneshmand, M. A., & Chan, J. L. (2025). Selective Angiography of Stimulant-Exposed Cardiac Donors Following Circulatory Death Does Not Impact Post-Transplant Outcomes. Journal of Clinical Medicine, 14(11), 3809. https://doi.org/10.3390/jcm14113809






