Clinical Features and Prognosis of Cervical Esophageal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Staging Workup
2.3. Surgery
2.4. Chemoradiotherapy
2.5. Histopathological Evaluation
2.6. Follow-Up After Treatment
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
3.2. Cervical Esophageal Cancer
3.3. Definitive Chemoradiotherapy
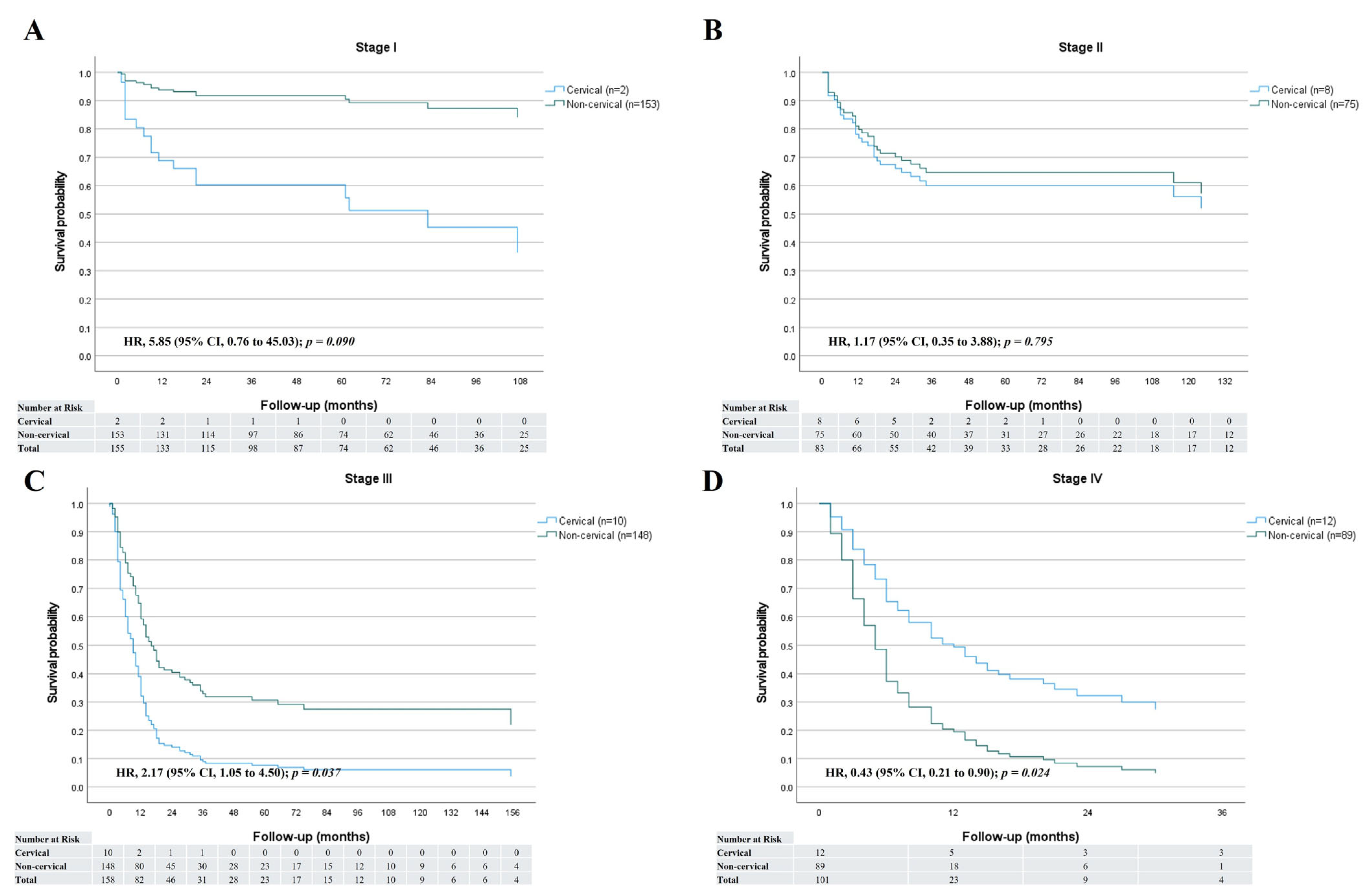
3.4. Survival
3.5. Representative Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Eisner, D.C. Esophageal cancer: Treatment advances and need for screening. JAAPA 2024, 37, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef]
- Liu, C.Q.; Ma, Y.L.; Qin, Q.; Wang, P.H.; Luo, Y.; Xu, P.F.; Cui, Y. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac. Cancer 2023, 14, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.D.; Cooper, S.L.; Armeson, K.; Garrett-Mayer, E.; Sharma, A. Cervical esophageal cancer: A population-based study. Head Neck 2015, 37, 808–814. [Google Scholar] [CrossRef]
- Hoeben, A.; Polak, J.; Van De Voorde, L.; Hoebers, F.; Grabsch, H.I.; de Vos-Geelen, J. Cervical esophageal cancer: A gap in cancer knowledge. Ann. Oncol. 2016, 27, 1664–1674. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Farjah, F.; Gerdes, H.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2023, 21, 393–422. [Google Scholar] [CrossRef] [PubMed]
- Obermannova, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef]
- Rice, T.W.; Gress, D.M.; Patil, D.T.; Hofstetter, W.L.; Kelsen, D.P.; Blackstone, E.H. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 304–317. [Google Scholar] [CrossRef]
- Rice, T.W.; Patil, D.T.; Blackstone, E.H. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann. Cardiothorac. Surg. 2017, 6, 119–130. [Google Scholar] [CrossRef]
- Japan Esophageal, S. Japanese Classification of Esophageal Cancer, 11th Edition: Part I. Esophagus 2017, 14, 1–36. [Google Scholar] [CrossRef]
- Chadwick, G.; Groene, O.; Hoare, J.; Hardwick, R.H.; Riley, S.; Crosby, T.D.; Hanna, G.B.; Cromwell, D.A. A population-based, retrospective, cohort study of esophageal cancer missed at endoscopy. Endoscopy 2014, 46, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Psyrri, A.; DiMaio, D. Human papillomavirus in cervical and head-and-neck cancer. Nat. Clin. Pract. Oncol. 2008, 5, 24–31. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Y.; Wang, X.; He, Z.; Weiss, N.S.; Madeleine, M.M.; Liu, F.; Tian, X.; Song, Y.; Pan, Y.; et al. Human papillomavirus infection and esophageal squamous cell carcinoma: A case-control study. Cancer Epidemiol. Biomarkers Prev. 2012, 21, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Syrjanen, K.J. HPV infections and oesophageal cancer. J. Clin. Pathol. 2002, 55, 721–728. [Google Scholar] [CrossRef]
- He, D.; Tsao, S.W.; Bu, H. [Human papillomavirus infection and esophageal squamous cell carcinoma]. Zhonghua Bing. Li Xue Za Zhi 1996, 25, 351–354. [Google Scholar]
- Ragin, C.C.; Taioli, E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int. J. Cancer 2007, 121, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Westra, W.H.; Taube, J.M.; Poeta, M.L.; Begum, S.; Sidransky, D.; Koch, W.M. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2008, 14, 366–369. [Google Scholar] [CrossRef]
- Gkika, E.; Gauler, T.; Eberhardt, W.; Stahl, M.; Stuschke, M.; Pottgen, C. Long-term results of definitive radiochemotherapy in locally advanced cancers of the cervical esophagus. Dis. Esophagus 2014, 27, 678–684. [Google Scholar] [CrossRef]
- Huang, S.H.; Lockwood, G.; Brierley, J.; Cummings, B.; Kim, J.; Wong, R.; Bayley, A.; Ringash, J. Effect of concurrent high-dose cisplatin chemotherapy and conformal radiotherapy on cervical esophageal cancer survival. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 735–740. [Google Scholar] [CrossRef]
- Stuschke, M.; Stahl, M.; Wilke, H.; Walz, M.K.; Oldenburg, A.R.; Stuben, G.; Jahnke, K.; Seeber, S.; Sack, H. Induction chemotherapy followed by concurrent chemotherapy and high-dose radiotherapy for locally advanced squamous cell carcinoma of the cervical oesophagus. Oncology 1999, 57, 99–105. [Google Scholar] [CrossRef]
- Caudell, J.J.; Gillison, M.L.; Maghami, E.; Spencer, S.; Pfister, D.G.; Adkins, D.; Birkeland, A.C.; Brizel, D.M.; Busse, P.M.; Cmelak, A.J.; et al. NCCN Guidelines(R) Insights: Head and Neck Cancers, Version 1.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Guler, O.C.; Onal, C. Oncological outcomes of squamous cell carcinoma of the cervical esophagus treated with definitive (chemo-)radiotherapy: A systematic review and meta-analysis. In regard to De Virgilio et al. J. Cancer Res. Clin. Oncol. 2022, 148, 2567–2568. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | |
|---|---|
| Total, n | 497 |
| Age, median (range) years | 68 (41~95) |
| Male, n (%) | 444 (89.3) |
| Location, n (%) | |
| Cervical | 32 (6.4) |
| Upper thoracic | 75 (15.1) |
| Mid-thoracic | 224 (45.1) |
| Lower thoracic | 166 (33.4) |
| Differentiation, n (%) | |
| Well | 105 (21.1) |
| Moderate | 198 (39.8) |
| Poorly | 102 (20.5) |
| Unknown | 92 (18.5) |
| T stage, n (%) | |
| 1 | 175 (35.2) |
| 2 | 56 (11.3) |
| 3 | 235 (47.3) |
| 4 | 31 (6.2) |
| Lymph node metastasis, n (%) | 275 (55.3) |
| Initially distant metastasis, n (%) | 77 (15.5) |
| Stage, n (%) | |
| I | 155 (31.2) |
| II | 83 (16.7) |
| III | 158 (31.8) |
| IV | 101 (20.3) |
| Main treatment, n (%) | |
| Operation | 229 (46.1) |
| Chemoradiotherapy or radiotherapy | 103 (20.7) |
| Endoscopic resection | 57 (11.5) |
| Chemotherapy | 38 (7.6) |
| None | 70 (14.1) |
| Cervical (n = 32) | Non-Cervical (n = 465) | p Value | |
|---|---|---|---|
| Age, median (range) years | 73 (46~87) | 67 (41~95) | 0.047 |
| Male, n (%) | 26 (81.3) | 418 (89.9) | 0.133 |
| Differentiation, n (%) | |||
| Well | 4 (12.5) | 101 (21.7) | 0.224 |
| Moderate | 12 (37.5) | 186 (40.0) | 0.887 |
| Poorly | 11 (34.4) | 91 (19.6) | 0.049 |
| Unknown | 5 (15.6) | 87 (18.7) | 0.664 |
| T stage, n (%) | |||
| 1 | 2 (6.2) | 173 (37.2) | 0.003 |
| 2 | 7 (21.9) | 49 (10.5) | 0.056 |
| 3 | 14 (43.8) | 221 (47.5) | 0.679 |
| 4 | 9 (28.1) | 22 (4.7) | <0.001 |
| Lymph node metastasis, n (%) | 21 (65.6) | 254 (54.6) | 0.229 |
| Initially distant metastasis, n (%) | 5 (15.6) | 72 (15.5) | 0.983 |
| Stage, n (%) | |||
| I | 2 (6.2) | 153 (32.9) | 0.007 |
| II | 8 (25.0) | 75 (16.1) | 0.198 |
| III | 10 (31.3) | 148 (31.8) | 0.946 |
| IV | 12 (37.5) | 89 (19.1) | 0.015 |
| Main treatment, n (%) | |||
| Operation | 1 (3.1) | 228 (49.0) | 0.001 |
| Chemoradiotherapy or radiotherapy | 22 (68.8) | 81 (17.4) | <0.001 |
| Endoscopic resection | 0 (0) | 57 (12.3) | 0.122 |
| Chemotherapy | 2 (6.2) | 36 (7.7) | 0.759 |
| None | 7 (21.9) | 63 (13.5) | 0.196 |
| Cervical (n = 22) | Non-Cervical (n = 73) | p Value | |
|---|---|---|---|
| Age, mean (range) years | 68 (46~87) | 72 (53~89) | 0.566 |
| Male, n (%) | 18 (81.8) | 69 (94.5) | 0.075 |
| Differentiation, n (%) | |||
| Well | 4 (18.2) | 13 (17.8) | 0.887 |
| Moderate | 9 (40.9) | 35 (47.9) | 0.562 |
| Poorly | 6 (27.3) | 10 (13.7) | 0.143 |
| Unknown | 3 (13.6) | 15 (20.5) | 0.472 |
| T stage, n (%) | |||
| 1 | 2 (9.1) | 9 (12.3) | 0.679 |
| 2 | 7 (31.8) | 14 (19.2) | 0.215 |
| 3 | 7 (31.8) | 44 (60.3) | 0.022 |
| 4 | 6 (27.3) | 6 (8.2) | 0.025 |
| Lymph node metastasis, n (%) | 12 (54.5) | 52 (71.2) | 0.148 |
| Initially distant metastasis, n (%) | 1 (4.5) | 5 (6.8) | 0.699 |
| Stage, n (%) | |||
| I | 2 (9.1) | 9 (12.3) | 0.679 |
| II | 7 (31.8) | 15 (20.5) | 0.276 |
| III | 7 (31.8) | 36 (49.3) | 0.153 |
| IV | 6 (27.3) | 13 (17.8) | 0.334 |
| Concurrent chemoradiotherapy, n (%) | 18 (81.8) | 61 (83.6) | 0.848 |
| Chemotherapy regimen | |||
| 5-Fluorouracil + Cisplatin | 12/18 (66.7) | 46/61 (75.4) | 0.463 |
| Paclitaxel + Carboplatin | 3/18 (16.7) | 12/61 (19.7) | 0.775 |
| Others | 3/18 (16.7) | 3/61 (4.9) | 0.119 |
| Radiotherapy alone, n (%) | 4 (18.2) | 12 (16.4) | 0.848 |
| Schedule completed, n (%) | 20 (90.9) | 63 (86.3) | 0.571 |
| Death during treatment, n (%) | 1 (4.5) | 2 (2.7) | 0.674 |
| Clinically complete response, n (%) | 14 (63.6) | 39 (53.4) | 0.340 |
| According to Stage | |||
| I | 1/2 (50.0) | 9/9 (100) | 0.112 |
| II | 6/7 (85.7) | 12/15 (80.0) | 0.747 |
| III | 2/7 (28.6) | 12/36 (33.3) | 0.806 |
| IV | 5/6 (83.3) | 6/13 (46.2) | 0.151 |
| recurrence after complete response | 2/14 (14.3) | 5/39 (12.8) | 0.890 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, D.-G.; Kim, K.; Liu, H.; Lee, S.S.; Lee, S.L. Clinical Features and Prognosis of Cervical Esophageal Cancer. J. Clin. Med. 2025, 14, 3803. https://doi.org/10.3390/jcm14113803
Ryu D-G, Kim K, Liu H, Lee SS, Lee SL. Clinical Features and Prognosis of Cervical Esophageal Cancer. Journal of Clinical Medicine. 2025; 14(11):3803. https://doi.org/10.3390/jcm14113803
Chicago/Turabian StyleRyu, Dae-Gon, Keekyoung Kim, Hongqun Liu, Samuel S. Lee, and Sangjune Laurence Lee. 2025. "Clinical Features and Prognosis of Cervical Esophageal Cancer" Journal of Clinical Medicine 14, no. 11: 3803. https://doi.org/10.3390/jcm14113803
APA StyleRyu, D.-G., Kim, K., Liu, H., Lee, S. S., & Lee, S. L. (2025). Clinical Features and Prognosis of Cervical Esophageal Cancer. Journal of Clinical Medicine, 14(11), 3803. https://doi.org/10.3390/jcm14113803






