Ovarian Reserve After Robotic Versus Laparoscopic Single-Site Ovarian Cystectomy for Mature Cystic Teratoma: A Prospective Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Procedures
2.2.1. Robotic Single-Site Ovarian Cystectomy (RO)
2.2.2. Laparoscopic Single-Site Ovarian Cystectomy (LO)
2.3. Data Analysis
2.4. Statistical Analysis
2.5. Ethical Approval and Trical Registration
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinha, R.; Sanjay, M.; Rupa, B.; Kumari, S. Robotic surgery in gynecology. J. Minimal Access Surg. 2015, 11, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Magrina, J.F. Robotic surgery in gynecology. Eur. J. Gynaecol. Oncol. 2007, 28, 77–82. [Google Scholar] [PubMed]
- Lawrie, T.A.; Liu, H.; Lu, D.; Dowswell, T.; Song, H.; Wang, L.; Shi, G. Robot-assisted surgery in gynaecology. Cochrane Database Syst. Rev. 2019, 4, CD011422. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Chang, C.S.; Noh, J.J.; Kim, T.J. Does Robot Assisted Laparoscopy (RAL) Have an Advantage in Preservation of Ovarian Reserve in Endometriosis Surgery? Comparison of Single-Port Access (SPA) RAL and SPA Laparoscopy. J. Clin. Med. 2023, 12, 4673. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, S.Y.; Jeong, K.; Yun, H.Y.; Chung, H.W. What is the role of robotic surgery in ovarian cystectomy with fertility preservation? J. Robot. Surg. 2023, 17, 2743–2747. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.S.; Lee, Y.S. Comparison of serum antimullerian hormone levels after robotic-assisted vs. laparoscopic approach for ovarian cystectomy in endometrioma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 249, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, H.; Li, D.; Qiu, L.; Dai, J.; Sun, D.; Zhang, J. Comparison of the impact of single-port laparoscopic and conventional laparoscopic ovarian cystectomy on the ovarian reserve in adult patients with benign ovarian cysts. Minim. Invasive Ther. Allied Technol. 2020, 29, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed]
- Su, H.I.; Maas, K.; Sluss, P.M.; Chang, R.J.; Hall, J.E.; Joffe, H. The impact of depot GnRH agonist on AMH levels in healthy reproductive-aged women. J. Clin. Endocrinol. Metab. 2013, 98, E1961–E1966. [Google Scholar] [CrossRef] [PubMed]
- Hariton, E.; Shirazi, T.N.; Douglas, N.C.; Hershlag, A.; Briggs, S.F. Anti-Mullerian hormone levels among contraceptive users: Evidence from a cross-sectional cohort of 27,125 individuals. Am. J. Obstet. Gynecol. 2021, 225, 515.e1–515.e10. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.S.; Wang, Y.L.; Chuang, F.C.; Sun, F.J. Effect of dienogest on serum anti-Mullerian hormone level after laparoscopic cystectomy of ovarian endometrioma. Taiwan. J. Obstet. Gynecol. 2023, 62, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, G.; Safinataj, M.; Shahesmaeili, A.; Allahqoli, L.; Salehiniya, H.; Alkatout, I. Effect of laparoscopic cystectomy on ovarian reserve in patients with ovarian cyst. Front. Endocrinol. 2022, 13, 964229. [Google Scholar] [CrossRef] [PubMed]
- Ergun, B.; Ozsurmeli, M.; Dundar, O.; Comba, C.; Kuru, O.; Bodur, S. Changes in Markers of Ovarian Reserve After Laparoscopic Ovarian Cystectomy. J. Minim. Invasive Gynecol. 2015, 22, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Hartono, E.; Budipramana, E.; Abdullah, N.; Tessy, T. Ovarian Cystectomy: Stitching or Cauterizing—A Comparison Study of Anti-Mullerian Hormone Level Pre- and Postoperatively. Gynecol. Minim. Invasive Ther. 2019, 8, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Baracat, C.M.F.; Abdalla-Ribeiro, H.S.A.; Araujo, R.; Bernando, W.M.; Ribeiro, P.A. The Impact on Ovarian Reserve of Different Hemostasis Methods in Laparoscopic Cystectomy: A Systematic Review and Meta-analysis. Rev. Bras. Ginecol. Obstet. 2019, 41, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Khoiwal, K.; Kapoor, N.; Gaurav, A.; Reddy, K.K.; Chaturvedi, J. The Effect of Body Mass Index on Peri-operative Parameters of Total Laparoscopic Hysterectomy: An Institutional Experience. Cureus 2021, 13, e15558. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, M.; Hamilton, K.M.; Schneyer, R.J.; Levin, G.; Weiss, Y.; Siedhoff, M.T.; Wright, K.N.; Meyer, R. The impact of body mass index on surgical complications in minimally invasive hysterectomy for uterine fibroids. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 305, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lim, S.W.; Choi, H.S.; Jeong, S.Y.; Oh, J.H.; Lim, S.B. The impact of obesity on outcomes of laparoscopic surgery for colorectal cancer in Asians. Surg. Endosc. 2010, 24, 1679–1685. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | LO (n = 43) | RO (n = 40) | p |
|---|---|---|---|
| Age, year | 30.4 ± 4.9 | 29.7 ± 4.9 | 0.490 |
| BMI, kg/m2 | 23.1 ± 3.6 | 21.7 ± 2.1 | 0.030 |
| Married | 0.951 | ||
| No | 32 (74.4) | 30 (75.0) | |
| Yes | 11 (25.6) | 10 (25.0) | |
| Nulliparous | 0.316 | ||
| No | 36 (83.7) | 37 (92.5) | |
| Yes | 7 (16.3) | 33 (72.5) | |
| Previous abdominal surgery | 0.435 | ||
| No | 38 (88.4) | 38 (95.0) | |
| Yes | 5 (11.6) | 2 (5.0) | |
| Peritoneal adhesion | 0.111 | ||
| No | 6 (14.0) | 1 (2.5) | |
| Yes | 37 (86.0) | 39 (97.5) | |
| Diameter of ovarian cyst, cm | 6.7 ± 2.2 | 7.1 ± 1.7 | 0.354 |
| Location of ovarian cyst | 0.677 | ||
| Unilateral | 39 (90.7) | 38 (95.0) | |
| Bilateral | 4 (9.3) | 2 (5.0) |
| Characteristics | LO (n = 43) | RO (n = 40) | p |
|---|---|---|---|
| Operation time, min | 70.0 ± 24.0 | 86.5 ± 26.7 | 0.002 |
| Estimated blood loss, mL | 64.2 ± 37.2 | 61.0 ± 30.8 | 0.673 |
| Hemoglobin decrement, g/dL | 2.0 ± 0.7 | 2.0 ± 0.8 | 0.675 |
| Concurrent surgery | 0.100 | ||
| No | 38 (90.5) | 31 (77.5) | |
| Myomectomy | 1 (2.4) | 1 (2.5) | |
| Paratubal cystectomy | 1 (2.4) | 3 (7.5) | |
| Hysteroscopy | 2 (4.8) | 5 (12.5) | |
| Hospital stay, days | 4.1 ± 0.7 | 4.0 ± 0 | 0.070 |
| Hemostasis methods | <0.001 | ||
| None | 0 (0) | 2 (5.0) | |
| Bipolar coagulation | 27 (62.8) | 5 (12.5) | |
| Suture | 16 (37.2) | 33 (82.5) | |
| Complications | 0.495 | ||
| None | 41 (95.3) | 40 (100) | |
| Reoperation within 1 week | 0 (0) | 0 (0) | |
| Ileus | 2 (4.7) | 0 (0) | |
| Fever > 3 days | 0 (0) | 0 (0) | |
| Ovarian reserve | |||
| Preoperative AMH level, ng/mL | 4.26 ± 2.04 | 4.09 ± 1.66 | 0.677 |
| Postoperative AMH level, ng/mL | 3.75 ± 1.91 | 3.90 ± 1.70 | 0.720 |
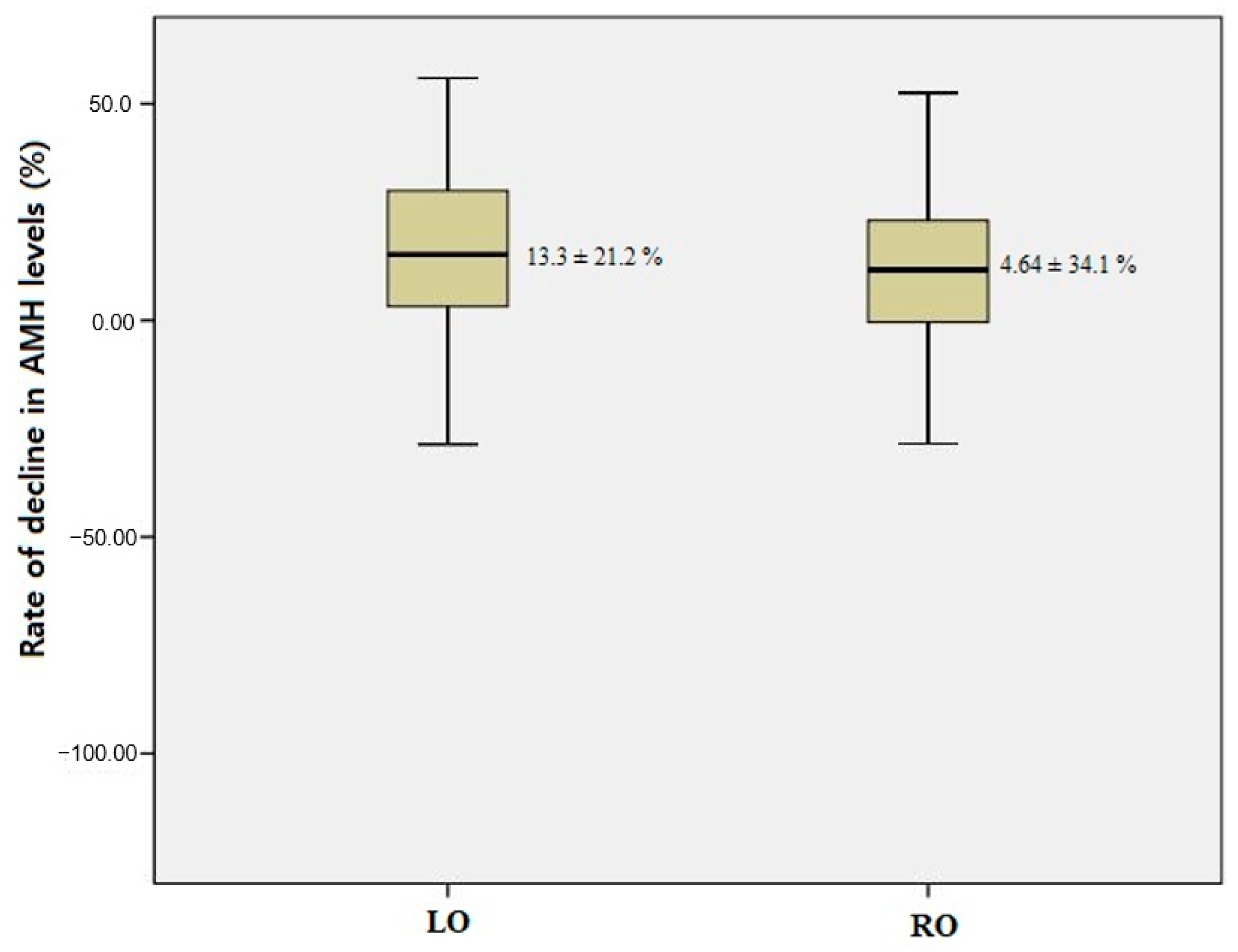
| Rate of decline in AMH level, % | 13.3 ± 21.2 | 4.64 ± 34.1 | 0.167 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Clinical Factors | OR(95% CI) | p | OR(95% CI) | p |
| Rate AMH decline ≥ 14.0 a | ||||
| RO (vs. LO) | 1.107(0.383–3.198) | 0.851 | ||
| BMI ≥ 21.9 kg/m2 a (vs. <21.9 kg/m2) | 1.107(0.383–3.198) | 0.184 | 0.547(0.218–1.373) | 0.199 |
| Suture (vs. bipolar coagulation) | 0.422(0.137–1.300) | 0.133 | 2.061(0.803–5.286) | 0.132 |
| OP time ≥ 70 min a (vs. <70 min) | 1.301(0.498–3.400) | 0.591 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, S.; Choi, S.H.; Lee, N.; Shim, S.H.; Kim, M.K.; Kim, M.-L.; Jung, Y.W.; Paek, J.Y.; Seong, S.J. Ovarian Reserve After Robotic Versus Laparoscopic Single-Site Ovarian Cystectomy for Mature Cystic Teratoma: A Prospective Comparative Study. J. Clin. Med. 2025, 14, 3800. https://doi.org/10.3390/jcm14113800
Won S, Choi SH, Lee N, Shim SH, Kim MK, Kim M-L, Jung YW, Paek JY, Seong SJ. Ovarian Reserve After Robotic Versus Laparoscopic Single-Site Ovarian Cystectomy for Mature Cystic Teratoma: A Prospective Comparative Study. Journal of Clinical Medicine. 2025; 14(11):3800. https://doi.org/10.3390/jcm14113800
Chicago/Turabian StyleWon, Seyeon, Su Hyeon Choi, Nara Lee, So Hyun Shim, Mi Kyoung Kim, Mi-La Kim, Yong Wook Jung, Jin Young Paek, and Seok Ju Seong. 2025. "Ovarian Reserve After Robotic Versus Laparoscopic Single-Site Ovarian Cystectomy for Mature Cystic Teratoma: A Prospective Comparative Study" Journal of Clinical Medicine 14, no. 11: 3800. https://doi.org/10.3390/jcm14113800
APA StyleWon, S., Choi, S. H., Lee, N., Shim, S. H., Kim, M. K., Kim, M.-L., Jung, Y. W., Paek, J. Y., & Seong, S. J. (2025). Ovarian Reserve After Robotic Versus Laparoscopic Single-Site Ovarian Cystectomy for Mature Cystic Teratoma: A Prospective Comparative Study. Journal of Clinical Medicine, 14(11), 3800. https://doi.org/10.3390/jcm14113800







