Protocols of Thiamine Supplementation: Clinically Driven Rationality vs. Biological Commonsense
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Collected Data
3.2. Bioavailability of Thiamine and Whole-Body Store
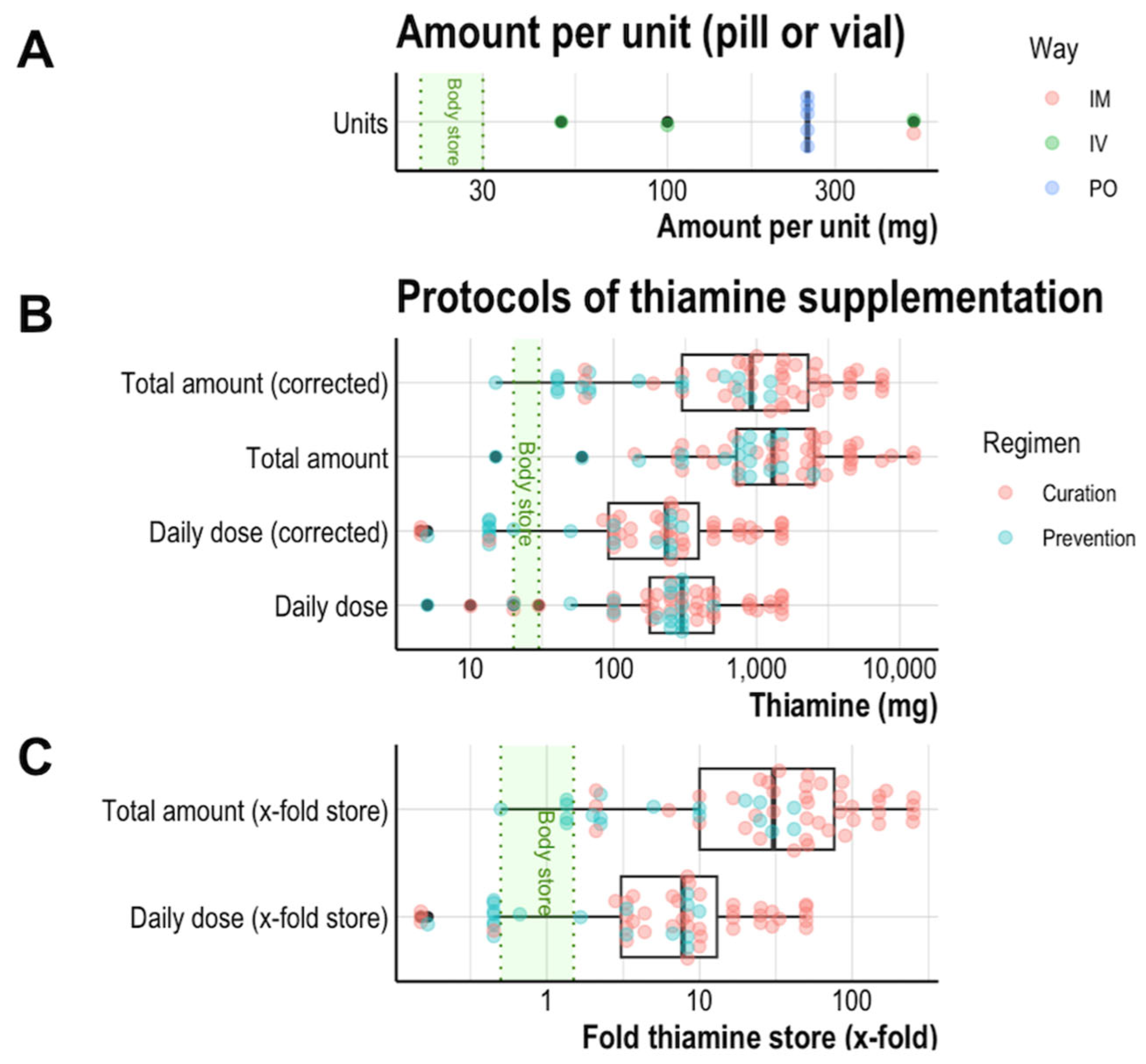
3.3. Amounts of Thiamine in Available Brands
3.4. Supplementation Regimens Deliver Very High Amounts
3.5. Clinical Expectation Is Reflected in Biological Supplementation
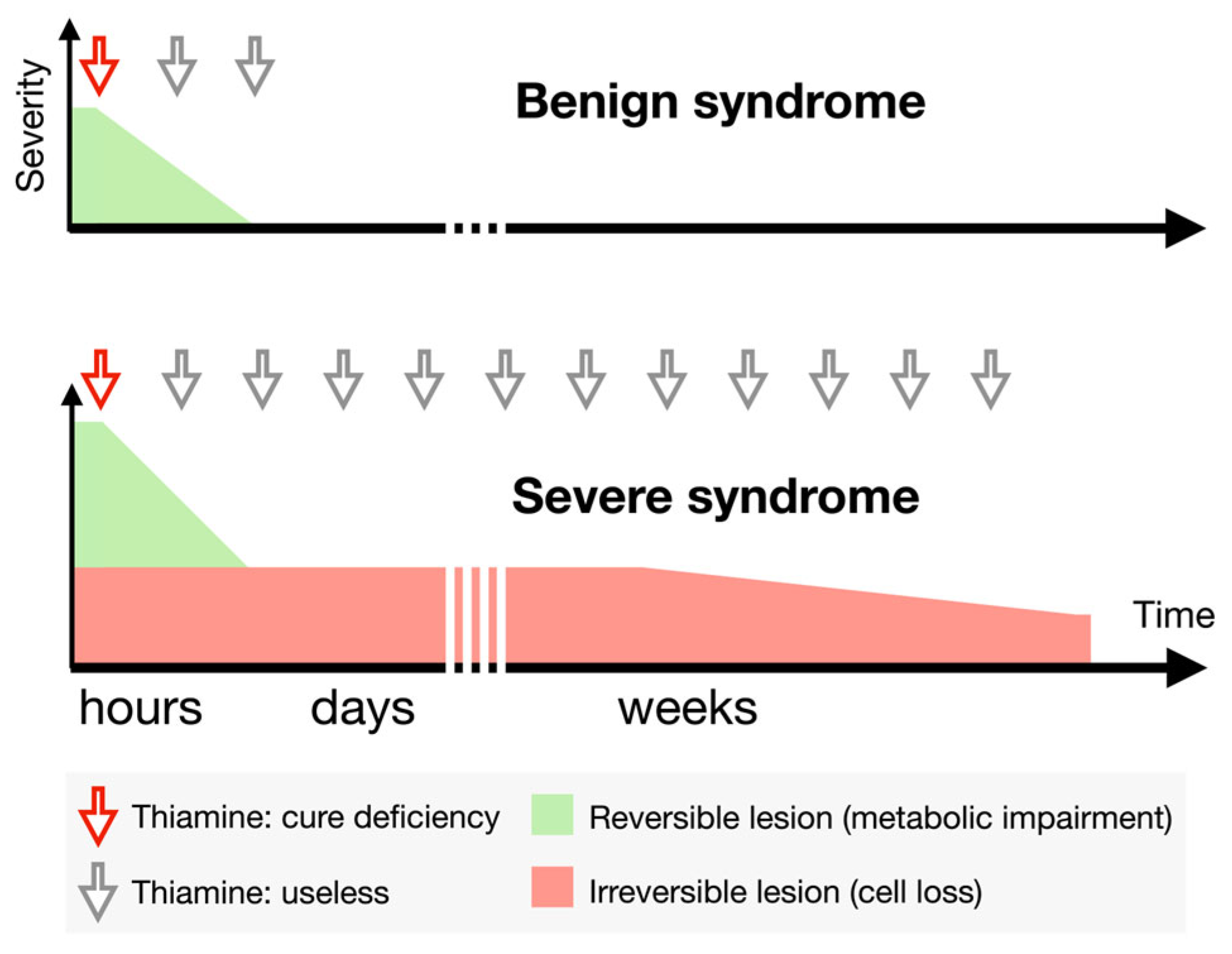
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smithline, H.A.; Donnino, M.; Greenblatt, D.J. Pharmacokinetics of High-Dose Oral Thiamine Hydrochloride in Healthy Subjects. BMC Clin. Pharmacol. 2012, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Blass, J.P. Iatrogenic Nutritional Deficiencies. Annu. Rev. Nutr. 1982, 2, 201–227. [Google Scholar] [CrossRef] [PubMed]
- Overton, E.; Emelyanova, A.; Bunik, V.I. Thiamine, Gastrointestinal Beriberi and Acetylcholine Signaling. Front. Nutr. 2025, 12, 1541054. [Google Scholar] [CrossRef]
- Thomson, A.D.; Leevy, C.M. Observations on the Mechanism of Thiamine Hydrochloride Absorption in Man. Clin. Sci. 1972, 43, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.D.; Baker, H.; Leevy, C.M. Patterns of 35S-Thiamine Hydrochloride Absorption in the Malnourished Alcoholic Patient. J. Lab. Clin. Med. 1970, 76, 34–45. [Google Scholar]
- Thomson, A.D. Mechanisms of Vitamin Deficiency in Chronic Alcohol Misusers and the Development of the Wernicke-Korsakoff Syndrome. Alcohol Alcohol. 2000, 35, 2. [Google Scholar] [CrossRef]
- Chataway, J.; Hardman, E. Thiamine in Wernicke’s Syndrome--How Much and How Long? Postgrad. Med. J. 1995, 71, 249. [Google Scholar] [CrossRef]
- Yoon, C.; Gedzior, J.; DePry, D. Wernicke–Korsakoff Syndrome: Focus on Low-Threshold Diagnosis and Prompt Treatment in the Primary Care Setting. Int. J. Psychiatry Med. 2019, 54, 172–180. [Google Scholar] [CrossRef]
- Weber, W.; Kewitz, H. Determination of Thiamine in Human Plasma and Its Pharmacokinetics. Eur. J. Clin. Pharmacol. 1985, 28, 213–219. [Google Scholar] [CrossRef]
- Baines, M.; Bligh, J.G.; Madden, J.S. Tissue Thiamin Levels of Hospitalised Alcoholics before and after Oral or Parenteral Vitamins. Alcohol Alcohol. 1988, 23, 49–52. [Google Scholar]
- Tallaksen, C.M.E.; Sande, A.; Bøhmer, T.; Bell, H.; Karlsen, J. Kinetics of Thiamin and Thiamin Phosphate Esters in Human Blood, Plasma and Urine after 50 Mg Intravenously or Orally. Eur. J. Clin. Pharmacol. 1993, 44, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.D. The Royal College of Physicians Report on Alcohol: Guidelines for Managing Wernicke’s Encephalopathy in the Accident and Emergency Department. Alcohol Alcohol. 2002, 37, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Alim, U.; Bates, D.; Langevin, A.; Werry, D.; Dersch-Mills, D.; Herman, R.J.; Mintz, M.; Ghosh, S. Thiamine Prescribing Practices for Adult Patients Admitted to an Internal Medicine Service. Can. J. Hosp. Pharm. 2017, 70, 179–187. [Google Scholar] [CrossRef]
- Agedal, K.J.; Steidl, K.E.; Burgess, J.L. An Overview of Type B Lactic Acidosis Due to Thiamine (B1) Deficiency. J. Pediatr. Pharmacol. Ther. 2023, 28, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Tanabe, Y.; Suzuki, K.; Kumakura, M.; Aizawa, Y. Shoshin Beriberi With Vasospastic Angina Pectoris. Possible Mechanism of Mid-Ventricular Obstruction. Circ. J. 2002, 66, 1070–1072. [Google Scholar] [CrossRef]
- Lei, Y.; Zheng, M.-H.; Huang, W.; Zhang, J.; Lu, Y. Wet Beriberi with Multiple Organ Failure Remarkably Reversed by Thiamine Administration: A Case Report and Literature Review. Medicine 2018, 97, e0010. [Google Scholar] [CrossRef]
- Giacalone, M.; Martinelli, R.; Abramo, A.; Rubino, A.; Pavoni, V.; Iacconi, P.; Giunta, F.; Forfori, F. Rapid Reversal of Severe Lactic Acidosis After Thiamine Administration in Critically Ill Adults: A Report of 3 Cases. Nut Clin. Pract. 2015, 30, 104–110. [Google Scholar] [CrossRef]
- Lim, M.S.; Win, W.; Von Essen, A.; Gannon, D.; Ramali, M. Lessons of the Month 1: Shoshin Beriberi: A Case Report of Fulminant Cardiovascular Collapse, Intractable Hyperlactatemia and Deteriorating Consciousness. Clin. Med. 2021, 21, e670–e672. [Google Scholar] [CrossRef]
- Dabar, G.; Harmouche, C.; Habr, B.; Riachi, M.; Jaber, B. Shoshin Beriberi in Critically-Ill Patients: Case Series. Nutr. J. 2015, 14, 51. [Google Scholar] [CrossRef]
- Kitamura, K.; Yamaguchi, T.; Tanaka, H.; Hashimoto, S.; Yang, M.; Takahashi, T. TPN-Induced Fulminant Beriberi: A Report on Our Experience and a Review of the Literature. Surg. Today 1996, 26, 769–776. [Google Scholar] [CrossRef]
- Rugilo, C.A.; Uribe Roca, M.C.; Zurru, M.C.; Capizzano, A.A.; Pontello, G.A.; Gatto, E.M. Proton MR Spectroscopy in Wernicke Encephalopathy. Am. J. Neuroradiol. 2003, 24, 952–955. [Google Scholar]
- Hazell, A.S.; Butterworth, R.F. Update of Cell Damage Mechanisms in Thiamine Deficiency: Focus on Oxidative Stress, Excitotoxicity and Inflammation. Alcohol Alcohol. 2009, 44, 141–147. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Mouri, H.; Jo, T.; Hashimoto, Y.; Matsui, H.; Fushimi, K.; Yasunaga, H.; Taniguchi, T. Clinical Features and Outcomes of Shoshin Beriberi: A Retrospective Nationwide Database Study. Int. Heart J. 2024, 65, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, M.L.; Bowden, S.C.; Whelan, G. Thiamin Treatment and Working Memory Function of Alcohol-Dependent People: Preliminary Findings. Alcohol. Clin. Exp. Res. 2001, 25, 112–116. [Google Scholar] [PubMed]
- Dingwall, K.M.; Delima, J.F.; Binks, P.; Batey, R.; Bowden, S.C. What Is the Optimum Thiamine Dose to Treat or Prevent Wernicke’s Encephalopathy or Wernicke–Korsakoff Syndrome? Results of a Randomized Controlled Trial. Alcohol. Clin. Exp. Res. 2022, 46, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Victor, M.; Adams, R.D.; Collins, G.H. The Wernicke-Korsakoff Syndrome. A Clinical and Pathological Study of 245 Patients, 82 with Post-Mortem Examinations. Contemp. Neurol. Ser. 1971, 7, 1–206. [Google Scholar]
- Tjong, E.; Peng, Y.-Y. Gastrointestinal Beriberi and Wernicke’s Encephalopathy Triggered by One Session of Heavy Drinking. Case Rep. Neurol. 2019, 11, 124–131. [Google Scholar] [CrossRef]
- Day, G.S.; Ladak, S.; Curley, K.; Farb, N.A.S.; Masiowski, P.; Pringsheim, T.; Ritchie, M.; Cheung, A.; Jansen, S.; Methot, L.; et al. Thiamine Prescribing Practices within University-affiliated Hospitals: A Multicenter Retrospective Review. J. Hosp. Med. 2015, 10, 246–253. [Google Scholar] [CrossRef]
- Wrenn, K.D.; Murphy, F.; Slovis, C.M. A Toxicity Study of Parenteral Thiamine Hydrochloride. Ann. Emerg. Med. 1989, 18, 867–870. [Google Scholar] [CrossRef]
- McLaughlin, K.; Joyal, K.; Lee, S.; Corrado, M.; Marquis, K.; Anger, K.; Szumita, P. Safety of Intravenous Push Thiamine Administration at a Tertiary Academic Medical Center. J. Am. Pharm. Assoc. 2020, 60, 598–601. [Google Scholar] [CrossRef]
- Aldhaeefi, M.; McLaughlin, K.; Goodberlet, M.; Szumita, P. Evaluation of the Safety of 500 Mg Intravenous Push Thiamine at a Tertiary Academic Medical Center. Sci. Prog. 2022, 105, 00368504221096539. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnan, M. Protocols of Thiamine Supplementation: Clinically Driven Rationality vs. Biological Commonsense. J. Clin. Med. 2025, 14, 3787. https://doi.org/10.3390/jcm14113787
Bonnan M. Protocols of Thiamine Supplementation: Clinically Driven Rationality vs. Biological Commonsense. Journal of Clinical Medicine. 2025; 14(11):3787. https://doi.org/10.3390/jcm14113787
Chicago/Turabian StyleBonnan, Mickael. 2025. "Protocols of Thiamine Supplementation: Clinically Driven Rationality vs. Biological Commonsense" Journal of Clinical Medicine 14, no. 11: 3787. https://doi.org/10.3390/jcm14113787
APA StyleBonnan, M. (2025). Protocols of Thiamine Supplementation: Clinically Driven Rationality vs. Biological Commonsense. Journal of Clinical Medicine, 14(11), 3787. https://doi.org/10.3390/jcm14113787





