Contrast-Induced Nephropathy (CIN) After Invasive Treatment of Acute Coronary Syndromes—Predictors, Short and Long-Term Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Sources
2.2. Definitions and Procedures
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Predictors of Contrast-Induced Nephropathy (CIN)
3.3. Predictors of 30-Day Mortality
3.4. Predictors of 365-Day Mortality
3.5. Predictors of Rehospitalization Due to Renal Complications
4. Discussion
4.1. Invasive Cardiology Procedures Requiring the Administration of Contrast Agents
4.2. Risk Factors and Pathophysiology of Contrast-Induced Nephropathy
4.3. Long-Term Outcomes and Prognostic Implications of CIN
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Ghali, W.A. Theophylline for prevention of contrast-induced nephropathy: A systematic review and meta-analysis. Arch. Intern. Med. 2005, 165, 1087–1093. [Google Scholar] [CrossRef]
- Zachura, M.; Piątek, Ł.; Kurzawski, J.; Janion, M. Coronary embolism causing acute myocardial infarction. Review of the literature. Med. Stud. 2016, 32, 131–135. [Google Scholar] [CrossRef]
- Kobo, O.; Abramov, D.; Davies, S.; Ahmed, S.B.; Sun, L.Y.; Mieres, J.H.; Parwani, P.; Siudak, Z.; Van Spall, H.G.C.; Mamas, M.A. CKD-Associated Cardiovascular Mortality in the United States: Temporal Trends From 1999 to 2020. Kidney Med. 2022, 5, 100597. [Google Scholar] [CrossRef]
- Kupisz-Urbańska, M.; Jankowski, P.; Topór-Mądry, R.; Chudzik, M.; Gąsior, M.; Gil, R.; Gryka, P.; Kalarus, Z.; Kubica, J.; Legutko, J.; et al. Survival in nonagenarians with acute myocardial infarction in 2014–2020: A nationwide analysis. Kardiol. Pol. 2023, 81, 1015–1017. [Google Scholar] [CrossRef]
- Xu, Z.R.; Chen, J.; Liu, Y.H.; Liu, Y.; Tan, N. The predictive value of the renal resistive index for contrast-induced nephropathy in patients with acute coronary syndrome. BMC Cardiovasc. Disord. 2019, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Sahinkus, S.; Aydin, E.; Aksoy, M.N.M.; Akcay, C.; Eynel, E.; Yaylaci, S. Association between Cardio-ankle Vascular Index and Contrast-induced Nephropathy. J. Coll. Physicians Surg. Pak. 2020, 30, 1251–1255. [Google Scholar]
- Chong, E.; Poh, K.K.; Liang, S.; Soon, C.Y.; Tan, H.-C. Comparison of risks and clinical predictors of contrast-induced nephropathy in patients undergoing emergency versus nonemergency percutaneous coronary interventions. J. Interv. Cardiol. 2010, 23, 451–459. [Google Scholar] [CrossRef]
- Rakowski, T.; Dziewierz, A.; Węgiel, M.; Siudak, Z.; Zasada, W.; Jąkała, J.; Dykla, D.; Matysek, J.; Surdacki, A.; Bartuś, S.; et al. Risk factors of contrast-induced nephropathy in patients with acute coronary syndrome. Kardiol. Pol. 2022, 80, 760–764. [Google Scholar] [CrossRef]
- KDIGO. Clinical Practice Guideline for Acute Kidney Injury. Section 4: Contrast-induced AKI. Kidney Int. Suppl. 2012, 2, 69–88. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC); Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Blömstrom-Lundqvist, C.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef] [PubMed]
- Kaliyaperumal, Y.; Sivadasan, S.; Aiyalu, R. Contrast-Induced Nephropathy: An Overview. Dr. Sulaiman Al Habib Med. J. 2023, 5, 118–127. [Google Scholar] [CrossRef]
- Siudak, Z.; Hawranek, M.; Kleczyński, P.; Bartuś, S.; Kusa, J.; Milewski, K.; Opolski, M.P.; Pawłowski, T.; Protasiewicz, M.; Smolka, G.; et al. Interventional cardiology in Poland in 2022. Annual summary report of the Association of Cardiovascular Interventions of the Polish Cardiac Society (AISN PTK) and Jagiellonian University Medical College. Postepy Kardiol. Interwencyjnej. 2023, 19, 82–85. [Google Scholar] [CrossRef]
- Tandar, A.; Sharma, V.; Ibrahim, M.; Jones, T.; Morgan, D.; Montzingo, C.; Lee, J.; Birgenheier, N.; Silverton, N.; Abraham, A.; et al. Preventing or Minimizing Acute Kidney Injury in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Invasive Cardiol. 2021, 33, E32–E39. [Google Scholar] [CrossRef]
- Tonchev, I.; Heberman, D.; Peretz, A.; Medvedovsky, A.T.; Gotsman, I.; Rashi, Y.; Poles, L.; Goland, S.; Perlman, G.Y.; Danenberg, H.D.; et al. Acute kidney injury after MitraClip implantation in patients with severe mitral regurgitation. Catheter. Cardiovasc. Interv. 2021, 97, E868–E874. [Google Scholar] [CrossRef]
- Magnocavallo, M.; Della Rocca, D.G.; Gianni, C.; Zagrodzky, W.; Lavalle, C.; Mohanty, S.; Chimenti, C.; Al-Ahmad, A.; Di Biase, L.; Horton, R.P.; et al. Zero contrast left atrial appendage occlusion and peridevice leak closure in patients with advanced kidney disease. Heart Rhythm. 2022, 19, 1013–1014. [Google Scholar] [CrossRef]
- Homma, K. Contrast-induced Acute Kidney Injury. Keio J. Med. 2016, 65, 67–73. [Google Scholar] [CrossRef] [PubMed]
- van der Molen, A.J.; Reimer, P.; Dekkers, I.A.; Bongartz, G.; Bellin, M.F.; Bertolotto, M.; Clement, O.; Heinz-Peer, G.; Stacul, F.; Webb, J.A.W.; et al. Post-contrast acute kidney injury—Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors: Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur. Radiol. 2018, 28, 2845–2855. [Google Scholar] [CrossRef]
- Aycock, R.D.; Westafer, L.M.; Boxen, J.L.; Majlesi, N.; Schoenfeld, E.M.; Bannuru, R.R. Acute Kidney Injury After Computed Tomography: A Meta-analysis. Ann. Emerg. Med. 2018, 71, 44–53. [Google Scholar] [CrossRef]
- Rudnick, M.R.; Leonberg-Yoo, A.K.; Litt, H.I.; Cohen, R.M.; Hilton, S.; Reese, P.P. The Controversy of Contrast-Induced Nephropathy With Intravenous Contrast: What Is the Risk? Am. J. Kidney Dis. 2020, 75, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sonhaye, L.; Kolou, B.; Tchaou, M.; Amadou, A.; Assih, K.; N’Timon, B.; Adambounou, K.; Agoda-Koussema, L.; Adjenou, K.; N’Dakena, K. Intravenous Contrast Medium Administration for Computed Tomography Scan in Emergency: A Possible Cause of Contrast-Induced Nephropathy. Radiol. Res. Pract. 2015, 2015, 805786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehran, R.; Aymong, E.D.; Nikolsky, E.; Lasic, Z.; Iakovou, I.; Fahy, M.; Mintz, G.S.; Lansky, A.J.; Moses, J.W.; Stone, G.W.; et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J. Am. Coll. Cardiol. 2004, 44, 1393–1399. [Google Scholar]
- Barbieri, L.; Verdoia, M.; Suryapranata, H.; De Luca, G.; Novara Atherosclerosis Study Group (NAS). Impact of vascular access on the development of contrast induced nephropathy in patients undergoing coronary angiography and/or percutaneous coronary intervention. Int. J. Cardiol. 2019, 275, 48–52. [Google Scholar] [CrossRef]
- Kolte, D.; Spence, N.; Puthawala, M.; Hyder, O.; Tuohy, C.P.; Davidson, C.B.; Sheldon, M.W.; Laskey, W.K.; Abbott, J.D. Association of radial versus femoral access with contrast-induced acute kidney injury in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction. Cardiovasc. Revascularization Med. 2016, 17, 546–551. [Google Scholar] [CrossRef]
- Liu, Y.H.; Jiang, L.; Duan, C.Y.; He, P.C.; Liu, Y.; Tan, N.; Chen, J.Y. Canada Acute Coronary Syndrome Score: A Preprocedural Risk Score for Contrast-Induced Nephropathy After Primary Percutaneous Coronary Intervention. Angiology 2017, 68, 782–789. [Google Scholar] [CrossRef]
- Januszek, R.; Bujak, K.; Kasprzycki, K.; Gąsior, M.; Bartuś, S. Prognosis of patients with renal failure one year following non-ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. Hellenic J. Cardiol. 2024, 76, 48–57. [Google Scholar] [CrossRef]
- Piątek, Ł.; Piątek, K.; Skrzypek, M.; Gąsior, M.; Janion, M.; Siudak, Z.; Sadowski, M. The impact of ECG at admission and a culprit lesion on 12-month outcomes in acute myocardial infarction—Analysis based on the PL-ACS Registry. Med. Stud. 2023, 39, 327–333. [Google Scholar] [CrossRef]
- Liu, T.; Lee, S.R. Poor Prognosis of Contrast-Induced Nephropathy during Long Term Follow Up. Chonnam Med. J. 2021, 57, 197–203. [Google Scholar] [CrossRef]
- Sato, A.; Aonuma, K.; Watanabe, M.; Hirayama, A.; Tamaki, N.; Tsutsui, H.; Murohara, T.; Ogawa, H.; Akasaka, T.; Yoshimura, M.; et al. CINC-J study investigators. Association of contrast-induced nephropathy with risk of adverse clinical outcomes in patients with cardiac catheterization: From the CINC-J study. Int. J. Cardiol. 2017, 227, 424–429. [Google Scholar] [CrossRef]
- Dimitriadis, K.; Vakka, A.; Pyrpyris, N.; Apostolos, A.; Beneki, E.; Stathopoulou, E.; Giannou, P.; Tsioufis, P.; Iliakis, P.; Aznaouridis, K.; et al. Efficacy of Chronic Use of Sodium-Glucose Co-transporter 2 Inhibitors on the Prevention of Contrast-Induced Acute Kidney Injury in Patients with Type 2 Diabetes Mellitus Following Coronary Procedures: A Systematic Review and Meta-Analysis. Am. J. Cardiovasc. Drugs 2025, 25, 57–69. [Google Scholar] [CrossRef]
- Leoncini, M.; Toso, A.; Maioli, M.; Tropeano, F.; Villani, S.; Bellandi, F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: Results from the PRATO-ACS Study (protective effect of rosuvastatin and antiplatelet therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome). J. Am. Coll. Cardiol. 2014, 63, 71–79. [Google Scholar] [PubMed]
- Han, Y.; Zhu, G.; Han, L.; Hou, F.; Huang, W.; Liu, H.; Gan, J.; Jiang, T.; Li, X.; Wang, W.; et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J. Am. Coll. Cardiol. 2014, 63, 62–70. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S. 2021 ACC/AHA/SCAI Guideline for coronary artery revascularization: A report of the American College of Cardiology/American heart association joint committee on clinical practice guidelines. Circulation 2022, 145, e21–e129. [Google Scholar]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Pranata, R.; Wahyudi, D.P. Prevention of Contrast-induced Nephropathy in Patients Undergoing Percutaneous Coronary Intervention. Curr. Cardiol. Rev. 2023, 20, E241023222628. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.M.; Doh, F.M.; Ko, K.I.; Kim, C.H.; Lee, M.J.; Oh, H.J.; Han, S.H.; Kim, B.S.; Yoo, T.-H.; Kang, S.-W.; et al. Diastolic dysfunction is associated with an increased risk of contrast-induced nephropathy: A retrospective cohort study. BMC Nephrol. 2013, 14, 146. [Google Scholar] [CrossRef][Green Version]
- Clec’h, C.; Razafimandimby, D.; Laouisset, M.; Chemouni, F.; Cohen, Y. Incidence and outcome of contrast-associated acute kidney injury in a mixed medical-surgical ICU population: A retrospective study. BMC Nephrol. 2013, 14, 31. [Google Scholar] [CrossRef]

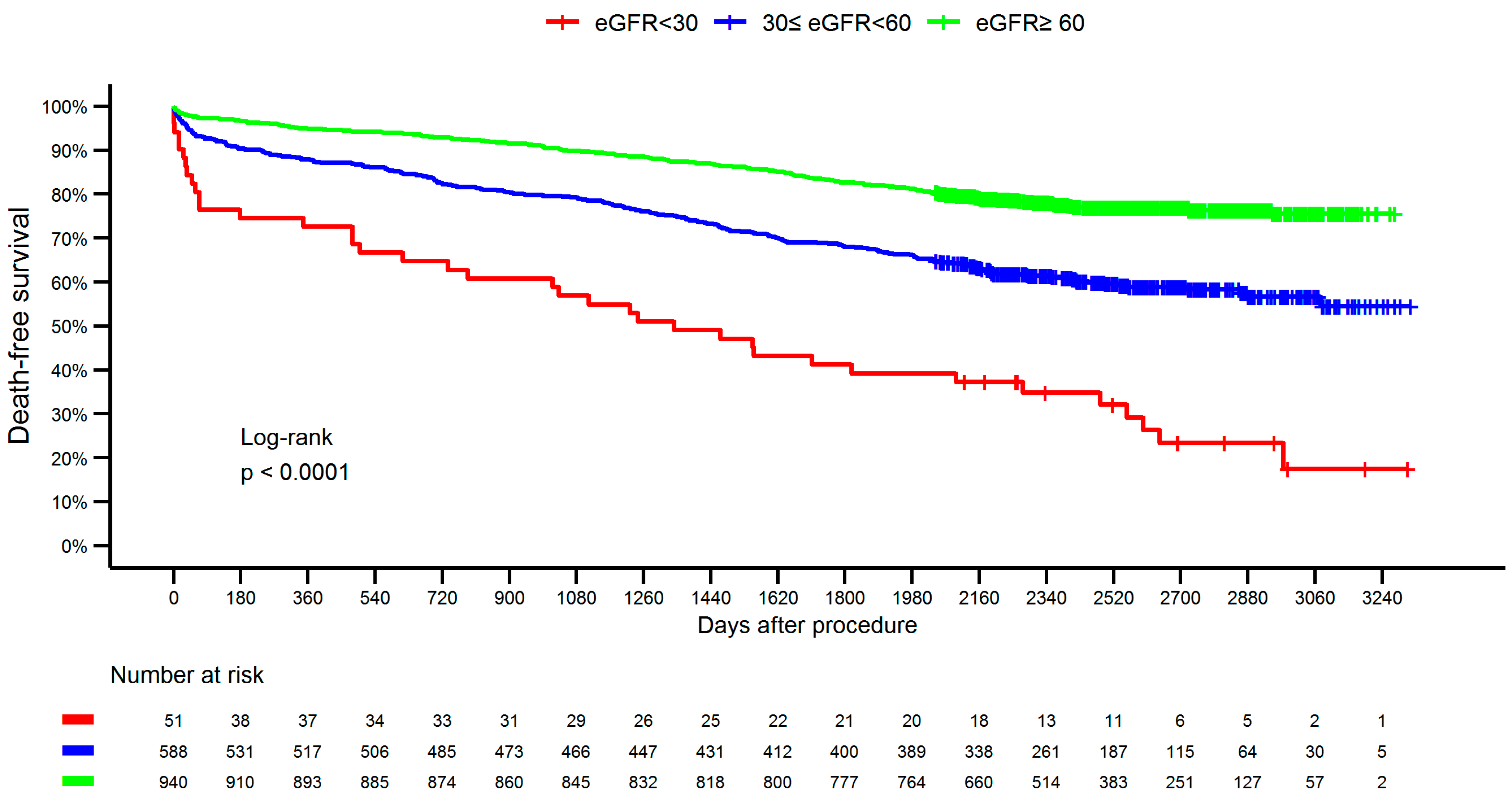
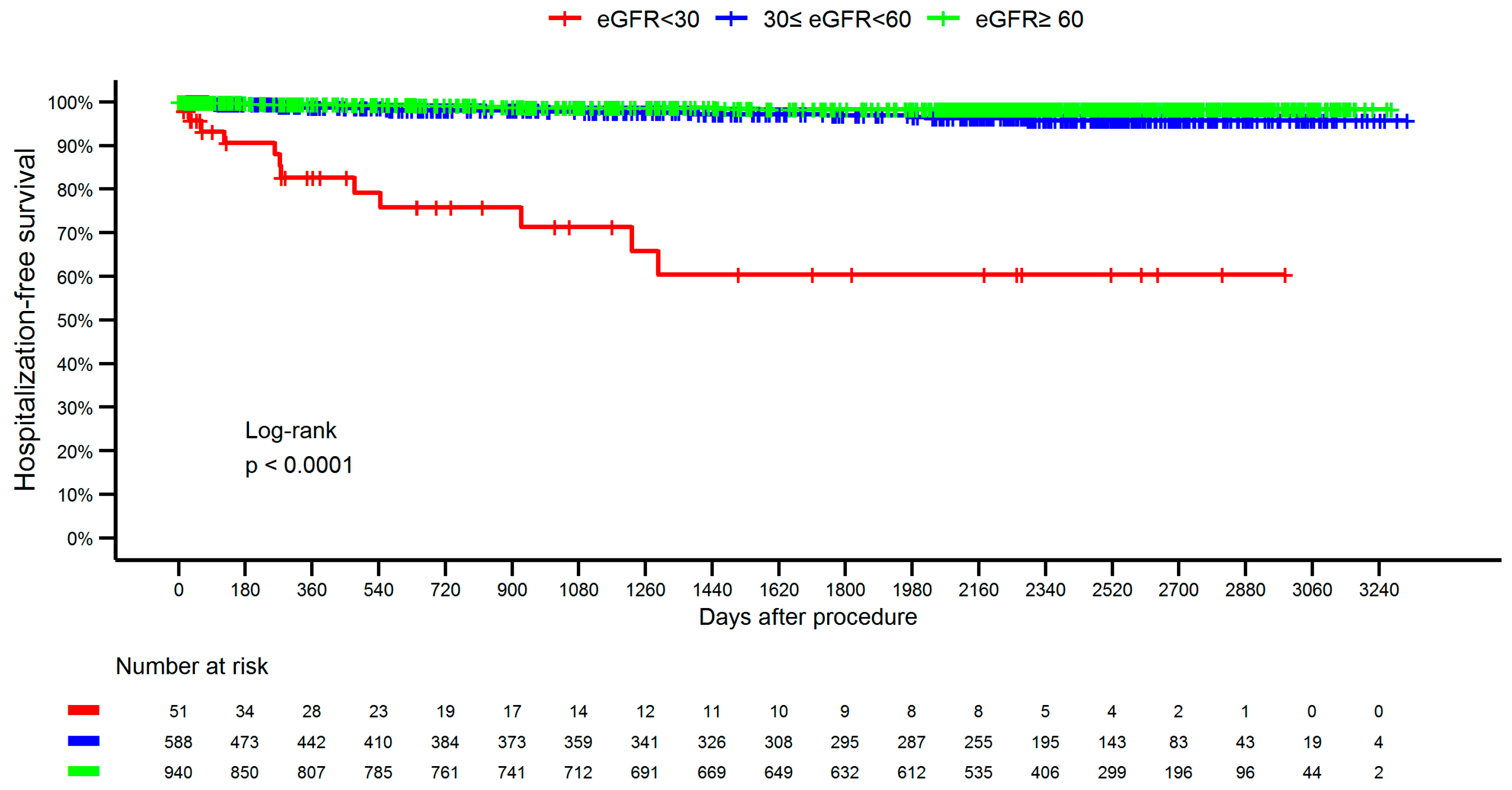
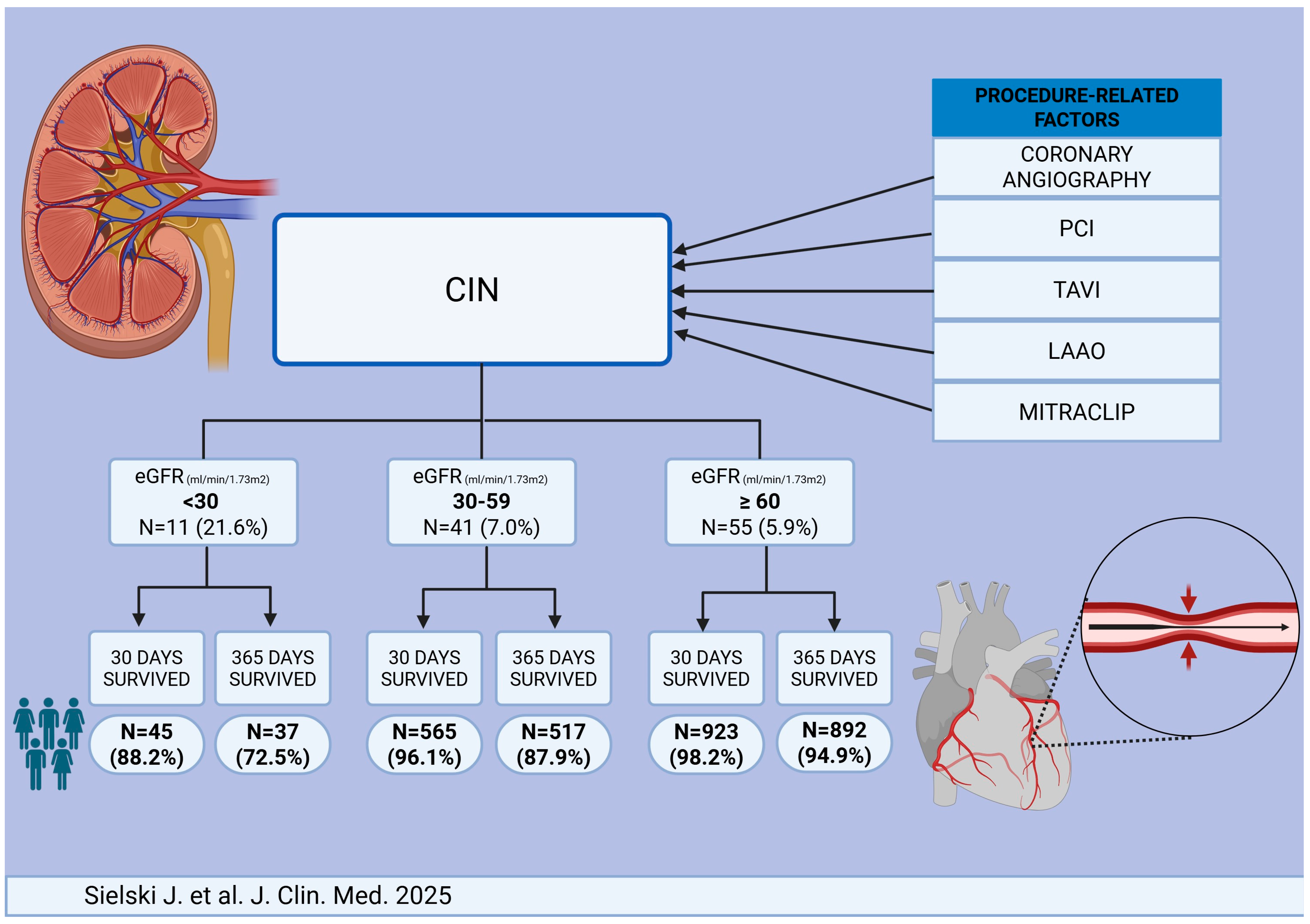
| Variable | <30 (N = 51) | 30–59 (N = 588) | ≥ 60 (N = 857) | Total (N = 1579) | p-Value | |
|---|---|---|---|---|---|---|
| Diagnosis n, (%) | NSTEMI | 10 (19.6) | 87 (14.8) | 129 (13.7) | 226 (14.3) | 0.009 |
| STEMI | 4 (7.8) | 58 (9.9) | 149 (15.9) | 211 (13.4) | ||
| UA | 37 (72.5) | 443 (75.3) | 662 (70.4) | 1142 (72.3) | ||
| Age, year | 75 (68–83) | 72 (66–79) | 63.0 (57–70) | 67 (60–75) | <0.001 | |
| Male gender n, (%) | 19 (37.3) | 267 (45.4) | 709 (75.4) | 995 (63.0) | <0.001 | |
| Weight, kg | 72 (64.5–80) | 76 (67.8–85) | 80.0 (70–90) | 79 (69–89) | <0.001 | |
| Diabetes mellitus n, (%) | 17 (33.3) | 119 (20.2) | 142 (15.1) | 278 (17.6) | <0.001 | |
| Contrast volume, mL | 100 (80–179.5) | 110 (80–190) | 120 (80–200) | 120 (80–200) | 0.25 | |
| Contrast volume, ml per kg | 1.6 (1.1–2.8) | 1.6 (1.1–2.4) | 1.6 (1.0–2.4) | 1.6 (1.1–2.4) | 0.78 | |
| Previous stroke n, (%) | 3 (5.9) | 21 (3.6) | 27 (2.9) | 51 (3.2) | 0.31 | |
| Previous MI n, (%) | 17 (33.3) | 147 (25.0) | 209 (22.2) | 373 (23.6) | 0.12 | |
| Previous PCI n, (%) | 12 (23.5) | 135 (23.0) | 174 (18.5) | 321 (20.3) | 0.09 | |
| Previous CABG n, (%) | 7 (13.7) | 39 (6.6) | 47 (5) | 93 (5.9) | 0.03 | |
| Smoking status n, (%) | 5 (9.8) | 72 (12.2) | 211 (22.4) | 288 (18.2) | <0.001 | |
| Arterial hypertension n, (%) | 40 (78.4) | 433 (73.6) | 637 (67.8) | 1110 (70.3) | 0.03 | |
| COPD n, (%) | 0 (0.0) | 1 (0.2) | 0 (0) | 1 (0.1) | 0.46 | |
| Vascular access n, (%) | Radial | 21 (43.8) | 384 (67.4) | 674 (74.2%) | 1079 (70.7) | <0.001 |
| other | 27 (56.2%) | 186 (32.6%) | 234 (25.8%) | 447 (29.3%) | ||
| Urea, mg/dl | 81 (64–108.5) | 46 (37–57) | 35 (29–41) | 38 (31–49) | <0.001 | |
| Glucose level on admission, md/dL | 139.0 (96.5–211.5) | 116.0 (94–158.8) | 110.0 (95–142) | 112.0 (95–150) | 0.007 | |
| Hemoglobin, G/dL | 11.4 (10.2–12.7) | 13.3 (12.2–14.4) | 14.2 (13.1–15.1) | 13.9 (12.6–14.8) | <0.001 | |
| Hematocrit (%) | 33.7 (31.5–37.8) | 39.4 (36.3–42.9) | 41.8 (38.8–44) | 40.8 (37.4–43.7) | <0.001 | |
| Red blood count, T/L | 3.8 (3.4–4.2) | 4.4 (4.1–4.8) | 4.7 (4.3–5) | 4.6 (4.2–4.9) | <0.001 | |
| White blood cells, G/L | 7.8 (6.2–10.7) | 8.3 (6.8–10.4) | 8.2 (6.8–10.1) | 8.2 (6.8–10.3) | 0.59 | |
| Platelets, G/L | 222 (183.5–258) | 215 (179–257) | 225.0 (188–268.5) | 222 (185–264) | 0.009 | |
| Troponin T hs, ng/L | 111.7 (52.7–469.3) | 31.1 (11.7–172.7) | 31.6 (9.5–178.3) | 34 (10.6–178.3) | <0.001 | |
| Total cholesterol, md/dL | 165.5 (127.5–196) | 171.0 (139–207) | 178.0 (149–215) | 175 (145–211) | 0.003 | |
| HDL cholesterol, md/dL | 40.0 (31.8–46) | 43 (36–52) | 43 (36–51) | 43 (36–51) | 0.05 | |
| Non-HDL cholesterol, md/dL | 130.5 (94.5–159.2) | 126 (96–162) | 135.0 (107.5–168) | 130 (102–165) | 0.003 | |
| LDL cholesterol, md/dL | 94 (71–118.5) | 101.5 (73–135) | 109.0 (83–141) | 106 (77–138) | 0.001 | |
| Triglicerydes, md/dL | 136 (101–185) | 120 (91–162) | 118.0 (89–159) | 120 (90–161) | 0.31 | |
| ALT, U/L | 21 (15–35.5) | 24.0 (17–34) | 26.0 (18–37) | 25 (18–36) | 0.02 | |
| AST, U/L | 28.5 (21.5–51.8) | 28.0 (22–41) | 29.0 (22–42) | 28 (22–42) | 0.95 | |
| Sodium, mmol/L | 139 (136.5–142) | 139 (138, 141) | 140.0 (138–141) | 140 (138–141) | 0.43 | |
| Potassium, mmol/L | 4.7 (4.3–5.4) | 4.3 (4.0–4.6) | 4.3 (4–4.6) | 4.3 (4.0–4.6) | <0.001 | |
| C-reactive protein, mg/L | 7.7 (2.4–22.4) | 5.1 (1.9–14.1) | 4.8 (1.7–19.4) | 5.0 (1.8–18.3) | 0.62 | |
| Baseline eGFR, ml/min/1.73 m2 | 24.1 (15.6–28.2) | 51.1 (44.8–56) | 71.9 (66–79.8) | 63.9 (52.7–73.8) | <0.001 | |
| Baseline creatinine [mg/dL] | 2.2 (1.8–3.6) | 1.2 (1.1–1.4) | 1.0 (0.9–1.1) | 1.0 (0.9–1.2) | <0.001 | |
| CIN n, (%) | 11 (21.6%) | 41 (7.0%) | 55 (5.9%) | 107 (6.8%) | <0.001 | |
| Death or hospitalization in 30 days n, (%) | 8 (15.7%) | 33 (5.6%) | 32 (3.4%) | 73 (4.6%) | 0.001 | |
| Death in 30 days n, (%) | 6 (11.8%) | 23 (3.9%) | 17 (1.8%) | 46 (2.9%) | <0.001 | |
| Death or hospitalization in 365 days n, (%) | 24 (47.1%) | 151 (25.7%) | 135 (14.4%) | 310 (19.6%) | <0.001 | |
| Death in 365 days n, (%) | 14 (27.5%) | 71 (12.1%) | 48 (5.1%) | 133 (8.4%) | <0.001 | |
| Hospitalization due to acute or chronic kidney disease in 30 days, n, (%) | 2 (3.9%) | 0 (0.0%) | 1 (0.1%) | 3 (0.2%) | 0.003 | |
| Hospitalization due to acute or chronic kidney disease in 365 days, n, (%) | 7 (13.7%) | 6 (1.0%) | 5 (0.5%) | 18 (1.1%) | <0.001 | |
| Variable | Univariable | Multivariable (FORWARD) | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Diagnosis | NSTEMI | Ref. level | NA | ||
| STEMI | 1.36 (0.62–2.99) | 0.44 | NA | ||
| UA | 1.34 (0.72–2.51) | 0.35 | NA | ||
| Age, per year | 1.03 (1.01–1.05) | 0.007 | NA | ||
| Gender [male] | 0.86 (0.58–1.29) | 0.48 | NA | ||
| Weight [kg] | 0.99 (0.98–1) | 0.18 | NA | ||
| Diabetes mellitus [yes] | 1.47 (0.92–2.35) | 0.11 | NA | ||
| Contrast volume per 10 mL increase | 1.75 (0.34–8.98) | 0.50 | NA | ||
| Previous stroke [yes] | 1.88 (0.78–4.52) | 0.16 | NA | ||
| Previous MI [yes] | 1.1 (0.7–1.73) | 0.69 | NA | ||
| Previous PCI [yes] | 0.89 (0.54–1.48) | 0.66 | NA | ||
| Previous CABG [yes] | 0.95 (0.4–2.22) | 0.90 | NA | ||
| Smoking status [yes] | 0.9 (0.53–1.52) | 0.69 | NA | ||
| Arterial hypertension [yes] | 0.9 (0.59–1.37) | 0.63 | NA | ||
| COPD [yes] | NA | NA | |||
| Vascular access | Radial | Ref. level | Ref. level | ||
| other | 2.06 (1.37–3.08) | <0.001 | 2.06 (1.37–3.08) | <0.001 | |
| Urea [mg/dl] | 1.02 (1.01–1.02) | <0.001 | NA | ||
| Glucose level on admission, per 10 units increase [md/dL] | 1.03 (1–1.05) | 0.02 | NA | ||
| Hemoglobin (g/dL) | 0.78 (0.7–0.87) | <0.001 | NA | ||
| Hematocrit (%) | 0.92 (0.88–0.95) | <0.001 | NA | ||
| Red blood count (T/L) | 0.47 (0.35–0.64) | <0.001 | NA | ||
| White blood cells (G/L) | 1.01 (0.99–1.03) | 0.3 | NA | ||
| Platelets, per 25 units increase (G/L) | 0.98 (0.91–1.05) | 0.58 | NA | ||
| Troponin T hs per 100 units increase (ng/L) | 1.02 (1–1.04) | 0.02 | NA | ||
| Total cholesterol per 10 units increase [md/dL] | 1 (0.96–1.04) | 0.87 | NA | ||
| HDL cholesterol [md/dL] | 0.99 (0.97–1.01) | 0.27 | NA | ||
| Non-HDL cholesterol per 10 units increase [md/dL] | 1 (0.96–1.04) | 0.96 | NA | ||
| LDL cholesterol per 10 units increase [md/dL] | 1 (0.95–1.05) | 0.96 | NA | ||
| Triglicerydes per 10 units increase [md/dL] | 1.01 (0.99–1.03) | 0.25 | NA | ||
| ALT per 5 units increase [U/L] | 1.01 (1–1.02) | 0.03 | NA | ||
| AST per 5 units increase [U/L] | 1.01 (1–1.02) | 0.009 | NA | ||
| Sodium [mmol/L] | 0.94 (0.89–1) | 0.06 | NA | ||
| Potassium [mmol/L] | 1.5 (0.99–2.25) | 0.05 | NA | ||
| C-reactive protein [mg/L] | 1.01 (1–1.01) | <0.001 | NA | ||
| Baseline eGFR [mL/min/1.73 m2] | 1 (0.99–1.01) | 0.95 | NA | ||
| Baseline eGFR per 5 units [mL/min/1.73 m2] | 1 (0.94–1.06) | 0.95 | NA | ||
| Baseline eGFR [mL/min/1.73 m2] | ≥60 | Ref. level | NA | ||
| 30–60 | 1.21 (0.79–1.83) | 0.38 | NA | ||
| <30 | 4.42 (2.15–9.1) | <0.001 | NA | ||
| Baseline creatinine [mg/dL] | 1.64 (1.29–2.1) | <0.001 | NA | ||
| Variables | Univariable | Multivariable (FORWARD) | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Diagnosis | NSTEMI | Ref. level | NA | ||
| STEMI | 0.61 (0.24–1.58) | 0.31 | NA | ||
| UA | 0.43 (0.22–0.87) | 0.02 | NA | ||
| Age, per year | 1.03 (1.01–1.05 | <0.001 | NA | ||
| Gender [male] | 0.83 (0.46–1.51) | 0.54 | NA | ||
| Weight [kg] | 0.98 (0.96–1) | 0.04 | NA | ||
| Diabetes mellitus [yes] | 0.7 (0.29–1.66) | 0.41 | NA | ||
| Contrast volume per 10 mL increase | 1.07 (0.08–14.06) | 0.96 | NA | ||
| Previous stroke [yes] | 4.96 (2–12.3) | <0.001 | 4.94 (1.58–15.51) | 0.006 | |
| Previous MI [yes] | 1.28 (0.67–2.47) | 0.45 | NA | ||
| Previous PCI [yes] | 0.58 (0.24–1.38) | 0.22 | NA | ||
| Previous CABG [yes] | 0.72 (0.17–3.02) | 0.65 | NA | ||
| Smoking status [yes] | 0.94 (0.43–2.04) | 0.88 | NA | ||
| Arterial hypertension [yes] | 1.2 (0.62–2.34) | 0.59 | NA | ||
| COPD [yes] | NA | NA | |||
| Vascular access | Radial | Ref. level | NA | ||
| other | 2.36 (1.27–4.4) | 0.007 | NA | ||
| Urea [mg/dL] | 1.03 (1.01–1.04) | <0.001 | NA | ||
| Glucose level on admission, per 10 units increase [md/dL] | 1.06 (1.04–1.09) | <0.001 | 1.07 (1.04–1.1) | <0.001 | |
| Hemoglobin [g/dl] | 0.76 (0.65–0.88) | <0.001 | 0.77 (0.65–0.92) | 0.003 | |
| Hematocrit (%) | 0.91 (0.86–0.96) | <0.001 | NA | ||
| Red blood count (T/L) | 0.5 (0.32–0.78) | 0.003 | NA | ||
| White blood cells (G/L) | 1.02 (1–1.03) | 0.06 | NA | ||
| Platelets, per 25 units increase (G/L) | 0.91 (0.81–1.02) | 0.12 | NA | ||
| Troponin T hs per 100 units increase (ng/L) | 1.04 (1.02–1.05) | <0.001 | NA | ||
| Total cholesterol per 10 units increase [md/dL] | 0.9 (0.83–0.96) | 0.002 | NA | ||
| HDL cholesterol [md/dL] | 0.97 (0.94–1) | 0.07 | NA | ||
| Non-HDL cholesterol per 10 units increase [md/dL] | 0.91 (0.85–0.98) | 0.02 | NA | ||
| LDL cholesterol per 10 units increase [md/dL] | 0.91 (0.84–0.99) | 0.02 | NA | ||
| Triglicerydes per 10 units increase [md/dL] | 0.99 (0.96–1.04) | 0.80 | NA | ||
| ALT per 5 units increase [U/L] | 1.02 (1.01–1.04) | 0.004 | NA | ||
| AST per 5 units increase [U/L] | 1.03 (1.01–1.04) | <0.001 | NA | ||
| Sodium [mmol/L] | 0.84 (0.78–0.9) | <0.001 | NA | ||
| Potassium [mmol/L] | 1.53 (0.84–2.8) | 0.17 | NA | ||
| C-reactive protein [mg/L] | 1.01 (1–1.02) | <0.001 | NA | ||
| Baseline eGFR [mL/min/1.73 m2] | 0.97 (0.95–0.98) | <0.001 | NA | ||
| Baseline eGFR per 5 units [mL/min/1.73 m2] | 0.85 (0.78–0.92) | <0.001 | NA | ||
| Baseline eGFR [mL/min/1.73 m2] | ≥60 | Ref. level | NA | ||
| 30–60 | 2.21 (1.17–4.17) | 0.01 | NA | ||
| <30 | 7.24 (2.72–19.24) | <0.001 | NA | ||
| Baseline creatinine [mg/dL] | 1.64 (1.29–2.1) | <0.001 | NA | ||
| CIN [yes] | 6.77 (3.49–13.13) | <0.001 | 5.64 (2.49–12.79) | <0.001 | |
| Variables | Univariable | Multivariable (FORWARD) | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Diagnosis | NSTEMI | Ref. level | Ref. level | ||
| STEMI | 0.56 (0.29–1.09) | 0.09 | NA | ||
| UA | 0.75 (0.4–1.01) | 0.05 | NA | ||
| Age [years] | 1.07 (1.05–1.09) | <0.001 | 1.05 (1.02–1.07) | <0.001 | |
| Gender [male] | 1.01 (0.7–1.45) | 0.97 | NA | ||
| Weight [kg] | 0.97 (0.96–0.99) | <0.001 | NA | ||
| Diabetes mellitus [yes] | 0.98 (0.61–1.56) | 0.92 | NA | ||
| Contrast volume per 10 mL increase | 1.88 (0.43–8.26) | 0.40 | |||
| Previous stroke [yes] | 2.43 (1.15–5.1) | 0.02 | 2.45 (1.02–5.89) | 0.046 | |
| Previous MI [yes] | 1.03 (0.68–1.56) | 0.90 | NA | ||
| Previous PCI [yes] | 0.59 (0.35–0.99) | 0.04 | NA | ||
| Previous CABG [yes] | 0.47 (0.17–1.31) | 0.15 | NA | ||
| Smoking status [yes] | 0.79 (0.56–1.1) | 0.45 | NA | ||
| Hypertension [yes] | 1.06 (0.81–1.4) | 0.62 | NA | ||
| COPD [yes] | NA | NA | |||
| Vascular access | Radial | Ref. level | NA | ||
| other | 1.51(1.03–2.21) | 0.03 | NA | ||
| Urea [mg/dL] | 1.03 (1.02–1.04) | <0.001 | NA | ||
| Glucose level on admission, per 10 units increase [md/dL] | 1.05 (1.03–1.07) | <0.001 | 1.05 (1.03–1.08) | <0.001 | |
| Hemoglobin [g/dL] | 0.72 (0.65–0.79) | <0.001 | 0.78 (0.7–0.88) | <0.001 | |
| Hematocrit (%) | 0.9 (0.87–0.93) | <0.001 | NA | ||
| Red blood count (T/L) | 0.41 (0.31–0.55) | <0.001 | NA | ||
| White blood cells (G/L) | 1.02 (1–1.04) | 0.09 | NA | ||
| Platelets, per 25 units increase (G/L) | 0.96 (0.9–1.03) | 0.28 | NA | ||
| Troponin T hs per 100 units increase (ng/L) | 1.03 (1.01–1.04) | <0.001 | NA | ||
| Total cholesterol per 10 units increase [md/dL] | 0.93 (0.89–0.96) | <0.001 | NA | ||
| HDL cholesterol [md/dL] | 0.98 (0.97–1.0) | 0.03 | NA | ||
| Non-HDL cholesterol per 10 units increase [md/dL] | 0.93 (0.9–0.98) | 0.002 | NA | ||
| LDL cholesterol per 10 units increase [md/dL] | 0.94 (0.9–0.98) | 0.006 | NA | ||
| Triglicerydes per 10 units increase [md/dL] | 0.96 (0.93–0.99) | 0.02 | NA | ||
| ALT per 5 units increase [U/L] | 1.01 (1–1.02) | 0.008 | NA | ||
| AST per 5 units increase [U/L] | 1.01 (1–1.02) | 0.001 | NA | ||
| Sodium [mmol/L] | 0.89 (0.85–0.94) | <0.001 | NA | ||
| Potassium [mmol/L] | 1.46 (1–2.12) | 0.046 | NA | ||
| C-reactive protein [mg/L] | 1.01 (1.01–1.01) | <0.001 | NA | ||
| Baseline eGFR [mL/min/1.73 m2] | 0.97 (0.96–0.98) | <0.001 | NA | ||
| Baseline eGFR [mL/min/1.73 m2] | ≥60 | Ref. level | NA | ||
| 30–59 | 2.55 (1.74–3.74) | <0.001 | NA | ||
| <30 | 7.03 (3.56–13.88) | <0.001 | NA | ||
| Baseline creatinine [mg/dL] | 1.77 (1.38–2.27) | <0.001 | NA | ||
| CIN [yes] | 3.85 (2.36–6.28) | <0.001 | 2.62 (1.42–4.84) | 0.002 | |
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Variables | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Diagnosis | NSTEMI | Ref. level | NA | ||
| STEMI | 0.53 (0.1–2.93) | 0.47 | NA | ||
| UA | 0.59 (0.19–1.84) | 0.36 | NA | ||
| Age, per year | 1 (0.96–1.04) | 0.90 | NA | ||
| Gender [male] | 2.07 (0.68–6.32) | 0.20 | NA | ||
| Weight [kg] | 0.97 (0.94–1) | 0.06 | NA | ||
| Diabetes mellitus [yes] | 1.34 (0.44–4.11) | 0.61 | NA | ||
| Contrast volume per 10 mL/kg increase | 7.18 (0.27–191.82) | 0.24 | NA | ||
| Previous stroke [yes] | NA | NA | |||
| Previous MI [yes] | 1.25 (0.44–3.52) | 0.68 | NA | ||
| Previous PCI [yes] | 0.78 (0.22–2.72) | 0.70 | NA | ||
| Previous CABG [yes] | NA | NA | |||
| Smoking status [yes] | 1.28 (0.42–3.93) | 0.66 | NA | ||
| Arterial hypertension [yes] | 0.66 (0.25–1.71) | 0.39 | NA | ||
| COPD [yes] | NA | NA | |||
| Vascular access | Radial | Ref. level | NA | ||
| other | 1.7 (0.64–4.5) | 0.28 | NA | ||
| Urea [mg/dL] | 1.04 (1.02–1.05) | <0.001 | NA | ||
| Glucose, per 10 units increase [md/dL] | 1 (0.93–1.07) | 0.97 | NA | ||
| Hemoglobin [g/dL] (G/dL) | 0.64 (0.52–0.79) | <0.001 | NA | ||
| Hematocrit (%) | 0.86 (0.8–0.93) | <0.001 | NA | ||
| Red blood count (T/L) | 0.26 (0.14–0.49) | <0.001 | NA | ||
| White blood cells (G/L) | 0.83 (0.67–1.02) | 0.08 | NA | ||
| Platelets, per 25 units increase (G/L) | 1.03 (0.89–1.2) | 0.68 | NA | ||
| Troponin T hs per 100 units increase (ng/L) | 1 (0.94–1.06) | 0.90 | NA | ||
| Total cholesterol per 10 units increase [md/dL] | 0.94 (0.84–1.04) | 0.23 | NA | ||
| HDL cholesterol [md/dL] | 0.95 (0.91–1) | 0.06 | NA | ||
| Non-HDL cholesterol per 10 units increase [md/dL] | 0.95 (0.85–1.06) | 0.40 | NA | ||
| LDL cholesterol per 10 units increase [md/dL] | 0.93 (0.82–1.05) | 0.24 | NA | ||
| Triglicerydes per 10 units increase [md/dL] | 1.02 (0.97–1.06) | 0.46 | NA | ||
| ALT per 5 units increase [U/L] | 0.82 (0.65–1.02) | 0.07 | NA | ||
| AST per 5 units increase [U/L] | 0.95 (0.85–1.06) | 0.39 | NA | ||
| Sodium [mmol/L] | 0.95 (0.82–1.09) | 0.46 | NA | ||
| Potassium [mmol/L] | 4.63 (2.12–10.13) | <0.001 | NA | ||
| C-reactive protein [mg/L] | 0.99 (0.97–1.02) | 0.58 | NA | ||
| Baseline eGFR [mL/min/1.73 m2] | 0.92 (0.9–0.95) | <0.001 | NA | ||
| Baseline eGFR per 5 units [mL/min/1.73 m2] | 0.67 (0.59–0.77) | <0.001 | NA | ||
| Baseline eGFR [mL/min/1.73 m2] | ≥60 | Ref. level | NA | ||
| 30–60 | 1.93 (0.59–6.35) | 0.28 | NA | ||
| <30 | 29.75 (9.08–97.48) | <0.001 | NA | ||
| Baseline creatinine [mg/dL] | 3.44 (2.4–4.93) | <0.001 | 3.44 (2.4–4.93) | <0.001 | |
| CIN [yes] | 4.04 (1.31–12.51) | 0.02 | NA | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sielski, J.; Kaziród-Wolski, K.; Piotrowska, A.M.; Jurczak, B.; Klasa, A.; Kozieł, K.; Ludew, M.; Maj, F.; Merchel, L.; Pytlak, K.; et al. Contrast-Induced Nephropathy (CIN) After Invasive Treatment of Acute Coronary Syndromes—Predictors, Short and Long-Term Outcome. J. Clin. Med. 2025, 14, 3725. https://doi.org/10.3390/jcm14113725
Sielski J, Kaziród-Wolski K, Piotrowska AM, Jurczak B, Klasa A, Kozieł K, Ludew M, Maj F, Merchel L, Pytlak K, et al. Contrast-Induced Nephropathy (CIN) After Invasive Treatment of Acute Coronary Syndromes—Predictors, Short and Long-Term Outcome. Journal of Clinical Medicine. 2025; 14(11):3725. https://doi.org/10.3390/jcm14113725
Chicago/Turabian StyleSielski, Janusz, Karol Kaziród-Wolski, Aleksandra M. Piotrowska, Bartłomiej Jurczak, Anna Klasa, Kacper Kozieł, Maciej Ludew, Filip Maj, Lena Merchel, Kamil Pytlak, and et al. 2025. "Contrast-Induced Nephropathy (CIN) After Invasive Treatment of Acute Coronary Syndromes—Predictors, Short and Long-Term Outcome" Journal of Clinical Medicine 14, no. 11: 3725. https://doi.org/10.3390/jcm14113725
APA StyleSielski, J., Kaziród-Wolski, K., Piotrowska, A. M., Jurczak, B., Klasa, A., Kozieł, K., Ludew, M., Maj, F., Merchel, L., Pytlak, K., Zabojszcz, M., & Siudak, Z. (2025). Contrast-Induced Nephropathy (CIN) After Invasive Treatment of Acute Coronary Syndromes—Predictors, Short and Long-Term Outcome. Journal of Clinical Medicine, 14(11), 3725. https://doi.org/10.3390/jcm14113725







