Abstract
Mitochondria play a central role in energy metabolism and continuously adapt through dynamic processes such as fusion and fission. When the balance between these processes is disrupted, it can lead to mitochondrial dysfunction and increased oxidative stress, contributing to the development and progression of various cardiometabolic diseases (CMDs). Their role is crucial in diabetes mellitus (DM), since their dysfunction drives β-cell apoptosis, immune activation, and chronic inflammation through excessive ROS production, worsening endogenous insulin secretion. Moreover, sympathetic nervous system activation and altered dynamics, contribute to hypertension through oxidative stress, impaired mitophagy, endothelial dysfunction, and cardiomyocyte hypertrophy. Furthermore, the role of mitochondria is catalytic in endothelial dysfunction through excessive reactive oxygen species (ROS) production, disrupting the vascular tone, permeability, and apoptosis, while impairing antioxidant defense and promoting inflammatory processes. Mitochondrial oxidative stress, resulting from an imbalance between ROS/Reactive nitrogen species (RNS) imbalance, promotes atherosclerotic alterations and oxidative modification of oxidizing low-density lipoprotein (LDL). Mitochondrial DNA (mtDNA), situated in close proximity to the inner mitochondrial membrane where ROS are generated, is particularly susceptible to oxidative damage. ROS activate redox-sensitive inflammatory signaling pathways, notably the nuclear factor kappa B (NF-κB) pathway, leading to the transcriptional upregulation of proinflammatory cytokines, chemokines, and adhesion molecules. This proinflammatory milieu promotes endothelial activation and monocyte recruitment, thereby perpetuating local inflammation and enhancing atherogenesis. Additionally, mitochondrial disruptions in heart failure promote further ischemic injury and excessive oxidative stress release and impair ATP production and Ca2⁺ dysregulation, contributing to cell death, fibrosis, and decreased cardiac performance. This narrative review aims to investigate the intricate relationship between mitochondrial dysfunction and CMDs.
1. Introduction
Mitochondria, commonly known as “the powerhouse” of the cell, are key organelles in eukaryotic cells, vitally responsible for energy transformation, generating large amounts of ATP for cellular metabolic processes, such as the tricarboxylic acid cycle (TCA) and oxidative phosphorylation (OXPHOS) [1]. Beyond their primary role in energy metabolism, mitochondria are highly involved in the pathogenesis of cardiometabolic diseases (CMD), including diabetes mellitus (DM), hypertension, dyslipidemia, and coronary heart disease, whose prevalence gradually increases, with the absence of sufficient physical activity, excessive drinking, and smoking [2].
Mitochondria dynamically reshape, having high plasticity, through fusion and fission cycles to meet the cell’s energy needs, by changing their shape, distribution, and size [3]. The dynamic balance of mitochondrial fission and fusion is vital for ensuring mitochondrial function, particularly when cells encounter metabolic or environmental stress, since fusion helps maintain mitochondrial function by mixing mitochondrial contents, optimizing ATP production, and fission is essential for removing damaged mitochondria and facilitating mitophagy [4]. An imbalance between mitochondrial fusion and fission causes mitochondrial fragmentation, dysfunctional oxidative phosphorylation, and increased ROS generation—characteristic markers of CMDs [5]. Mitochondrial dynamics show an imbalance in DM, atherosclerosis, and hypertension, with impaired fusion/fission balance, defective mitophagy, excessive mitochondrial fragmentation and oxidative stress presented in patients with CMDs [5]. Proteins involved in mitochondrial dynamics such as DRP1 (dynamin-related protein 1), MFN1/2 (mitofusin 1/2), and OPA1 (optic atrophy protein 1) regulate the balance between mitochondrial fission and fusion. DRP1-mediated fission is critical for the removal of damaged mitochondria via mitophagy, but excessive activity contributes to mitochondrial fragmentation and metabolic inflexibility. Reduced MFN2 expression, as observed in hypertensive and diabetic models, impairs mitochondrial connectivity and energy efficiency. OPA1, which mediates inner mitochondrial membrane fusion, is essential for maintaining cristae integrity and respiratory capacity [6,7]. Dysregulation of these dynamics leads to excessive reactive oxygen species (ROS) production, impaired mitochondrial membrane potential, and defective ATP synthesis, promoting disease development and progression [8,9,10].
Mitochondrial dysfunction in cardiometabolic diseases can arise from two major sources: primary mitochondrial defects and secondary mitochondrial dysfunction. Primary defects are often genetic in origin, involving mutations in mitochondrial or nuclear DNA that encode mitochondrial proteins [11]. These hereditary conditions can lead to systemic mitochondrial disorders that may predispose individuals to cardiometabolic complications. In contrast, secondary dysfunction arises due to external metabolic stressors such as chronic overnutrition, sedentary behavior, or oxidative stress, which compromise mitochondrial bioenergetics and signaling [12]. Understanding these distinctions is essential to dissecting the mechanistic complexity and to developing targeted therapeutic strategies.
With the rising prevalence of cardiometabolic diseases projected to increase significantly in the coming years, this narrative review aims to explore the intricate relationship between mitochondrial dysfunction and CMDs. Research for this review was conducted through a comprehensive search of the PubMed and Scopus databases, focusing on the most recent and cutting-edge evidence from observational studies, randomized controlled trials, and clinical investigations; these emerging data shed light on future therapeutic approaches.
2. Mitochondrial Dysfunction and Diabetes Mellitus
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disorder marked by T-cell-mediated destruction of pancreatic β-cells, leading to impaired insulin secretion in the pancreatic islets of Langerhans [13,14]. As the disease progresses, the number of damaged pancreatic β-cells exceeds 75%, resulting in β-cell dysfunction, triggering hyperglycemia and the subsequent need for insulin administration [13]. The biosynthesis and exocytosis of insulin are dependent on mitochondrial adenosine-5′ triphosphate (ATP), energy generated by mitochondria [15,16]. Mitochondrial activity, particularly ROS generation, contributes to β-cell apoptosis during the autoimmune-mediated T1DM development [17]. Mice fed a high-fat high-sucrose diet showed increased ROS production, significant mitochondrial dysfunction in skeletal muscle, and higher insulin resistance [18]. In particular, the production of mitochondrial ROS (mtROS) is highly associated with endoplasmic reticulum (ER) stress-mediated β-cell death, with NF-κB signaling acting as a major pathway driving this interaction [19]. In vitro and in vivo studies have shown that myocytes that contain mitochondria-enriched extracellular vesicles exposed to pathogenetic conditions negatively demonstrated impaired insulin signaling and insulin-stimulated glucose uptake, developing further glucose intolerance [20]. Furthermore, the excessive generation of mtROS promotes macrophage polarization from the M2 anti-inflammatory phenotype to the M1 proinflammatory phenotype, leading to the secretion of cytokines including IL-1β, IL-12, and TNF-α, contributing to the progression of autoimmunity and persistent inflammation in pancreatic β-cells [21,22]. Additionally, CD4+ and CD8+ T-cells play a pivotal role in β-cell destruction, since mtROS affects antigen presentation by dendritic cells, by activating mitochondrial apoptosis via perforin–granzyme mechanisms and Fas–Fas ligand interactions [23]. T1DM progression is further enhanced by the excessive release of IL-1β and IL-18 cytokines, which is promoted by the mitochondria-derived damage-associated molecular patterns (DAMPs), such as oxidized mtDNA and activated NLRP3 inflammasome [24]. Enhanced NLRP3 inflammasome activation is associated with aggravated mitochondrial damage, in conditions of Sirtuin 3 (SIRT3) deficiency, which is protective against mitochondrial dysfunction, especially in diabetic mice with cardiomyopathy [25]. Furthermore, the expression of MHC class I and II molecules is upregulated by IFN-γ, enhancing antigen presentation and promoting autoreactive T-cell infiltration into the islets, promoting further β-cell destruction [26]. Mitophagy also plays a crucial role in the regulation of inflammation, since the process of the defective mitochondria is removed, contributing to downregulation of inflammatory signaling pathways and low-grade chronic inflammation, which is pivotal to the progression of T1DM [27]. Mitochondrial dysfunction shifts drive macrophage polarization toward a proinflammatory state, further activating NLRP3 and NF-κB pathways that promote a perpetuating chronic inflammation, increased cell death, and lower endogenous insulin secretion [28].
3. Mitochondrial Dysfunction and Subclinical Cardiovascular Disease
3.1. Mitochondrial Dysfunction and Arterial Hypertension
Alterations in mitochondrial dynamics have been associated with activation of the sympathetic nervous system, which is a crucial pathogenetic mechanism for the regulation of arterial hypertension [29]. Cardiometabolic-induced hypertension is characterized by the excessive activation of specific signaling pathways, including the renin–angiotensin–aldosterone system (RAAS), Ca2+-activated protein phosphatase calcineurin, and the sympathetic nervous system (SNS) [30].
Heart function is dependent on mitochondrial activity, as cardiomyocytes are characterized by a high mitochondrial density, likely due to their need for a continuous and substantial supply of ATP to support their repetitive contractions and the activity of various ion transporters [31]. Hypertension appears to be promoted by cardiomyocyte and cardiac hypertrophy, since increased left ventricular (LV) pressure and volume overload increase afterload, requiring the heart to generate greater force to pump blood [32]. Changes in mitochondrial fusion and fission processes have been linked to various pathological heart conditions [33,34]. In a recent study of cultured neonatal rat cardiomyocytes, epinephrine administration promoted mitochondrial fission, decreased mitochondrial mean volume, and increased the number of mitochondria per cell, showing that increased SNS activation promotes mitochondrial dysfunction [35]. Moreover, a shift towards increased mitochondrial fragmentation was reported in studies in which phenylephrine was administered to induce cardiac hypertrophy [36]. Furthermore, excessive phenylephrine and SNS activation induced a decrease in messenger RNA (mRNA) levels of the mitochondrial fusion protein MFN2 in hypertensive rat models, which demonstrates the pivotal role of mitochondrial dysregulation in arterial hypertension [36]. Moreover, in human umbilical vein endothelial cells, exposure to angiotensin II showed an increased mitochondrial permeability transition pore opening, elevating ROS production, which demonstrates the pivotal role of endothelial mitochondria in hypertension development [37]. Another study, in which mice infused with angiotensin II significantly attenuated the development of hypertension after treatment with the mitochondrial fission inhibitor factors, showed that excessive mitochondrial fission contributes to hypertension [37]. Notably, in hypertension, mitochondrial oxidative damage is induced by the activation of angiotensin (Ang) II, leading to reduced endothelial nitric oxide (NO) levels and increased vascular oxidative stress [38]. Furthermore, Ang II-mediated protein kinase-C activity results in excess production of O2− and H2O2 that primarily leads to increased ROS production [39]. Mitochondrial quality control (MQC) mechanisms also play a catalytic role in the angiogenic response of endothelial cells to vascular endothelial growth factor (VEGF), since the knock-down of Mitofusin 1 (MFN1) is involved in mitochondrial fusion [40]. Another mechanism that regulates hypertension is mitophagy, resulting in reduced inflammation and ROS production [41]. Hypertensive models show impaired ATG-5-mediated mitophagy during Ang II-induced hypertension and impaired mitochondrial dynamics, respectively, such as reduced mitochondrial mass and structure, mitochondrial size, and osmotic swelling, which disrupts the mitochondrial OXPHOS efficiency [42]. As a result, mitochondrial dysfunction is highly involved in the pathogenesis of hypertension through several pathways that seem to affect mitochondrial dynamics, leading to increased oxidative stress, endothelial dysfunction, and cardiomyocyte hypertrophy (Figure 1).
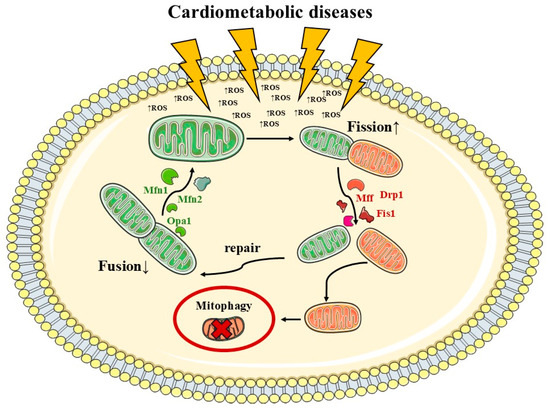
Figure 1.
Cardiometabolic diseases’ impact on mitochondrial dynamics. Mitochondrial dynamics are highly affected by oxidative stress and metabolic impairments, causing an imbalance between fusion and fission. Pathophysiology caused by excessive (↑) fission and reduced (↓) fusion. Mitochondrial fission is regulated by proteins such as dynamin-related protein 1 (Drp1), mitochondrial fission factor (Mff), and mitochondrial fission 1 protein (Fis1), and fusion is orchestrated by the mitofusin-1 (Mfn1), Mfn2, and optic atrophy protein 1 (Opa1).
3.2. Mitochondrial Dysfunction and Endothelial Dysfunction
The endothelium plays a crucial role in the vasculature, since it modulates vascular homeostasis by regulating the vascular tone and the vascular permeability of anti-inflammatory, antioxidant, anti-proliferative and anti-thrombotic factors [43]. Endothelial dysfunction and cardiometabolic diseases are interconnected in a bidirectional manner.
Mitochondria are essential for ATP production, cellular metabolic control, and regulation of apoptosis [44]. Their role in maintaining a balance between the production of NO and the concentration of Ca2+ is pivotal, since it facilitates the release of NO through the activation of eNOS [45]. Moreover, endothelial homeostasis is severely disrupted by the toxic byproducts of aerobic metabolism, ROS, which are mainly produced by mitochondria [46]. Disturbances in Ca2+ balance, resulting in ROS overproduction, have been associated with the opening of the membrane permeability transition pore (mPTP) and disruption in vascular permeability [47]. Moreover, intracellular Ca2+ signaling is essential for controlling the vasomotor activity of vascular smooth muscle cells (VSMCs). Mitochondria are key regulators of intracellular calcium levels through their ability to take up and recycle Ca2⁺ [48]. Evidence from preclinical research suggests that mitochondrial Ca2+ dynamics influence both the function and viability of vascular cells. In endothelial cells, hexokinase influences mitochondrial Ca2⁺ balance by blocking the voltage-dependent anion channel. In coronary endothelial cells, hyperglycemia-induced hexokinase upregulation decreases mitochondrial Ca2⁺ and limits ROS production [49]. These disruptions can cause permanent damage to the endothelial cellular architecture and trigger the process of apoptosis or programmed cell death [50].
Furthermore, the generation of ROS and oxidative stress facilitates leukocyte adhesion molecules. Mitochondrial-induced endothelial dysfunction results in excessive ROS production and NADPH oxidase (NOX) and e-NOS dysregulation, along with a decline in antioxidant defense mechanisms [51]. The overactivation of NOX generates excessive ROS, being a contributing factor to endothelial cell dysfunction [52]. Additionally, mitochondria-derived ROS, which can take place at complex I or complex III, are essential in regulating cell signaling pathways in endothelial cells. More specifically:
- Complex I (NADH: ubiquinone oxidoreductase) dysfunction often leads to impaired electron flow and increased electron leakage to oxygen, generating superoxide radicals. This dysfunction is particularly relevant in insulin-resistant states, where excessive ROS can impair insulin receptor signaling and reduce glucose uptake in peripheral tissues [18,53].
- Complex II (succinate dehydrogenase) contributes less directly to ROS production but plays a key role in linking the TCA cycle to the electron transport chain. Mutations or reduced activity can disrupt both energy production and metabolic flexibility [53,54].
- Complex III (cytochrome bc1 complex) can also leak electrons to oxygen, especially during reverse electron transport, which is a major source of ROS in endothelial cells under hyperglycemic conditions. This contributes to vascular oxidative stress, endothelial dysfunction, and atherosclerosis [55].
- Complex IV (cytochrome c oxidase) deficiency leads to the incomplete reduction of oxygen, impaired ATP synthesis, and further ROS accumulation, which has been linked to impaired myocardial energetics and contractility in heart failure [56].
- Complex V (ATP synthase) dysfunction primarily affects ATP production, but emerging evidence suggests it also modulates mitochondrial membrane potential and may influence ROS indirectly [57].
The electron transport chain serves as the primary generator of mitochondrial reactive oxygen species (mtROS) [58]. Excessive mtROS release has been proven to contribute both to acute and chronic impairment of endothelial function by altering the signaling of key proteins involved in vascular regulation [59,60]. Notably, endothelial cells are exposed not only to endogenous oxidative species but also to numerous exogenous sources of reactive species, which exacerbate ROS-induced endothelial dysfunction [54]. During atherosclerosis, activated neutrophils release large quantities of ROS in areas where the endothelium is damaged [54]. Animal studies have demonstrated that, in aged mice, treatment with mitochondrial-targeted antioxidants improved vascular endothelial function [61].
Mitochondria also have a catalytic role in the regulation of endogenous mitochondrial antioxidant defense. One mechanism of maintaining redox homeostasis and excessive mitochondria-derived ROS is superoxide dismutase (SOD) isozymes, which catalyze the conversion of O2− into H2O2 and increase the NO bioavailability [62]. In particular, SOD2 is a key endogenous mitochondrial antioxidant mechanism when considering vascular function and enhanced NO bioavailability, due to its vasodilatory properties [63]. Sirtuin-3 (SIRT-3), a mitochondrial localized deacetylase, seems to modulate SOD2 activity and the antioxidant defense system, respectively [64].
3.3. Mitochondrial Dysfunction and Atherosclerosis
The mitochondrial respiratory chain efficiently produces energy, with over 98% of electrons used for energy and only 1–2% generating ROS. Oxidative stress occurs when the organism cannot neutralize the overproduction of ROS and reactive nitrogen species (RNS) [65]. Metabolic disease-associated mitochondrial oxidative stress results from an imbalance between elevated ROS/RNS generation and inadequate antioxidant protection [53]. Elevated oxidative stress, resulting from impaired antioxidant mechanisms, can facilitate the progression of atherosclerotic disease.
Moreover, oxidative stress is highly involved in atherogenesis by the oxidation of low-density lipoprotein (LDL) resulting in oxLDL [55,66]. Alterations in the structure of lipoprotein particles can affect lipid, carbohydrate, and protein sections of LDL particles, resulting in modifications of density, size, and other LDL-like particles such as small dense LDL (sdLDL), characterized by reduced antioxidant activity [67]. Such particles remain in the arterial wall for an extended period due to interactions with tissue elements like glycans, leading to a buildup of areas prone to atherosclerosis [67,68]. This prolonged oxidative stress contributes to the development of inflammation in endothelial cells, whose role is crucial for the process of atherosclerosis [66,69].
Furthermore, mitochondrial DNA (mtDNA) is highly affected by ROS/RNS-induced damage, as a result of prolonged low-grade inflammation [70]. The absence of histones and limited repair mechanisms make mtDNA more vulnerable to damage from ROS, contributing to oxidative damage to the respiratory chain and lipid peroxidation [71,72]. Impaired mitochondrial genome function affects mitochondrial physiology and ATP production, contributing to increased ROS levels and enhanced mitochondrial apoptosis [73].
The role of macrophages is also pivotal in the progression of atherosclerotic lesions, since their activation either classically (M1) or activated macrophages (M2) contributes to destabilization of the atherosclerotic fibrous cap region and lipid accumulation in the atheromatic plaques [74]. Mitochondrial oxidative metabolism supports macrophage alternative activation, with both M1 and M2 reducing oxygen consumption and damaging mtDNA [75]. M1 macrophages produce large numbers of proinflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-12, IL-18, and IL-23, which is induced by their polarization and is highly affected by mitochondrial ROS above physiological levels [76,77]. Several mutations in mtDNA have been found to contribute to the proinflammatory response in monocytes, changing the activation of monocyte-derived macrophages through mitochondrial dysfunction in atherosclerosis [78] (Figure 2).

Figure 2.
Overview of the vascular consequences of mitochondrial dysfunction. Mitochondrial impairment contributes to endothelial dysfunction via reduced nitric oxide (NO) bioavailability, endothelial nitric oxide synthase (eNOS) dysregulation, decreased superoxide dismutase 2 (SOD2) activity, and diminished sirtuin-3 (SIRT3) function. It promotes atherosclerosis by enhancing oxidized LDL (oxLDL) accumulation, sustaining chronic low-grade inflammation, and promoting alternative macrophage activation. In the pathogenesis of arterial hypertension, mitochondrial dysfunction leads to increased sympathetic nervous system (SNS) activity, heightened angiotensin II (Ang II) signaling, and increased mitochondrial permeability transition pore (MPTP) opening. In heart failure, it contributes through calcium (Ca2⁺) overload, accumulation of mitochondrial DNA (mtDNA) mutations, and impaired ATP production.
3.4. Mitochondrial Dysfunction and Clinical Cardiovascular Disease
The cardiomyocyte mitochondria are divided into two distinct populations: one adjacent to the sarcolemma and the other enclosed within the contractile apparatus [79]. In cardiac muscle cells, both mitochondrial groups maintain electrical connectivity, allowing the transfer of electrical signals between mitochondria [80]. The mitochondrial structure is dynamically controlled by fusion and fission proteins, responding to changes in cardiac pathology [81]. For instance, giant mitochondria are observed in some cardiomyopathies and in response to dietary, drug, or toxin exposure [82]. Moreover, mitochondrial biochemical alterations are significant contributors to heart failure [83].
Ischemic myocardial injury caused by an abrupt coronary occlusion triggers ischemic injury, leading to tissue hypoxia and ATP depletion [65]. Hypoxia from impaired blood flow lowers oxidative phosphorylation, decreases cellular ATP levels, and leads to mitochondrial membrane depolarization [84]. Moreover, ischemia-induced hypoxia causes a switch to anaerobic glycolysis in the myocardium, which lowers intracellular pH due to lactate buildup, a byproduct of anaerobic respiration [85]. Cardiac ischemia–reperfusion injury reintroduction to oxygen-rich blood initiates an uncontrolled ROS-driven response, worsening necrosis for up to three days post-reperfusion [86]. Mitochondrial damage during ischemia-reperfusion injury causes a mitochondrial Ca2+ overload and unregulated ROS production, which leads to mitochondrial permeability transition pore opening and subsequent cell death [87]. Notably, the heart has low ATP levels due to its high demand and rapid turnover of ATP. Cellular ATP is primarily produced by oxidative phosphorylation (OXPHOS), a process driven by the electron transport chain on the inner mitochondrial membrane [88]. Decreased ATP production and impaired OXPHOS in the ischemic heart lead to a decline in cardiac performance [89].
Moreover, mitochondria are essential not only for ATP generation but also for metabolism, Ca2+ homeostasis, lipid synthesis, and redox regulation in the heart [90]. Changes in metabolism can lead to heterogeneity in heart failure presentations and progression. Heart failure with preserved ejection fraction (HFpEF) involves oxidative stress as a key regulator, triggering inflammation, fibrosis, and altered Ca2+ homeostasis [91]. Mitochondrial impairment and citric acid cycle abnormalities in diastolic HF correlate with protein hyperacetylation, a process reversible with nicotinamide riboside [92]. At the core of mitochondrial metabolism lies the tricarboxylic acid (TCA) cycle. In particular, in insulin-resistant states or diabetic hearts, there is increased reliance on fatty acid oxidation at the expense of glucose oxidation, leading to a reduced efficiency of the TCA cycle and ATP yield per oxygen molecule consumed, thus promoting metabolic stress [93]. Impaired TCA cycle flux leads to accumulation of metabolic intermediates and electron carriers, contributing to oxidative damage, inflammation, and endothelial dysfunction in both the heart and vasculature [94]. In HFpEF, altered TCA cycle enzyme activity has been linked to protein hyperacetylation that impairs the function of key TCA cycle enzymes, further exacerbating metabolic inefficiency [95]. Metabolic inflexibility and mitochondrial stress further promote the secretion of proinflammatory cytokines and profibrotic mediators, contributing to adverse cardiac remodeling. Furthermore, impaired Ca2⁺ regulation is a key characteristic of heart failure. The impact of mitochondrial Ca2+ dynamics in heart failure remains debated, with some evidence suggesting mitochondrial Ca2⁺ overload is damaging, while other studies indicate potential benefits [96,97,98]. The mitochondrial Ca2⁺ uniporter (MCU) facilitates Ca2⁺ uptake, while the Na⁺/Ca2⁺ exchanger (NCLX) mediates efflux, both playing crucial roles in mitochondrial Ca2⁺ homeostasis [99]. Studies suggest that mitochondrial Ca2⁺ overload can be detrimental, contributing to oxidative stress and cardiomyocyte death, but in some models, increased mitochondrial Ca2⁺ enhances energy production, indicating a complex and context-dependent role in heart failure [100,101,102]. Mitochondrial ROS (mtROS) also play a dual role in heart failure, contributing to both physiological and pathological processes. Excessive mtROS in the failing heart lead to mitochondrial damage, opening of the mitochondrial permeability transition pore (mPTP), and cell death, while impairing mitochondrial function and biogenesis [103,104]. Antioxidant systems, such as peroxiredoxin (Prx) and glutathione peroxidase (Gpx), help mitigate ROS damage, but dysfunction in these systems, along with mitochondrial NADPH supply issues, exacerbates oxidative stress, impairs ATP production, and worsens heart failure [57,105,106]. In addition, mtROS contribute to a variety of cellular dysfunctions, including protein and lipid damage, inflammation, and impaired mitochondrial signaling [107]. Finally, mitochondrial DNA (mtDNA) is crucial in heart failure (HF), with studies showing a >40% reduction in mtDNA content and impaired replication in failing hearts, hindering mitochondrial biogenesis [56,108]. MtDNA is uniquely vulnerable to damage, and its repair capacity is significantly limited compared to nuclear DNA, due to the lack of protective histones, which, in the nucleus, serve to protect DNA from oxidative damage and regulate transcription [109]. The mitochondrial inner membrane is a primary site of ROS production. mtDNA lies in close proximity to the electron transport chain, increasing the risk of oxidative lesions [109]. A 30-year study, the Atherosclerosis Risk in Communities, revealed an inverse relationship between mtDNA copy number and heart failure risk [110]. Due to the lack of histones and limited epigenetic control, mtDNA is exclusively impacted by oxidative stress, unlike the nuclear genome [111]. These findings show that several mitochondrial mechanisms have been identified that contribute to heart failure before ATP depletion occurs (Table 1).

Table 1.
Mechanisms by which mitochondrial dysfunction affects cardiometabolic diseases.
4. Other Mitochondrial Factors and Cardiometabolic Disease
4.1. Mitochondrial Open Reading Frame of the 12S rRNA Type-c (MOTS-c)
The mitochondrial open reading frame of the 12S rRNA type-c (MOTS-c) consists of 16 amino acids forming an α-helical structure [123]. In animal models, MOTS-c enhances insulin sensitivity in skeletal muscle while protecting against diet-induced obesity and insulin resistance [124]. Studies have shown that MOTS-c levels were significantly lower in young obese males, an inverse correlation with insulin resistance in lean individuals, and found to be lower in patients with type 1 and type 2 diabetes [125,126]. Furthermore, MOTS-c shows protective effects in CVD, since studies suggest improving cardiac function and pathological ventricular remodeling by activating pathways such as AMPK that mimic the benefits of aerobic exercise, though its precise target in cardiomyocytes remains unclear [127]. In diabetic rats, MOTS-c demonstrated protection against myocardial mitochondrial damage and cardiac function, with beneficial effects on diabetic cardiomyopathy [128]. Moreover, MOTS-c has anti-inflammatory effects by regulating cytokine levels through AMPK activation and increasing anti-inflammatory cytokines while decreasing proinflammatory cytokines [129,130]. MOTS-c is the first peptide encoded that has been included in clinical trials for its protective effects, showing the pivotal role of mitochondria in therapeutics and drug targets [131].
4.2. Damage-Associated Molecular Patterns (DAMPs)
Damage-associated molecular patterns (DAMPs) are internal danger signals that are released from cells undergoing damage or death, which activate the innate immune response by binding to pattern recognition receptors (PRRs) [132]. In DM patients, chronic hyperglycemia through IL-1β secretion from various cell types, including β cells, triggers a strong danger signal that impairs insulin secretion, promotes β-cell death, and contributes to insulin resistance [133]. The regulation of proinflammatory cytokine processing and secretion is primarily controlled by inflammasomes in cardiometabolic diseases, especially from nucleotide-binding domain and leucine-rich repeat protein 3 (NLRP3) inflammasome, which plays a crucial role in T2DM [134]. High glucose levels activate NLRP3 inflammasomes, which exacerbate β-cell dysfunction and insulin resistance in both T1DM and T2DM, enhancing inflammation and impairing insulin signaling [135]. Moreover, DAMPs enhance endothelial dysfunction, since they contribute to the promotion of foam cell formation and lipid accumulation, affecting atherosclerotic plaques and vascular inflammation [136]. Mitochondrial DNA (mtDNA) and other mitochondrial components are recognized as mitochondrial damage-associated molecular patterns (mtDAMPs) that lead to the progression of inflammation and atherosclerotic plaque formation, contributing to atherosclerosis, insulin resistance, and hypertension [133]. Medium-chain acylcarnitines (MCACs) and long-chain acylcarnitines (LCACs) predict major adverse cardiovascular events (MACE) in type 2 diabetes patients (TECOS/EXSCEL cohorts, p < 0.01). Elevated dicarboxylacylcarnitines associate with 32% increased MACE risk after multivariable adjustment (HR 1.32, 95% CI 1.12–1.55) [137]. Finally, several other proteins and mitochondrial elements have been related to mitochondrial dynamics, quality control, and signaling, and they play pivotal roles in the development and progression of cardiometabolic diseases (Table 2).

Table 2.
Proteins and mitochondrial elements involved in mitochondrial dysfunction in cardiometabolic diseases.
5. Potential Treatment Targets
Several therapeutic agents have been developed to target mitochondrial dysfunction. MitoQ, a mitochondria-targeted antioxidant, has shown efficacy in reducing vascular oxidative stress in both animal models and early-phase clinical trials [143]. NAD+ precursors such as nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) improve mitochondrial function by enhancing SIRT1/3 activity, boosting mitochondrial biogenesis and antioxidant defense. However, limitations such as short half-lives, tissue-specific bioavailability, and variability in clinical responses necessitate further investigation through randomized controlled trials.
In addition to MitoQ and NAD+ boosters, other agents like elamipretide (SS-31) have gained attention for stabilizing cardiolipin and improving mitochondrial respiration in failing hearts [144]. Clinical trials have demonstrated that elamipretide can enhance left ventricular function and mitochondrial efficiency in patients with heart failure with reduced ejection fraction (HFrEF) [145]. Moreover, natural compounds such as resveratrol and coenzyme Q10 have shown promise in modulating mitochondrial oxidative stress and inflammation, although the results have been inconsistent in larger clinical studies [146,147].
Gene therapy approaches and mitochondrial transplantation are emerging frontiers. For example, adenoviral-mediated overexpression of PGC-1α and TFAM in preclinical models has improved mitochondrial biogenesis and attenuated cardiac remodeling [148]. While still experimental, mitochondrial transfer techniques have been successfully applied in preclinical settings to rescue ischemic tissues and may offer therapeutic potential in the context of CMDs [149].
6. Limitations of the Review
This review is primarily based on the published literature and does not include a systematic meta-analysis of the data. While effort was made to include high-quality human and preclinical studies, there may be publication bias favoring positive findings. In addition, many mechanistic insights are derived from animal models, which may not fully replicate human cardiometabolic disease pathology. The rapidly evolving nature of mitochondrial therapeutics means that some recent advances may not be comprehensively captured.
7. Conclusions
Mitochondrial dysfunction plays a fundamental role in the development and progression of CMDs by regulating oxidative stress, inflammatory responses, and cellular homeostasis, with disruptions in mitochondrial dynamics also contributing to several complications. Excessive mtROS and RNS production, overproduction of proinflammatory cytokines, reduced endothelial nitric oxide secretion, and mtDNA alterations are crucial for the imbalances in mitochondrial homeostasis, whose future research may pave the way for innovative strategies to combat CMDs and improve long-term cardiometabolic health. Future research should address key knowledge gaps, including the precise role of mitochondrial calcium homeostasis, tissue-specific responses to mitochondrial stress, and the bidirectional crosstalk between mitochondria and nuclear epigenetics in CMDs. Moreover, integrating mitochondrial profiling into precision medicine frameworks could enable tailored therapeutic approaches based on mitochondrial phenotypes. Large-scale longitudinal human studies incorporating mitochondrial biomarkers are needed to validate their utility in risk stratification and treatment monitoring.
Author Contributions
Conceptualization, L.P., S.L. and A.K.; methodology, E.K. (Emmanouil Korakas), J.T., E.K. (Eva Kassi) and E.O.; writing—original draft preparation, L.P. and S.L.; writing—review and editing, L.P., S.L., A.K., E.K. (Emmanouil Korakas), J.T., E.K. (Eva Kassi), E.O., I.I. and V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Heine, K.B.; Hood, W.R. Mitochondrial behaviour, morphol, and animal performance. Biol. Rev. Camb. Philos. Soc. 2020, 95, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef]
- Chandhok, G.; Lazarou, M.; Neumann, B. Structure, function, and regulation of mitofusin-2 in health and disease. Biol. Rev. Camb. Philos. Soc. 2018, 93, 933–949. [Google Scholar] [CrossRef]
- Tokuyama, T.; Yanagi, S. Role of Mitochondrial Dynamics in Heart Diseases. Genes 2023, 14, 1876. [Google Scholar] [CrossRef]
- Shirakabe, A.; Zhai, P.; Ikeda, Y.; Saito, T.; Maejima, Y.; Hsu, C.P.; Nomura, M.; Egashira, K.; Levine, B.; Sadoshima, J. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation 2016, 133, 1249–1263. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.; Torres, G.; Wu, S.; Ouyang, C.; Xie, Z.; Zou, M.H. Metformin Suppresses Diabetes-Accelerated Atherosclerosis via the Inhibition of Drp1-Mediated Mitochondrial Fission. Diabetes 2017, 66, 193–205. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Pliouta, L.; Katogiannis, K.; Maratou, E.; Thymis, J.; Michalopoulou, E.; Prentza, V.; Katsanaki, E.; Vlachomitros, D.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Neurohumoral and Mitochondrial Activation in Patients With Diabetes. J. Am. Heart Assoc. 2025, 14, e039129. [Google Scholar] [CrossRef]
- Niyazov, D.M.; Kahler, S.G.; Frye, R.E. Primary Mitochondrial Disease and Secondary Mitochondrial Dysfunction: Importance of Distinction for Diagnosis and Treatment. Mol. Syndromol. 2016, 7, 122–137. [Google Scholar] [CrossRef]
- Valenti, D.; Vacca, R.A. Primary and Secondary Mitochondrial Diseases: Etiologies and Therapeutic Strategies. J. Clin. Med. 2022, 11, 4209. [Google Scholar] [CrossRef]
- Blagov, A.V.; Summerhill, V.I.; Sukhorukov, V.N.; Popov, M.A.; Grechko, A.V.; Orekhov, A.N. Type 1 diabetes mellitus: Inflammation, mitophagy, and mitochondrial function. Mitochondrion 2023, 72, 11–21. [Google Scholar] [CrossRef]
- Derella, C.C.; Thomas, J.; Harris, R.A. Women Have Greater Endothelin-B Receptor Function and Lower Mitochondrial Capacity Compared to Men With Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2023, 108, 2561–2568. [Google Scholar] [CrossRef]
- Kaufman, B.A.; Li, C.; Soleimanpour, S.A. Mitochondrial regulation of beta-cell function: Maintaining the momentum for insulin release. Mol. Asp. Med. 2015, 42, 91–104. [Google Scholar] [CrossRef]
- Lu, A.; Chu, C.; Mulvihill, E.; Wang, R.; Liang, W. ATP-sensitive K(+) channels and mitochondrial permeability transition pore mediate effects of hydrogen sulfide on cytosolic Ca(2+) homeostasis and insulin secretion in beta-cells. Pflugers Arch. 2019, 471, 1551–1564. [Google Scholar] [CrossRef]
- Padgett, L.E.; Broniowska, K.A.; Hansen, P.A.; Corbett, J.A.; Tse, H.M. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann. N. Y. Acad. Sci. 2013, 1281, 16–35. [Google Scholar] [CrossRef]
- Bonnard, C.; Durand, A.; Peyrol, S.; Chanseaume, E.; Chauvin, M.A.; Morio, B.; Vidal, H.; Rieusset, J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Investig. 2008, 118, 789–800. [Google Scholar] [CrossRef]
- Ge, X.; Tang, P.; Rong, Y.; Jiang, D.; Lu, X.; Ji, C.; Wang, J.; Huang, C.; Duan, A.; Liu, Y.; et al. Exosomal miR-155 from M1-polarized macrophages promotes EndoMT and impairs mitochondrial function via activating NF-kappaB signaling pathway in vascular endothelial cells after traumatic spinal cord injury. Redox Biol. 2021, 41, 101932. [Google Scholar] [CrossRef]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef]
- Amrani, A.; Verdaguer, J.; Thiessen, S.; Bou, S.; Santamaria, P. IL-1alpha, IL-1beta, and IFN-gamma mark beta cells for Fas-dependent destruction by diabetogenic CD4(+) T lymphocytes. J. Clin. Investig. 2000, 105, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Ming, S.; Tian, J.; Ma, K.; Pei, C.; Li, L.; Wang, Z.; Fang, Z.; Liu, M.; Dong, H.; Li, W.; et al. Oxalate-induced apoptosis through ERS-ROS-NF-kappaB signalling pathway in renal tubular epithelial cell. Mol. Med. 2022, 28, 88. [Google Scholar] [CrossRef]
- Savinov, A.Y.; Tcherepanov, A.; Green, E.A.; Flavell, R.A.; Chervonsky, A.V. Contribution of Fas to diabetes development. Proc. Natl. Acad. Sci. USA 2003, 100, 628–632. [Google Scholar] [CrossRef]
- Carlos, D.; Costa, F.R.; Pereira, C.A.; Rocha, F.A.; Yaochite, J.N.; Oliveira, G.G.; Carneiro, F.S.; Tostes, R.C.; Ramos, S.G.; Zamboni, D.S.; et al. Mitochondrial DNA Activates the NLRP3 Inflammasome and Predisposes to Type 1 Diabetes in Murine Model. Front. Immunol. 2017, 8, 164. [Google Scholar] [CrossRef]
- Song, S.; Ding, Y.; Dai, G.L.; Zhang, Y.; Xu, M.T.; Shen, J.R.; Chen, T.T.; Chen, Y.; Meng, G.L. Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation. Acta Pharmacol. Sin. 2021, 42, 230–241. [Google Scholar] [CrossRef]
- Marroqui, L.; Dos Santos, R.S.; Op de Beeck, A.; Coomans de Brachene, A.; Marselli, L.; Marchetti, P.; Eizirik, D.L. Interferon-alpha mediates human beta cell HLA class I overexpression, endoplasmic reticulum stress and apoptosis, three hallmarks of early human type 1 diabetes. Diabetologia 2017, 60, 656–667. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef]
- Pearson, G.; Chai, B.; Vozheiko, T.; Liu, X.; Kandarpa, M.; Piper, R.C.; Soleimanpour, S.A. Clec16a, Nrdp1, and USP8 Form a Ubiquitin-Dependent Tripartite Complex That Regulates beta-Cell Mitophagy. Diabetes 2018, 67, 265–277. [Google Scholar] [CrossRef]
- Weiss, E.; de la Pena-Ramirez, C.; Aguilar, F.; Lozano, J.J.; Sanchez-Garrido, C.; Sierra, P.; Martin, P.I.; Diaz, J.M.; Fenaille, F.; Castelli, F.A.; et al. Sympathetic nervous activation, mitochondrial dysfunction and outcome in acutely decompensated cirrhosis: The metabolomic prognostic models (CLIF-C MET). Gut 2023, 72, 1581–1591. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Nicolas-Avila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martinez, L.; Sanchez-Diaz, M.; Diaz-Garcia, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109.e23. [Google Scholar] [CrossRef]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The Transition From Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail. 2017, 5, 543–551. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, D.D.; Qiu, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’Brien, K.D.; Tian, R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020, 130, 6054–6063. [Google Scholar] [CrossRef]
- Murray, K.O.; Ludwig, K.R.; Darvish, S.; Coppock, M.E.; Seals, D.R.; Rossman, M.J. Chronic mitochondria antioxidant treatment in older adults alters the circulating milieu to improve endothelial cell function and mitochondrial oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H187–H194. [Google Scholar] [CrossRef]
- Pennanen, C.; Parra, V.; Lopez-Crisosto, C.; Morales, P.E.; Del Campo, A.; Gutierrez, T.; Rivera-Mejias, P.; Kuzmicic, J.; Chiong, M.; Zorzano, A.; et al. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. J. Cell Sci. 2014, 127, 2659–2671. [Google Scholar] [CrossRef]
- Fang, L.; Moore, X.L.; Gao, X.M.; Dart, A.M.; Lim, Y.L.; Du, X.J. Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life Sci. 2007, 80, 2154–2160. [Google Scholar] [CrossRef]
- Preston, K.J.; Kawai, T.; Torimoto, K.; Kuroda, R.; Nakayama, Y.; Akiyama, T.; Kimura, Y.; Scalia, R.; Autieri, M.V.; Rizzo, V.; et al. Mitochondrial fission inhibition protects against hypertension induced by angiotensin II. Hypertens. Res. 2024, 47, 1338–1349. [Google Scholar] [CrossRef]
- Yao, Y.; Cao, Y.; Xu, Y.; Chen, G.; Liu, Y.; Jiang, H.; Fan, R.; Qin, W.; Wang, X.; Chai, H.; et al. CARMA3 Deficiency Aggravates Angiotensin II-Induced Abdominal Aortic Aneurysm Development Interacting Between Endoplasmic Reticulum and Mitochondria. Can. J. Cardiol. 2023, 39, 1449–1462. [Google Scholar] [CrossRef]
- Tashkandi, A.J.; Gorman, A.; McGoldrick Mathers, E.; Carney, G.; Yacoub, A.; Setyaningsih, W.A.W.; Kuburas, R.; Margariti, A. Metabolic and Mitochondrial Dysregulations in Diabetic Cardiac Complications. Int. J. Mol. Sci. 2025, 26, 3016. [Google Scholar] [CrossRef]
- Jiang, Y.; Krantz, S.; Qin, X.; Li, S.; Gunasekara, H.; Kim, Y.M.; Zimnicka, A.; Bae, M.; Ma, K.; Toth, P.T.; et al. Caveolin-1 controls mitochondrial damage and ROS production by regulating fission—Fusion dynamics and mitophagy. Redox Biol. 2022, 52, 102304. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2023, 19, 401–414. [Google Scholar] [CrossRef]
- Ritz, P.; Berrut, G. Mitochondrial function, energy expenditure, aging and insulin resistance. Diabetes Metab. 2005, 31, 5S67–5S73. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Cai, G.; Lin, F.; Wu, D.; Lin, C.; Chen, H.; Wei, Y.; Weng, H.; Chen, Z.; Wu, M.; Huang, E.; et al. Rosmarinic Acid Inhibits Mitochondrial Damage by Alleviating Unfolded Protein Response. Front. Pharmacol. 2022, 13, 859978. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, S.; Ji, Z.; Xu, H.; Zhao, W.; Xia, Z.; Xu, R. Mechanistic study of mtROS-JNK-SOD2 signaling in bupivacaine-induced neuron oxidative stress. Aging 2020, 12, 13463–13476. [Google Scholar] [CrossRef]
- Jamar, N.H.; Kritsiligkou, P.; Grant, C.M. The non-stop decay mRNA surveillance pathway is required for oxidative stress tolerance. Nucleic Acids Res. 2017, 45, 6881–6893. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Dubinin, M.V.; Belosludtseva, N.V.; Mironova, G.D. Mitochondrial Ca2+ Transport: Mechanisms, Molecular Structures, and Role in Cells. Biochemistry 2019, 84, 593–607. [Google Scholar] [CrossRef]
- Kannurpatti, S.S. Mitochondrial calcium homeostasis: Implications for neurovascular and neurometabolic coupling. J. Cereb. Blood Flow Metab. 2017, 37, 381–395. [Google Scholar] [CrossRef]
- Pan, M.; Han, Y.; Basu, A.; Dai, A.; Si, R.; Willson, C.; Balistrieri, A.; Scott, B.T.; Makino, A. Overexpression of hexokinase 2 reduces mitochondrial calcium overload in coronary endothelial cells of type 2 diabetic mice. Am. J. Physiol. Cell Physiol. 2018, 314, C732–C740. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Dubinin, M.V. Diabetes Mellitus, Mitochondrial Dysfunction and Ca(2+)-Dependent Permeability Transition Pore. Int. J. Mol. Sci. 2020, 21, 6559. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Cross-Talk between NADPH Oxidase and Mitochondria: Role in ROS Signaling and Angiogenesis. Cells 2020, 9, 1849. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Davidson, S.M.; Duchen, M.R. Endothelial mitochondria: Contributing to vascular function and disease. Circ. Res. 2007, 100, 1128–1141. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Sobenin, I.A. Modified and Dysfunctional Lipoproteins in Atherosclerosis: Effectors or Biomarkers? Curr. Med. Chem. 2019, 26, 1512–1524. [Google Scholar] [CrossRef]
- Karamanlidis, G.; Nascimben, L.; Couper, G.S.; Shekar, P.S.; del Monte, F.; Tian, R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ. Res. 2010, 106, 1541–1548. [Google Scholar] [CrossRef]
- Ku, H.J.; Ahn, Y.; Lee, J.H.; Park, K.M.; Park, J.W. IDH2 deficiency promotes mitochondrial dysfunction and cardiac hypertrophy in mice. Free Radic. Biol. Med. 2015, 80, 84–92. [Google Scholar] [CrossRef]
- Qi, W.; Lu, C.; Huang, H.; Zhang, W.; Song, S.; Liu, B. (+)-Usnic Acid Induces ROS-dependent Apoptosis via Inhibition of Mitochondria Respiratory Chain Complexes and Nrf2 Expression in Lung Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 876. [Google Scholar] [CrossRef]
- Kahveci, A.S.; Barnatan, T.T.; Kahveci, A.; Adrian, A.E.; Arroyo, J.; Eirin, A.; Harris, P.C.; Lerman, A.; Lerman, L.O.; Torres, V.E.; et al. Oxidative Stress and Mitochondrial Abnormalities Contribute to Decreased Endothelial Nitric Oxide Synthase Expression and Renal Disease Progression in Early Experimental Polycystic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 1994. [Google Scholar] [CrossRef]
- Sun, J.; Huang, X.; Niu, C.; Wang, X.; Li, W.; Liu, M.; Wang, Y.; Huang, S.; Chen, X.; Li, X.; et al. aFGF alleviates diabetic endothelial dysfunction by decreasing oxidative stress via Wnt/beta-catenin-mediated upregulation of HXK2. Redox Biol. 2021, 39, 101811. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Robinson, A.T.; Rossman, M.J.; Seals, D.R.; Edwards, D.G. Mitochondrial contributions to vascular endothelial dysfunction, arterial stiffness, and cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H2080–H2100. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Pedraza-Chaverri, J. Mitochondrial Redox Signaling and Oxidative Stress in Kidney Diseases. Biomolecules 2021, 11, 1144. [Google Scholar] [CrossRef]
- Dosunmu-Ogunbi, A.; Yuan, S.; Reynolds, M.; Giordano, L.; Sanker, S.; Sullivan, M.; Stolz, D.B.; Kaufman, B.A.; Wood, K.C.; Zhang, Y.; et al. SOD2 V16A amplifies vascular dysfunction in sickle cell patients by curtailing mitochondria complex IV activity. Blood 2022, 139, 1760–1765. [Google Scholar] [CrossRef]
- Wu, H.; Feng, L.; Wu, H.; Wang, L.; Xu, H.; Fu, F. Synergistic effects of PS-NPs and Cd on ovarian toxicity in adolescent rats: Ferroptosis by induction of mitochondrial redox imbalance via the SIRT3-SOD2/Gpx4 pathway. Ecotoxicol. Environ. Saf. 2025, 290, 117622. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef]
- Lampsas, S.; Xenou, M.; Oikonomou, E.; Pantelidis, P.; Lysandrou, A.; Sarantos, S.; Goliopoulou, A.; Kalogeras, K.; Tsigkou, V.; Kalpis, A.; et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules 2023, 28, 969. [Google Scholar] [CrossRef]
- Viola, M.; Karousou, E.; D’Angelo, M.L.; Moretto, P.; Caon, I.; Luca, G.; Passi, A.; Vigetti, D. Extracellular Matrix in Atherosclerosis: Hyaluronan and Proteoglycans Insights. Curr. Med. Chem. 2016, 23, 2958–2971. [Google Scholar] [CrossRef]
- Manninen, S.; Lankinen, M.; Erkkila, A.; Nguyen, S.D.; Ruuth, M.; de Mello, V.; Oorni, K.; Schwab, U. The effect of intakes of fish and Camelina sativa oil on atherogenic and anti-atherogenic functions of LDL and HDL particles: A randomized controlled trial. Atherosclerosis 2019, 281, 56–61. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodriguez-Manas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Wu, R.; Tan, Q.; Niu, K.; Zhu, Y.; Wei, D.; Zhao, Y.; Fang, H. MMS19 localizes to mitochondria and protects the mitochondrial genome from oxidative damage. Biochem. Cell Biol. 2018, 96, 44–49. [Google Scholar] [CrossRef]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial dysfunction in type 2 diabetes mellitus: An organ-based analysis. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef]
- Salnikova, D.; Orekhova, V.; Grechko, A.; Starodubova, A.; Bezsonov, E.; Popkova, T.; Orekhov, A. Mitochondrial Dysfunction in Vascular Wall Cells and Its Role in Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8990. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.Z.; Rabinovitch, P.S.; Tabas, I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappaB-mediated inflammation in macrophages. Circ. Res. 2014, 114, 421–433. [Google Scholar] [CrossRef]
- Zhou, Y.; Que, K.T.; Zhang, Z.; Yi, Z.J.; Zhao, P.X.; You, Y.; Gong, J.P.; Liu, Z.J. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018, 7, 4012–4022. [Google Scholar] [CrossRef]
- Oikonomou, E.; Tsaplaris, P.; Anastasiou, A.; Xenou, M.; Lampsas, S.; Siasos, G.; Pantelidis, P.; Theofilis, P.; Tsatsaragkou, A.; Katsarou, O.; et al. Interleukin-1 in Coronary Artery Disease. Curr. Top. Med. Chem. 2022, 22, 2368–2389. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Zhelankin, A.V.; Kolmychkova, K.I.; Mitrofanov, K.Y.; Kubekina, M.V.; Ivanova, E.A.; Sobenin, I.A. Susceptibility of monocytes to activation correlates with atherogenic mitochondrial DNA mutations. Exp. Mol. Pathol. 2015, 99, 672–676. [Google Scholar] [CrossRef]
- Tseng, W.W.; Wei, A.C. Kinetic Mathematical Modeling of Oxidative Phosphorylation in Cardiomyocyte Mitochondria. Cells 2022, 11, 4020. [Google Scholar] [CrossRef]
- Morad, M.; Zhang, X.H. Mechanisms of spontaneous pacing: Sinoatrial nodal cells, neonatal cardiomyocytes, and human stem cell derived cardiomyocytes. Can. J. Physiol. Pharmacol. 2017, 95, 1100–1107. [Google Scholar] [CrossRef]
- Hoppel, C.L.; Tandler, B.; Fujioka, H.; Riva, A. Dynamic organization of mitochondria in human heart and in myocardial disease. Int. J. Biochem. Cell Biol. 2009, 41, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Shami, G.J.; Griffiths, L.; Lal, S.; Irving, H.; Braet, F. Giant mitochondria in cardiomyocytes: Cellular architecture in health and disease. Basic. Res. Cardiol. 2023, 118, 39. [Google Scholar] [CrossRef]
- Yu, H.; Yu, M.; Li, Z.; Zhang, E.; Ma, H. Identification and analysis of mitochondria-related key genes of heart failure. J. Transl. Med. 2022, 20, 410. [Google Scholar] [CrossRef]
- Toglia, P.; Ullah, G. Mitochondrial dysfunction and role in spreading depolarization and seizure. J. Comput. Neurosci. 2019, 47, 91–108. [Google Scholar] [CrossRef]
- Murphy, E.; Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008, 88, 581–609. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijevic, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- She, R.; Liu, D.; Liao, J.; Wang, G.; Ge, J.; Mei, Z. Mitochondrial dysfunctions induce PANoptosis and ferroptosis in cerebral ischemia/reperfusion injury: From pathology to therapeutic potential. Front. Cell. Neurosci. 2023, 17, 1191629. [Google Scholar] [CrossRef]
- Saraste, M. Oxidative phosphorylation at the fin de siecle. Science 1999, 283, 1488–1493. [Google Scholar] [CrossRef]
- Ashrafian, H. Cardiac energetics in congestive heart failure. Circulation 2002, 105, e44–e45. [Google Scholar] [CrossRef]
- Nguyen, B.Y.; Ruiz-Velasco, A.; Bui, T.; Collins, L.; Wang, X.; Liu, W. Mitochondrial function in the heart: The insight into mechanisms and therapeutic potentials. Br. J. Pharmacol. 2019, 176, 4302–4318. [Google Scholar] [CrossRef]
- Lozhkin, A.; Vendrov, A.E.; Ramos-Mondragon, R.; Canugovi, C.; Stevenson, M.D.; Herron, T.J.; Hummel, S.L.; Figueroa, C.A.; Bowles, D.E.; Isom, L.L.; et al. Mitochondrial oxidative stress contributes to diastolic dysfunction through impaired mitochondrial dynamics. Redox Biol. 2022, 57, 102474. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Deng, Y.; Yang, L.; Ou, W.; Xie, M.; Ding, L.; Jiang, C.; Yu, H.; Li, Q.; et al. Mitochondrial protein hyperacetylation underpins heart failure with preserved ejection fraction in mice. J. Mol. Cell. Cardiol. 2022, 165, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Rabinovitch, P.S. Cardiac aging in mice and humans: The role of mitochondrial oxidative stress. Trends Cardiovasc. Med. 2009, 19, 213–220. [Google Scholar] [CrossRef]
- Trammell, S.A.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef]
- Bayeva, M.; Gheorghiade, M.; Ardehali, H. Mitochondria as a therapeutic target in heart failure. J. Am. Coll. Cardiol. 2013, 61, 599–610. [Google Scholar] [CrossRef]
- Williams, G.S.; Boyman, L.; Lederer, W.J. Mitochondrial calcium and the regulation of metabolism in the heart. J. Mol. Cell. Cardiol. 2015, 78, 35–45. [Google Scholar] [CrossRef]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef]
- Diaz-Juarez, J.; Suarez, J.; Cividini, F.; Scott, B.T.; Diemer, T.; Dai, A.; Dillmann, W.H. Expression of the mitochondrial calcium uniporter in cardiac myocytes improves impaired mitochondrial calcium handling and metabolism in simulated hyperglycemia. Am. J. Physiol. Cell Physiol. 2016, 311, C1005–C1013. [Google Scholar] [CrossRef]
- Ji, L.; Liu, F.; Jing, Z.; Huang, Q.; Zhao, Y.; Cao, H.; Li, J.; Yin, C.; Xing, J.; Li, F. MICU1 Alleviates Diabetic Cardiomyopathy Through Mitochondrial Ca(2+)-Dependent Antioxidant Response. Diabetes 2017, 66, 1586–1600. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, V.; Oikonomou, E.; Anastasiou, A.; Lampsas, S.; Zakynthinos, G.E.; Kalogeras, K.; Katsioupa, M.; Kapsali, M.; Kourampi, I.; Pesiridis, T.; et al. Molecular Mechanisms and Therapeutic Implications of Endothelial Dysfunction in Patients with Heart Failure. Int. J. Mol. Sci. 2023, 24, 4321. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.K.; Gerasimenko, J.V.; Thorne, C.; Ferdek, P.; Pozzan, T.; Tepikin, A.V.; Petersen, O.H.; Sutton, R.; Watson, A.J.; Gerasimenko, O.V. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J. Biol. Chem. 2009, 284, 20796–20803. [Google Scholar] [CrossRef]
- Bernardi, P.; Di Lisa, F. The mitochondrial permeability transition pore: Molecular nature and role as a target in cardioprotection. J. Mol. Cell. Cardiol. 2015, 78, 100–106. [Google Scholar] [CrossRef]
- Wang, F.; Travins, J.; Lin, Z.; Si, Y.; Chen, Y.; Powe, J.; Murray, S.; Zhu, D.; Artin, E.; Gross, S.; et al. A small molecule inhibitor of mutant IDH2 rescues cardiomyopathy in a D-2-hydroxyglutaric aciduria type II mouse model. J. Inherit. Metab. Dis. 2016, 39, 807–820. [Google Scholar] [CrossRef]
- Oikonomou, E.; Theofilis, P.; Lampsas, S.; Katsarou, O.; Kalogeras, K.; Marinos, G.; Tsatsaragkou, A.; Anastasiou, A.; Lysandrou, A.; Gounaridi, M.I.; et al. Current Concepts and Future Applications of Non-Invasive Functional and Anatomical Evaluation of Coronary Artery Disease. Life 2022, 12, 1803. [Google Scholar] [CrossRef]
- Sam, F.; Kerstetter, D.L.; Pimental, D.R.; Mulukutla, S.; Tabaee, A.; Bristow, M.R.; Colucci, W.S.; Sawyer, D.B. Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. J. Card. Fail. 2005, 11, 473–480. [Google Scholar] [CrossRef]
- Ganotopoulou, A.; Korakas, E.; Pliouta, L.; Kountouri, A.; Pililis, S.; Lampsas, S.; Ikonomidis, I.; Rallidis, L.S.; Papazafiropoulou, A.; Melidonis, A.; et al. Association Between Plasma ADAMTS-7 Levels and Diastolic Dysfunction in Patients with Type 2 Diabetes Mellitus. Medicina 2024, 60, 1981. [Google Scholar] [CrossRef]
- Alencar, R.R.; Batalha, C.; Freire, T.S.; de Souza-Pinto, N.C. Enzymology of mitochondrial DNA repair. Enzymes 2019, 45, 257–287. [Google Scholar] [CrossRef]
- Hong, Y.S.; Longchamps, R.J.; Zhao, D.; Castellani, C.A.; Loehr, L.R.; Chang, P.P.; Matsushita, K.; Grove, M.L.; Boerwinkle, E.; Arking, D.E.; et al. Mitochondrial DNA Copy Number and Incident Heart Failure: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2020, 141, 1823–1825. [Google Scholar] [CrossRef]
- Mongelli, A.; Mengozzi, A.; Geiger, M.; Gorica, E.; Mohammed, S.A.; Paneni, F.; Ruschitzka, F.; Costantino, S. Mitochondrial epigenetics in aging and cardiovascular diseases. Front. Cardiovasc. Med. 2023, 10, 1204483. [Google Scholar] [CrossRef] [PubMed]
- Ronn, T.; Ofori, J.K.; Perfilyev, A.; Hamilton, A.; Pircs, K.; Eichelmann, F.; Garcia-Calzon, S.; Karagiannopoulos, A.; Stenlund, H.; Wendt, A.; et al. Genes with epigenetic alterations in human pancreatic islets impact mitochondrial function, insulin secretion, and type 2 diabetes. Nat. Commun. 2023, 14, 8040. [Google Scholar] [CrossRef]
- Li, W.; Bai, Z.; Liu, J.; Tang, Y.; Yin, C.; Jin, M.; Mu, L.; Li, X. Mitochondrial ROS-dependent CD4(+)PD-1(+)T cells are pathological expansion in patients with primary immune thrombocytopenia. Int. Immunopharmacol. 2023, 122, 110597. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, P.; Xu, Y.; Tian, W.; Jing, L.; Lv, Q.; Zhao, Y.; Wang, H.; Shao, Q. Mitochondrial Quality Control Orchestrates the Symphony of B Cells and Plays Critical Roles in B Cell-Related Diseases. J. Immunol. Res. 2024, 2024, 5577506. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, Y.; Zhou, X. The role of mitophagy in innate immune responses triggered by mitochondrial stress. Cell Commun. Signal. 2020, 18, 186. [Google Scholar] [CrossRef]
- Jiang, R.Q.; Li, Q.Q.; Sheng, R. Mitochondria associated ER membranes and cerebral ischemia: Molecular mechanisms and therapeutic strategies. Pharmacol. Res. 2023, 191, 106761. [Google Scholar] [CrossRef]
- Kotani, K.; Tsuzaki, K.; Sakane, N. The relationship between gamma-glutamyltransferase (GGT), bilirubin (Bil) and small dense low-density lipoprotein (sdLDL) in asymptomatic subjects attending a clinic for screening dyslipidaemias. Ann. Acad. Med. Singap. 2014, 43, 216–219. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538. [Google Scholar] [CrossRef]
- Tsai, C.F.; Chen, G.W.; Chen, Y.C.; Shen, C.K.; Lu, D.Y.; Yang, L.Y.; Chen, J.H.; Yeh, W.L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients 2021, 14, 67. [Google Scholar] [CrossRef]
- Cobo, I.; Tanaka, T.N.; Chandra Mangalhara, K.; Lana, A.; Yeang, C.; Han, C.; Schlachetzki, J.; Challcombe, J.; Fixsen, B.R.; Sakai, M.; et al. DNA methyltransferase 3 alpha and TET methylcytosine dioxygenase 2 restrain mitochondrial DNA-mediated interferon signaling in macrophages. Immunity 2022, 55, 1386–1401.e10. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva, B.; Podlesniy, P.; Miloshev, G.; Georgieva, M. Yeast Chromatin Mutants Reveal Altered mtDNA Copy Number and Impaired Mitochondrial Membrane Potential. J. Fungi 2023, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Xin, Y.; Tian, G.; Zhou, J.; Liu, X. Mitochondrial ROS-Modulated mtDNA: A Potential Target for Cardiac Aging. Oxidative Med. Cell. Longev. 2020, 2020, 9423593. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.S.; Min, S.H.; Lee, C.; Cho, Y.M. Mitochondrial-encoded MOTS-c prevents pancreatic islet destruction in autoimmune diabetes. Cell Rep. 2021, 36, 109447. [Google Scholar] [CrossRef]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef]
- Cataldo, L.R.; Fernandez-Verdejo, R.; Santos, J.L.; Galgani, J.E. Plasma MOTS-c levels are associated with insulin sensitivity in lean but not in obese individuals. J. Investig. Med. 2018, 66, 1019–1022. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Bettahi, I.; Jerobin, J.; Chandra, P.; Abi Khalil, C.; Skarulis, M.; Atkin, S.L.; Abou-Samra, A.B. Mitochondrial-Derived Peptides Are Down Regulated in Diabetes Subjects. Front. Endocrinol. 2019, 10, 331. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, M.; Pan, Y.; Liang, M.; Fu, Y.; Duan, Y.; Tang, M.; Laher, I.; Li, S. The mitochondrial signaling peptide MOTS-c improves myocardial performance during exercise training in rats. Sci. Rep. 2021, 11, 20077. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Pang, X.; Ma, J.; Yuan, J.; Pan, Y.; Fu, Y.; Laher, I.; Li, S. MOTS-c repairs myocardial damage by inhibiting the CCN1/ERK1/2/EGR1 pathway in diabetic rats. Front. Nutr. 2022, 9, 1060684. [Google Scholar] [CrossRef]
- Yin, X.; Jing, Y.; Chen, Q.; Abbas, A.B.; Hu, J.; Xu, H. The intraperitoneal administration of MOTS-c produces antinociceptive and anti-inflammatory effects through the activation of AMPK pathway in the mouse formalin test. Eur. J. Pharmacol. 2020, 870, 172909. [Google Scholar] [CrossRef]
- Yang, B.; Yu, Q.; Chang, B.; Guo, Q.; Xu, S.; Yi, X.; Cao, S. MOTS-c interacts synergistically with exercise intervention to regulate PGC-1alpha expression, attenuate insulin resistance and enhance glucose metabolism in mice via AMPK signaling pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166126. [Google Scholar] [CrossRef]
- Wei, M.; Gan, L.; Sha, X.; Lu, H.; Jiang, Y.; Lei, X.; Xu, C.; Ruan, B.; Wang, L.; Lu, Z. Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem. Biophys. Res. Commun. 2016, 476, 412–419. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Wenceslau, C.F.; McCarthy, C.G.; Szasz, T.; Spitler, K.; Goulopoulou, S.; Webb, R.C.; Working Group on DAMPs in Cardiovascular Disease. Mitochondrial damage-associated molecular patterns and vascular function. Eur. Heart J. 2014, 35, 1172–1177. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef]
- Loo, Y.M.; Gale, M., Jr. Immune signaling by RIG-I-like receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Regan, J.A.; Mentz, R.J.; Nguyen, M.; Green, J.B.; Truby, L.K.; Ilkayeva, O.; Newgard, C.B.; Buse, J.B.; Sourij, H.; Sjostrom, C.D.; et al. Mitochondrial metabolites predict adverse cardiovascular events in individuals with diabetes. JCI Insight 2023, 8, e168563. [Google Scholar] [CrossRef]
- Tian, X.Y.; Ma, S.; Tse, G.; Wong, W.T.; Huang, Y. Uncoupling Protein 2 in Cardiovascular Health and Disease. Front. Physiol. 2018, 9, 1060. [Google Scholar] [CrossRef]
- Jin, J.Y.; Wei, X.X.; Zhi, X.L.; Wang, X.H.; Meng, D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 2021, 42, 655–664. [Google Scholar] [CrossRef]
- Lin, J.; Duan, J.; Wang, Q.; Xu, S.; Zhou, S.; Yao, K. Mitochondrial Dynamics and Mitophagy in Cardiometabolic Disease. Front. Cardiovasc. Med. 2022, 9, 917135. [Google Scholar] [CrossRef]
- Canadas-Garre, M.; Maqueda, J.J.; Banos-Jaime, B.; Hill, C.; Skelly, R.; Cappa, R.; Brennan, E.; Doyle, R.; Godson, C.; Maxwell, A.P.; et al. Mitochondrial related variants associated with cardiovascular traits. Front. Physiol. 2024, 15, 1395371. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, S.; Pugin, J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front. Immunol. 2018, 9, 832. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cocheme, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Szeto, H.H. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 2014, 171, 2029–2050. [Google Scholar] [CrossRef]
- Chatfield, K.C.; Sparagna, G.C.; Chau, S.; Phillips, E.K.; Ambardekar, A.V.; Aftab, M.; Mitchell, M.B.; Sucharov, C.C.; Miyamoto, S.D.; Stauffer, B.L. Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC Basic Transl. Sci. 2019, 4, 147–157. [Google Scholar] [CrossRef]
- Novelle, M.G.; Wahl, D.; Dieguez, C.; Bernier, M.; de Cabo, R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef]
- Rosenfeldt, F.L.; Haas, S.J.; Krum, H.; Hadj, A.; Ng, K.; Leong, J.Y.; Watts, G.F. Coenzyme Q10 in the treatment of hypertension: A meta-analysis of the clinical trials. J. Hum. Hypertens. 2007, 21, 297–306. [Google Scholar] [CrossRef]
- Riehle, C.; Abel, E.D. PGC-1 proteins and heart failure. Trends Cardiovasc. Med. 2012, 22, 98–105. [Google Scholar] [CrossRef]
- Cowan, D.B.; Yao, R.; Akurathi, V.; Snay, E.R.; Thedsanamoorthy, J.K.; Zurakowski, D.; Ericsson, M.; Friehs, I.; Wu, Y.; Levitsky, S.; et al. Intracoronary Delivery of Mitochondria to the Ischemic Heart for Cardioprotection. PLoS ONE 2016, 11, e0160889. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).