Pars Plana Vitrectomy and ILM Peeling for Refractory Diabetic Macular Edema Without Vitreomacular Traction
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
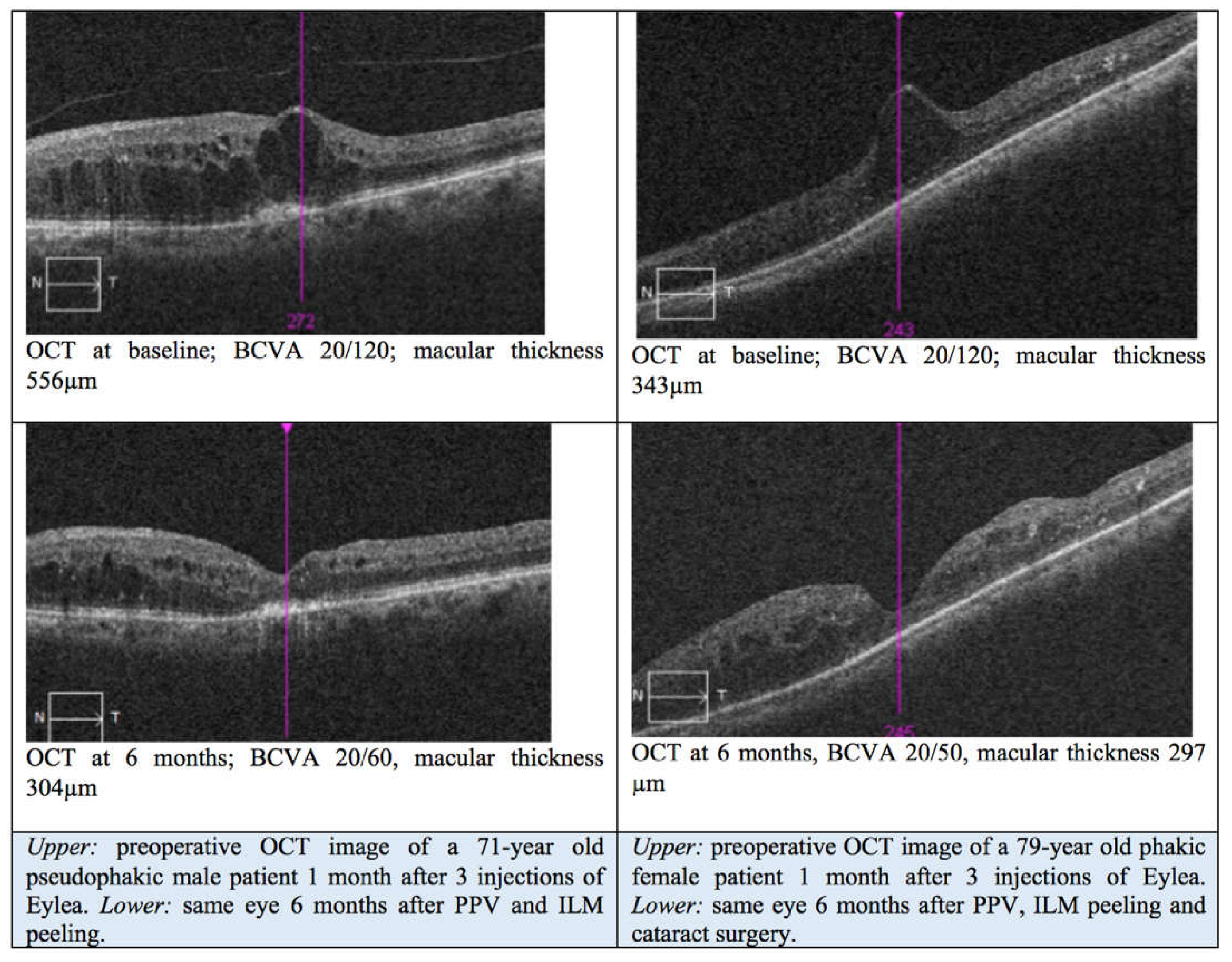
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCVA | best-corrected distance visual acuity |
| CMT | central macular thickness |
| DME | diabetic macular edema |
| ERM | epiretinal membranes |
| ILM | internal limiting membrane |
| IVI | intravitreal injection |
| PPV | pars plana vitrectomy |
| PVD | posterior vitreous detachment |
| SD | standard deviation |
| SD-OCT | spectral-domain optical coherence tomography |
| VMT | vitreomacular traction |
References
- Ciulla, T.A.; Amador, A.G.; Zinman, B. Diabetic Retinopathy and Diabetic Macular Edema: Pathophysiology, Screening, and Novel Therapies. Diabetes Care 2003, 26, 2653–2664. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Bressler, N.M.; Doan, Q.V.; Gleeson, M.; Danese, M.; Bower, J.K.; Selvin, E.; Dolan, C.; Fine, J.; Colman, S.; et al. Prevalence of and Risk Factors for Diabetic Macular Edema in the United States. JAMA Ophthalmol. 2014, 132, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J. Mechanisms of Retinal Fluid Accumulation and Blood-Retinal Barrier Breakdown. Dev Ophthalmol 2017, 58, 11–20. [Google Scholar] [CrossRef]
- Massin, P.; Duguid, G.; Erginay, A.; Haouchine, B.; Gaudric, A. Optical Coherence Tomography for Evaluating Diabetic Macular Edema before and after Vitrectomy. Am. J. Ophthalmol. 2003, 135, 169–177. [Google Scholar] [CrossRef]
- Hikichi, T.; Fujio, N.; Akiba, J.; Azuma, Y.; Takahashi, M.; Yoshida, A. Association between the Short-Term Natural History of Diabetic Macular Edema and the Vitreomacular Relationship in Type II Diabetes Mellitus. Ophthalmology 1997, 104, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.R.H.; Dowell, N.G.; Jackson, T.L.; Tofts, P.S.; Hughes, E.H. Measuring the Effect of Pars Plana Vitrectomy on Vitreous Oxygenation Using Magnetic Resonance Imaging. Invest. Ophthalmol. Vis. Sci. 2013, 54, 2028–2034. [Google Scholar] [CrossRef]
- Stefánsson, E. Physiology of Vitreous Surgery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 147–163. [Google Scholar] [CrossRef]
- Gandorfer, A.; Messmer, E.M.; Ulbig, M.W.; Kampik, A. Resolution of Diabetic Macular Edema after Surgical Removal of the Posterior Hyaloid and the Inner Limiting Membrane. Retina 2000, 20, 126–133. [Google Scholar] [CrossRef]
- Kulikov, A.N.; Sosnovskii, S.V.; Berezin, R.D.; Maltsev, D.S.; Oskanov, D.H.; Gribanov, N.A. Vitreoretinal Interface Abnormalities in Diabetic Macular Edema and Effectiveness of Anti-VEGF Therapy: An Optical Coherence Tomography Study. Clin. Ophthalmol. 2017, 11, 1995–2002. [Google Scholar] [CrossRef]
- Jackson, T.L.; Nicod, E.; Angelis, A.; Grimaccia, F.; Pringle, E.; Kanavos, P. PARS PLANA VITRECTOMY FOR DIABETIC MACULAR EDEMA: A Systematic Review, Meta-Analysis, and Synthesis of Safety Literature. Retina 2017, 37, 886–895. [Google Scholar] [CrossRef]
- Laidlaw, D.A.H. Vitrectomy for Diabetic Macular Oedema. Eye 2008, 22, 1337–1341. [Google Scholar] [CrossRef]
- Dehghan, M.-H.; Salehipour, M.; Naghib, J.; Babaeian, M.; Karimi, S.; Yaseri, M. Pars Plana Vitrectomy with Internal Limiting Membrane Peeling for Refractory Diffuse Diabetic Macular Edema. J. Ophthalmic Vis. Res. 2010, 5, 162–167. [Google Scholar]
- Mukai, R.; Matsumoto, H.; Akiyama, H. Surgical Outcomes of Vitrectomy for Intractable Diabetic Macular Edema. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 363–368. [Google Scholar] [CrossRef]
- Ranno, S.; Vujosevic, S.; Mambretti, M.; Metrangolo, C.; Alkabes, M.; Rabbiolo, G.; Govetto, A.; Carini, E.; Nucci, P.; Radice, P. Role of Vitrectomy in Nontractional Refractory Diabetic Macular Edema. J. Clin. Med. 2023, 12, 2297. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Liu, H.; Wang, L.-N.; Ding, Y.-Z.; Luan, J. Efficacy and Safety of Vitrectomy with Internal Limiting Membrane Peeling for Diabetic Macular Edema: A Meta-Analysis. Int. J. Ophthalmol. 2018, 11, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Yanyali, A.; Horozoglu, F.; Celik, E.; Nohutcu, A.F. Long-Term Outcomes of Pars Plana Vitrectomy with Internal Limiting Membrane Removal in Diabetic Macular Edema. Retina 2007, 27, 557–566. [Google Scholar] [CrossRef]
- Hoerauf, H.; Brüggemann, A.; Muecke, M.; Lüke, J.; Müller, M.; Stefánsson, E.; Hammes, H.-P.; Weiss, C. Pars Plana Vitrectomy for Diabetic Macular Edema. Internal Limiting Membrane Delamination vs Posterior Hyaloid Removal. A Prospective Randomized Trial. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 997–1008. [Google Scholar] [CrossRef][Green Version]
- Kunikata, H.; Abe, T.; Kinukawa, J.; Nishida, K. Preoperative Factors Predictive of Postoperative Decimal Visual Acuity ≥ 1.0 Following Surgical Treatment for Idiopathic Epiretinal Membrane. Clin. Ophthalmol. 2011, 5, 147–154. [Google Scholar] [CrossRef]
- Guo, J.; Bi, X.; Chen, S.-N.; Chen, S.; He, G.-H.; Wu, B.; Zhang, W.; Wang, J. Efficacy of Internal Limiting Membrane Peeling for Diabetic Macular Edema after Preoperative Anti-Vascular Endothelial Growth Factor Injection. Int. J. Ophthalmol. 2020, 13, 1758–1764. [Google Scholar] [CrossRef]
- Mitamura, Y.; Hirano, K.; Baba, T.; Yamamoto, S. Correlation of Visual Recovery with Presence of Photoreceptor Inner/Outer Segment Junction in Optical Coherence Images after Epiretinal Membrane Surgery. Br. J. Ophthalmol. 2009, 93, 171–175. [Google Scholar] [CrossRef]
- Hillenkamp, J.; Saikia, P.; Gora, F.; Sachs, H.G.; Lohmann, C.P.; Roider, J.; Bäumler, W.; Gabel, V.-P. Macular Function and Morphology after Peeling of Idiopathic Epiretinal Membrane with and without the Assistance of Indocyanine Green. Br. J. Ophthalmol. 2005, 89, 437–443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gunay, B.O.; Erdogan, G. Evaluation of Macular Changes in the Long Term after Pars Plana Vitrectomy with Internal Limiting Membrane Peeling for Diabetic Macular Edema. Ophthalmologica 2021, 244, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.A.; Qin, H.; Apte, R.S.; Beck, R.R.; Bressler, N.M.; Browning, D.J.; Danis, R.P.; Glassman, A.R.; Googe, J.M.; Kollman, C.; et al. Vitrectomy Outcomes in Eyes with Diabetic Macular Edema and Vitreomacular Traction. Ophthalmology 2010, 117, 1087–1093.e3. [Google Scholar] [CrossRef]
- Nawrocka, Z.A.; Nawrocki, J. Vitrectomy in Diabetic Macular Edema: A Swept-Source OCT Angiography Study. Ophthalmol. Sci. 2022, 2, 100207. [Google Scholar] [CrossRef]
- Bressler, N.M.; Beaulieu, W.T.; Glassman, A.R.; Blinder, K.J.; Bressler, S.B.; Jampol, L.M.; Melia, M.; Wells, J.A. 3rd Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 257–269. [Google Scholar] [CrossRef]
- Guo, H.; Li, W.; Nie, Z.; Zhang, X.; Jiao, M.; Bai, S.; Duan, N.; Li, X.; Hu, B. Microinvasive Pars Plana Vitrectomy Combined with Internal Limiting Membrane Peeling versus Anti-VEGF Intravitreal Injection for Treatment-Naïve Diabetic Macular Edema (VVV-DME Study): Study Protocol for a Randomized Controlled Trial. Trials 2023, 24, 685. [Google Scholar] [CrossRef]
- Stefánsson, E. Ocular Oxygenation and the Treatment of Diabetic Retinopathy. Surv. Ophthalmol. 2006, 51, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Kadonosono, K.; Itoh, N.; Ohno, S. Perifoveal Microcirculation before and after Vitrectomy for Diabetic Cystoid Macular Edema. Am. J. Ophthalmol. 2000, 130, 740–744. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Huang, S.; Jia, B.; Lu, L.; Fu, J. Microstructural and Hemodynamic Changes in the Fundus after Pars Plana Vitrectomy for Different Vitreoretinal Diseases. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 1977–1992. [Google Scholar] [CrossRef]
- Christoforidis, J.B.; D’Amico, D.J. Surgical and Other Treatments of Diabetic Macular Edema: An Update. Int. Ophthalmol. Clin. 2004, 44, 139–160. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Burgeson, R.E.; Butkowski, R.J.; Couchman, J.R.; Zardi, L.; Ninomiya, Y.; Sado, Y.; Huang, Z.S.; Nesburn, A.B.; Kenney, M.C. Basement Membrane Abnormalities in Human Eyes with Diabetic Retinopathy. J. Histochem. Cytochem. 1996, 44, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Sorgente, N.; Goodnight, R.; Ryan, S.J. Alterations in the Distribution of Fibronectin and Laminin in the Diabetic Human Eye. Invest. Ophthalmol. Vis. Sci. 1987, 28, 515–521. [Google Scholar]
- Matsunaga, N.; Ozeki, H.; Hirabayashi, Y.; Shimada, S.; Ogura, Y. Histopathologic Evaluation of the Internal Limiting Membrane Surgically Excised from Eyes with Diabetic Maculopathy. Retina 2005, 25, 311–316. [Google Scholar] [CrossRef]
- Kishi, S.; Demaria, C.; Shimizu, K. Vitreous Cortex Remnants at the Fovea after Spontaneous Vitreous Detachment. Int. Ophthalmol. 1986, 9, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kalvoda, J.; Dusková, J.; Kubena, A.; Povýsil, C.; Kalvodová, B. Morphometry of Surgically Removed Internal Limiting Membrane during Vitrectomy in Diabetic Macular Edema. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1307–1314. [Google Scholar] [CrossRef]
- Stefaniotou, M.; Aspiotis, M.; Kalogeropoulos, C.; Christodoulou, A.; Psylla, M.; Ioachim, E.; Alamanos, I.; Psilas, K. Vitrectomy Results for Diffuse Diabetic Macular Edema with and without Inner Limiting Membrane Removal. Eur. J. Ophthalmol. 2004, 14, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Tachi, N.; Ogino, N. Vitrectomy for Diffuse Macular Edema in Cases of Diabetic Retinopathy. Am. J. Ophthalmol. 1996, 122, 258–260. [Google Scholar] [CrossRef]
- Wolf, S.; Schnurbusch, U.; Wiedemann, P.; Grosche, J.; Reichenbach, A.; Wolburg, H. Peeling of the Basal Membrane in the Human Retina: Ultrastructural Effects. Ophthalmology 2004, 111, 238–243. [Google Scholar] [CrossRef]
- Adelman, R.; Parnes, A.; Michalewska, Z.; Parolini, B.; Boscher, C.; Ducournau, D. Strategy for the Management of Diabetic Macular Edema: The European Vitreo-Retinal Society Macular Edema Study. Biomed Res. Int. 2015, 2015, 352487. [Google Scholar] [CrossRef]
- Nakajima, T.; Roggia, M.F.; Noda, Y.; Ueta, T. EFFECT OF INTERNAL LIMITING MEMBRANE PEELING DURING VITRECTOMY FOR DIABETIC MACULAR EDEMA: Systematic Review and Meta-Analysis. Retina 2015, 35, 1719–1725. [Google Scholar] [CrossRef]
- Rosenblatt, B.J.; Shah, G.K.; Sharma, S.; Bakal, J. Pars Plana Vitrectomy with Internal Limiting Membranectomy for Refractory Diabetic Macular Edema without a Taut Posterior Hyaloid. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Michalewska, Z.; Stewart, M.W.; Landers, M.B., 3rd; Bednarski, M.; Adelman, R.A.; Nawrocki, J. Vitrectomy in the Management of Diabetic Macular Edema in Treatment-Naïve Patients. Can. J. Ophthalmol. 2018, 53, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Rush, R.B.; Rush, S.W. Pars Plana Vitrectomy with Internal Limiting Membrane Peeling for Treatment-Naïve Diabetic Macular Edema: A Prospective, Uncontrolled Pilot Study. Clin. Ophthalmol. 2021, 15, 2619–2624. [Google Scholar] [CrossRef]
- Iglicki, M.; Lavaque, A.; Ozimek, M.; Negri, H.P.; Okada, M.; Chhablani, J.; Busch, C.; Loewenstein, A.; Zur, D. Biomarkers and Predictors for Functional and Anatomic Outcomes for Small Gauge Pars Plana Vitrectomy and Peeling of the Internal Limiting Membrane in Naïve Diabetic Macular Edema: The VITAL Study. PLoS ONE 2018, 13, e0200365. [Google Scholar] [CrossRef]
- Chhablani, J.K.; Kim, J.S.; Cheng, L.; Kozak, I.; Freeman, W. External Limiting Membrane as a Predictor of Visual Improvement in Diabetic Macular Edema after Pars Plana Vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Ivastinovic, D.; Haas, A.; Weger, M.; Seidel, G.; Mayer-Xanthaki, C.; Lindner, E.; Guttmann, A.; Wedrich, A. Vitrectomy for Diabetic Macular Edema and the Relevance of External Limiting Membrane. BMC Ophthalmology 2021, 21, 334. [Google Scholar] [CrossRef]
- Pessoa, B.; Leite, J.; Ferreira, A.; Ramalhão, J.; Poças, J.; José, D.; Coelho, C.; Figueira, J.; Meireles, A.; Beirão, J.M. Oct Biomarkers for Early Prognosis in Diabetic Macular Edema Treatment with Ranibizumab. Eur. J. Ophthalmol. 2024, 34, 1141–1148. [Google Scholar] [CrossRef]
- Sen, S.; Ramasamy, K.; Sivaprasad, S. Indicators of Visual Prognosis in Diabetic Macular Oedema. J. Pers. Med. 2021, 11, 449. [Google Scholar] [CrossRef]
- Nishijima, K.; Murakami, T.; Hirashima, T.; Uji, A.; Akagi, T.; Horii, T.; Ueda-Arakawa, N.; Muraoka, Y.; Yoshimura, N. Hyperreflective Foci in Outer Retina Predictive of Photoreceptor Damage and Poor Vision after Vitrectomy for Diabetic Macular Edema. Retina 2014, 34, 732–740. [Google Scholar] [CrossRef]
- Nanegrungsunk, O.; Patikulsila, D.; Sadda, S.R. Ophthalmic Imaging in Diabetic Retinopathy: A Review. Clin. Exp. Ophthalmol. 2022, 50, 1082–1096. [Google Scholar] [CrossRef]
- Uji, A.; Murakami, T.; Suzuma, K.; Yoshitake, S.; Arichika, S.; Ghashut, R.; Fujimoto, M.; Yoshimura, N. INFLUENCE OF VITRECTOMY SURGERY ON THE INTEGRITY OF OUTER RETINAL LAYERS IN DIABETIC MACULAR EDEMA. Retina 2018, 38, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Uji, A.; Murakami, T.; Unoki, N.; Ogino, K.; Horii, T.; Yoshitake, S.; Dodo, Y.; Yoshimura, N. Parallelism for Quantitative Image Analysis of Photoreceptor-Retinal Pigment Epithelium Complex Alterations in Diabetic Macular Edema. Invest. Ophthalmol. Vis. Sci. 2014, 55, 3361–3367. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Nishijima, K.; Kita, M.; Oh, H.; Tsujikawa, A.; Yoshimura, N. Association between Foveal Photoreceptor Status and Visual Acuity after Resolution of Diabetic Macular Edema by Pars Plana Vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.J.; Laidlaw, A.; Hammond, C.; Muqit, M.M.K.; Steel, D.; Dinah, C.; Lee, E.; Hillier, R.; Almeida, G.; Hussain, R.; et al. Vitrectomy as an Adjunct to Treat-and-Extend Anti-VEGF Injections for Diabetic Macular Edema: The Vitrectomy in Diabetic Macular Oedema (VIDEO) Randomized Clinical Trial. JAMA Ophthalmol. 2024, 142, 837–844. [Google Scholar] [CrossRef]
| Baseline Characteristics | 15 Patients (15 Eyes) |
|---|---|
| Gender | |
| Female (%) | 7 (47%) |
| Male (%) | 8 (53%) |
| Age in years | 65.5 ± 8.5 (50–79) |
| Diabetes treatment | |
| Oral (%) | 3 (20%) |
| Insulin (%) | 12 (80%) |
| BCVA in logMAR [mean ± SD (range)] | 0.69 ± 0.27 (0.30–1.30) |
| Intravitreal injections | |
| Anti-VEGF (%) | 12 (80%) |
| Dexamethasone (%) | 1 (7%) |
| Anti-VEGF + Dexamethasone (%) | 2 (14%) |
| DME duration in months [mean ± SD (range)] | 10.9 ± 8.4 (3.1–32.5) |
| Lens status | |
| Phakic (%) | 6 (40%) |
| Pseudophakic (%) | 9 (60%) |
| CMT in µm [mean ± SD (range)] | 457 ± 114 (305–816) |
| Abnormal IS/OS junction | 11 (73%) |
| Normal OCT | Abnormal OCT | p-Value | |
|---|---|---|---|
| Age in years (mean ± SD) | 67.3 ± 9.5 | 64.8 ± 8.5 | 0.66 |
| Preoperative BCVA in logMAR (mean ± SD) | 0.55 ± 0.17 | 0.75 ± 0.28 | 0.28 |
| CMT in µm (mean ± SD) | 396 ± 129 | 479 ± 146 | 0.41 |
| Duration of DME in months (mean ± SD) | 5.6 ± 3.5 | 12.9 ± 9.0 | 0.08 |
| BCVA at 1 month in logMAR (mean ± SD) | 0.15 ± 0.10 | 0.60 ± 0.21 | 0.001 |
| CMT at 1 month in µm (mean ± SD) | 334 ± 37 | 336 ± 133 | 0.45 |
| BCVA at 6 months in logMAR (mean ± SD) | 0.13 ± 0.19 | 0.53 ± 0.25 | 0.01 |
| CMT at 6 months in µm (mean ± SD) | 296 ± 28 | 304 ± 79 | 1 |
| BCVA improvement at 6 months in logMAR (mean ± SD) | 0.43 ± 0.05 | 0.22 ± 0.17 | 0.06 |
| CMT improvement at 6 months in µm (mean ± SD) | 101 ± 120 | 175 ± 152 | 0.66 |
| Baseline | 1-Month Follow-Up | 6-Month Follow-Up | |
|---|---|---|---|
| CMT in µm (mean ± SD) | 457 ± 114 | 336 ± 112; p = 0.035 | 302 ± 68; p = 0.001, p * = 0.089 |
| BCVA in logMAR (mean ± SD) | 0.69 ± 0.27 | 0.48 ± 0.28; p = 0.013 | 0.42 ± 0.29; p < 0.001, p * = 0.33 |
| Improvement ≥ 2 ETDRS lines (%) | 10 (67) | 10 (67) | |
| Improvement 0–2 ETDRS lines (%) | 0 (0) | 4 (27) | |
| No change (%) | 2 (13) | 1 (7) | |
| Worsening 0–2 ETDRS lines (%) | 2 (13) | 0 (0) | |
| Worsening ≥ 2 ETDRS lines (%) | 1 (7) | 0 (0) | |
| BCVA in logMAR in pseudophakic eyes (n = 9) (mean ± SD) | 0.64 ± 0.21 | 0.48 ± 0.29; p = 0.15 | 0.40 ± 0.26; p = 0.02, p * = 0.43 |
| Improvement ≥ 2 ETDRS lines (%) | 6 (67) | 6 (67) | |
| Improvement 0–2 ETDRS lines (%) | 0 (0) | 2 (22) | |
| No change (%) | 1 (11) | 1 (11) | |
| Worsening 0–2 ETDRS lines (%) | 1 (11) | 0 | |
| Worsening ≥ 2 ETDRS lines (%) | 1 (11) | 0 |
| Duration of DME | Preoperative BCVA | Preoperative CMT | |
|---|---|---|---|
| Preoperative BCVA [Pearson correlation coefficient (p-value)] | 0.78 (0.001) | 1 | 0.21 (0.46) |
| BCVA at 1 month [Pearson correlation coefficient (p-value)] | 0.52 (0.048) | 0.42 (0.12) | 0.27 (0.32) |
| BCVA at 6 months [Pearson correlation coefficient (p-value)] | 0.73 (0.002) | 0.82 (<0.001) | 0.41(0.13) |
| CMT at baseline [Pearson correlation coefficient (p-value)] | 0.28 (0.32) | 0.21 (0.46) | 1 |
| CMT at 1 month [Pearson correlation coefficient (p-value)] | 0.19 (0.52) | 0.03 (0.93) | 0.01 (0.96) |
| CMT at 6 months [Pearson correlation coefficient (p-value)] | −0.01 (0.96) | −0.43 (0.11) | 0.21 (0.46) |
| BCVA improvement at 6 months [Pearson correlation coefficient (p-value)] | −0.04 (0.90) | 0.15 (0.59) | −0.37 (0.17) |
| CMT improvement at 6 months [Pearson correlation coefficient (p-value)] | 0.28 (0.32) | 0.41 (0.13) | 0.89 (<0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
el-Khoury, S.; Ngo, C.; Muraine, M.; Abdelmassih, Y.; Portmann, A. Pars Plana Vitrectomy and ILM Peeling for Refractory Diabetic Macular Edema Without Vitreomacular Traction. J. Clin. Med. 2025, 14, 3686. https://doi.org/10.3390/jcm14113686
el-Khoury S, Ngo C, Muraine M, Abdelmassih Y, Portmann A. Pars Plana Vitrectomy and ILM Peeling for Refractory Diabetic Macular Edema Without Vitreomacular Traction. Journal of Clinical Medicine. 2025; 14(11):3686. https://doi.org/10.3390/jcm14113686
Chicago/Turabian Styleel-Khoury, Sylvain, Chloe Ngo, Marc Muraine, Youssef Abdelmassih, and Alexandre Portmann. 2025. "Pars Plana Vitrectomy and ILM Peeling for Refractory Diabetic Macular Edema Without Vitreomacular Traction" Journal of Clinical Medicine 14, no. 11: 3686. https://doi.org/10.3390/jcm14113686
APA Styleel-Khoury, S., Ngo, C., Muraine, M., Abdelmassih, Y., & Portmann, A. (2025). Pars Plana Vitrectomy and ILM Peeling for Refractory Diabetic Macular Edema Without Vitreomacular Traction. Journal of Clinical Medicine, 14(11), 3686. https://doi.org/10.3390/jcm14113686







