Influence of Fetal-Type Posterior Cerebral Artery on Morphological Characteristics and Rupture Risk of Posterior Communicating Artery Aneurysms: A Radiomics Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Patient Consent
2.2. Study Population and Clinical Characteristics
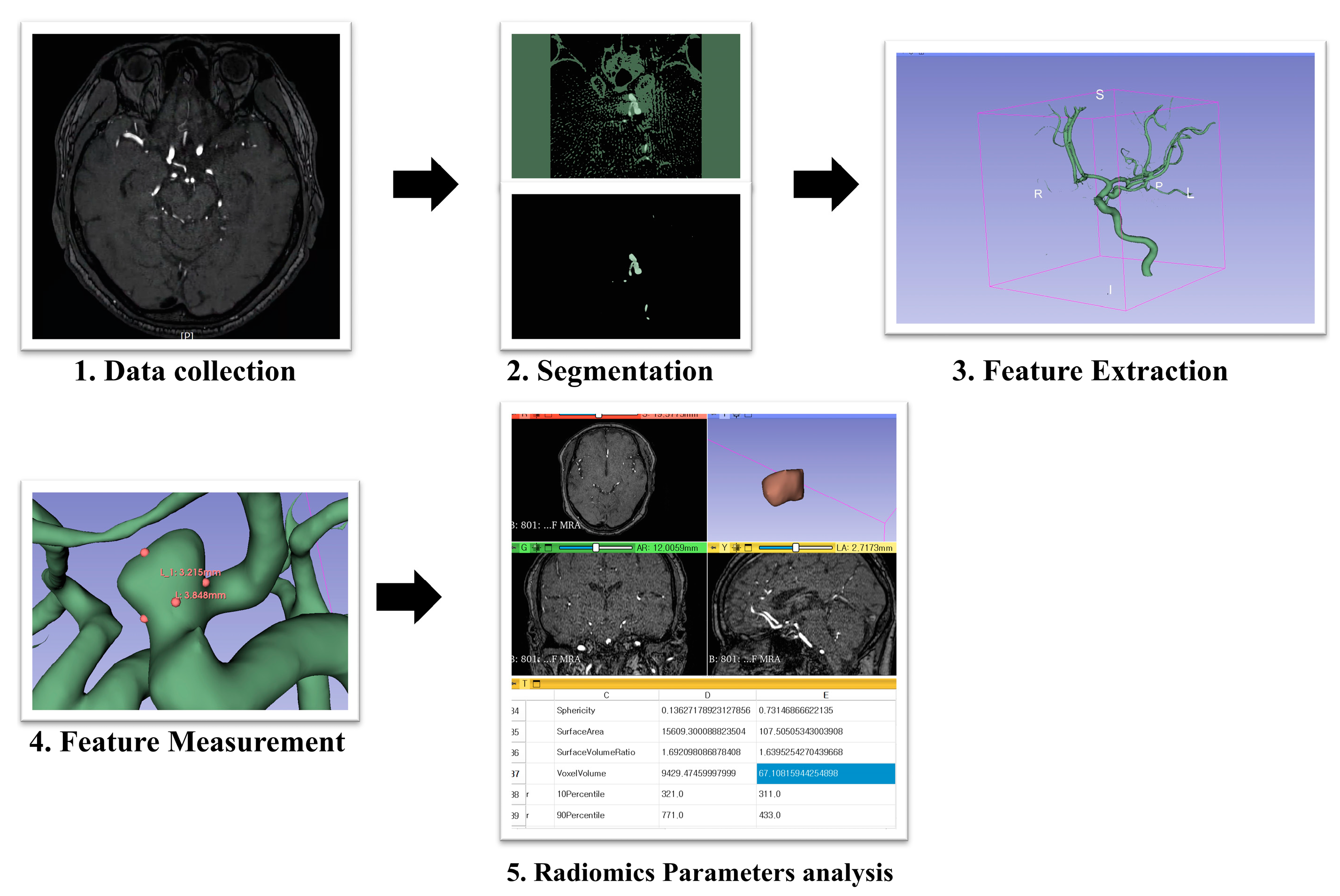
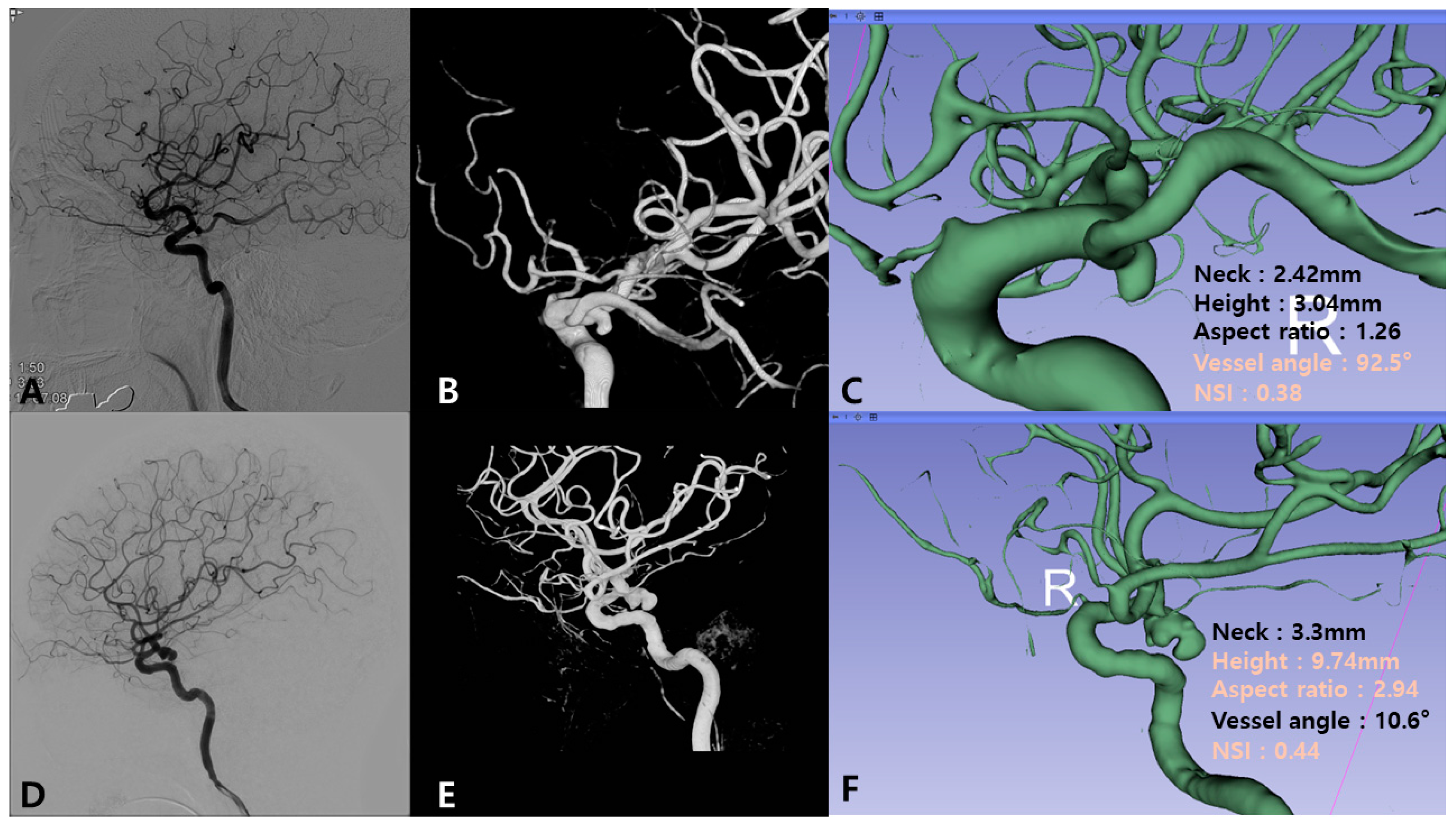
2.3. Radiological Evaluation and Parameter Measurement
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics and Univariate Analysis of Ruptured Status
3.2. Univariate Analysis of Morphological Parameters
3.3. Univariate Analysis of Radiomics Factors
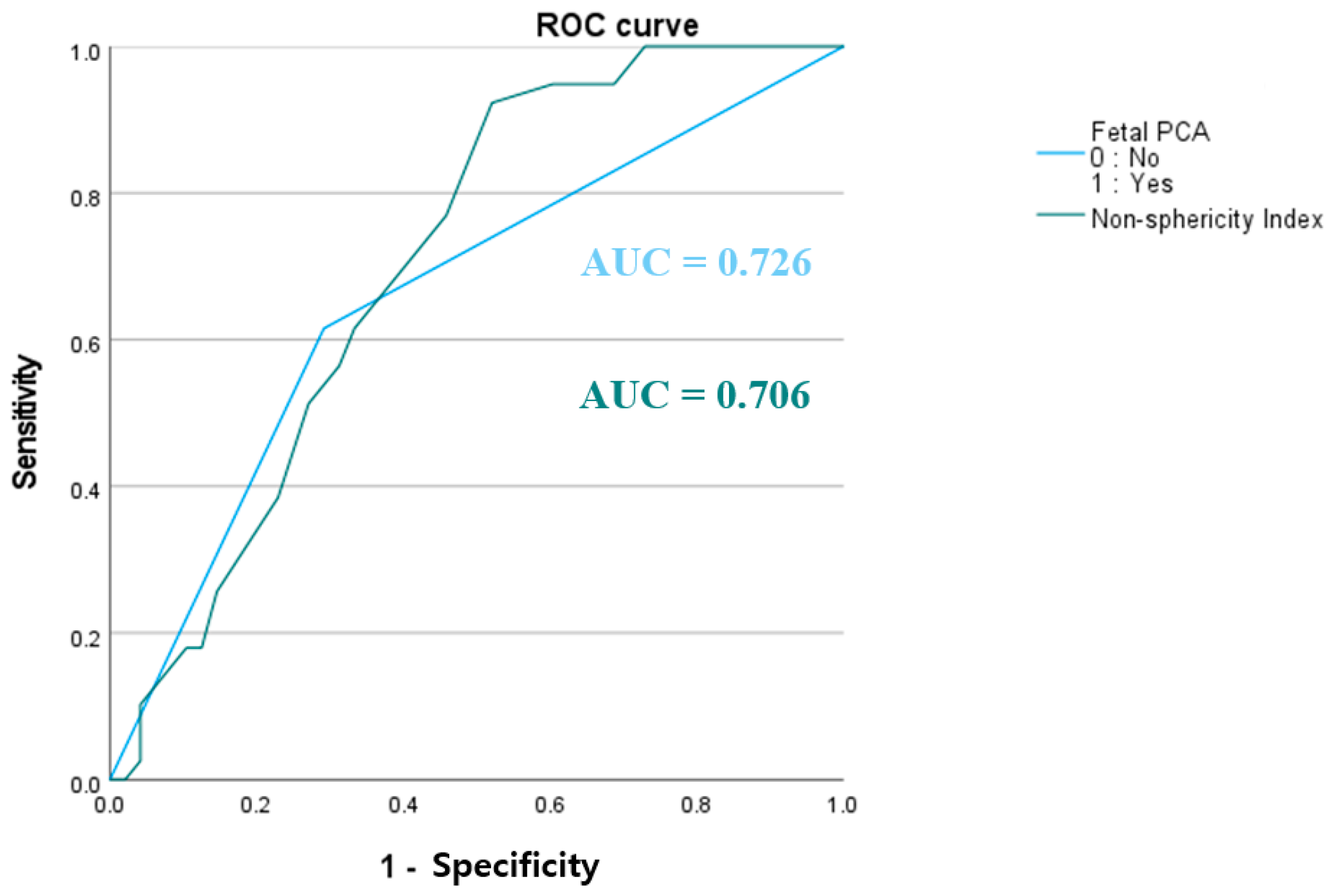
3.4. Association Between Fetal PCA and Aneurysm Rupture
3.5. Differences Based on Fetal PCA Status
3.6. Multivariate Logistic Regression Analysis
4. Discussion
4.1. Impact of Fetal PCA on Aneurysm Rupture Risk
4.2. Differential Influence of Parameters on Rupture Risk Based on Fetal PCA Status
4.3. Utility of Geometric Radiomics Parameters
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCoA | posterior communicating artery |
| PCA | posterior cerebral artery |
| DSA | digital subtraction angiography |
| MRA | magnetic resonance angiography |
| NSI | non-sphericity index |
| ROC | receiver operating characteristic |
| IAs | intracranial aneurysms |
| DM | diabetes mellitus |
| AUC | area under the curve |
References
- Golshani, K.; Ferrell, A.; Zomorodi, A.; Smith, T.P.; Britz, G.W. A review of the management of posterior communicating artery aneurysms in the modern era. Surg. Neurol. Int. 2010, 1, 88. [Google Scholar] [CrossRef] [PubMed]
- UCAS Japan Investigators; Morita, A.; Kirino, T.; Hashi, K.; Aoki, N.; Fukuhara, S.; Hashimoto, N.; Nakayama, T.; Sakai, M.; Teramoto, A.; et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N. Engl. J. Med. 2012, 366, 2474–2482. [Google Scholar] [CrossRef] [PubMed]
- Korja, M.; Silventoinen, K.; Laatikainen, T.; Jousilahti, P.; Salomaa, V.; Kaprio, J. Cause-specific mortality of 1-year survivors of subarachnoid hemorrhage. Neurology 2013, 80, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.J.; Doddasomayajula, R.; Mut, F.; Detmer, F.; Pritz, M.B.; Hamzei-Sichani, F.; Brinjikji, W.; Kallmes, D.F.; Jimenez, C.M.; Putman, C.M.; et al. Angioarchitectures and hemodynamic characteristics of posterior communicating artery aneurysms and their association with rupture status. AJNR Am. J. Neuroradiol. 2017, 38, 2111–2118. [Google Scholar] [CrossRef]
- Juvela, S.; Porras, M.; Heiskanen, O. Natural history of unruptured intracranial aneurysms: A long-term follow-up study. J. Neurosurg. 1993, 79, 174–182. [Google Scholar] [CrossRef]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J., III; Meissner, I.; Brown, R.D.; Piepgras, D.G.; Forbes, G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Arjal, R.K.; Zhu, T.; Zhou, Y. The study of fetal-type posterior cerebral circulation on multislice CT angiography and its influence on cerebral ischemic strokes. Clin. Imaging 2014, 38, 221–225. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wan, Y. Is fetal-type posterior cerebral artery a risk factor for intracranial aneurysm as analyzed by multislice CT angiography? Exp. Ther. Med. 2018, 15, 838–846. [Google Scholar] [CrossRef]
- Wentland, A.L.; Rowley, H.A.; Vigen, K.K.; Field, A.S. Fetal origin of the posterior cerebral artery produces left-right asymmetry on perfusion imaging. AJNR Am. J. Neuroradiol. 2010, 31, 448–453. [Google Scholar] [CrossRef]
- Uchino, A.; Saito, N.; Takahashi, M.; Okano, N.; Tanisaka, M. Variations of the posterior cerebral artery diagnosed by MR angiography at 3 tesla. Neuroradiology 2016, 58, 141–146. [Google Scholar] [CrossRef]
- Le, T.T.G.; Ryu, S.W.; Yoon, J.J.; Nam, T.; Ryu, J. Assessing the impact of fetal-type posterior cerebral artery variations on cerebral hemodynamics. Phys. Fluids 2024, 36, 101901. [Google Scholar] [CrossRef]
- Xu, Z.; Kim, B.S.; Lee, K.S.; Choi, J.H.; Shin, Y.S. Morphological and clinical risk factors for the rupture of posterior communicating artery aneurysms: Significance of fetal-type posterior cerebral artery. Neurol. Sci. 2019, 40, 2377–2382. [Google Scholar] [CrossRef]
- Tanaka, K.; Furukawa, K.; Ishida, F.; Suzuki, H. Hemodynamic differences of posterior communicating artery aneurysms between adult and fetal types of posterior cerebral artery. Acta Neurochir. 2023, 165, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Feng, Z.; Wang, C.; Cao, W.; Fang, Y.; Karmonik, C.; Liu, J.; Huang, Q. Morphological risk factors for rupture of small (<7 mm) posterior communicating artery aneurysms. World Neurosurg. 2016, 87, 311–315. [Google Scholar] [CrossRef]
- Arrambide-Garza, F.J.; Alvarez-Lozada, L.A.; de León-Gutiérrez, H.; Villarreal-Silva, E.E.; Alvarez-Villalobos, N.A.; Quiroga-Garza, A.; Elizondo-Omaña, R.E.; Guzman-Lopez, S. Fetal-type posterior cerebral artery and association of rupture in posterior communicating artery aneurysms: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2023, 231, 107815. [Google Scholar] [CrossRef]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.L.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.; Chong, W.; Duan, C.Z.; Zhang, X.; Morgan, M.; Qian, Y. A preliminary investigation of radiomics differences between ruptured and unruptured intracranial aneurysms. Eur. Radiol. 2021, 31, 2716–2725. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, P.; Jiang, Y.; Ge, H.; Li, S.; Jin, H.; Li, Y. Prediction of aneurysm stability using a machine learning model based on PyRadiomics-derived morphological features. Stroke 2019, 50, 2314–2321. [Google Scholar] [CrossRef]
- Maniaci, A.; Lavalle, S.; Gagliano, C.; Lentini, M.; Masiello, E.; Parisi, F.; Iannella, G.; Cilia, N.D.; Salerno, V.; Cusumano, G.; et al. The integration of radiomics and artificial intelligence in modern medicine. Life 2024, 14, 1248. [Google Scholar] [CrossRef]
- Dhar, S.; Tremmel, M.; Mocco, J.; Kim, M.; Yamamoto, J.; Siddiqui, A.H.; Hopkins, L.N.; Meng, H. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 2008, 63, 185–196, discussion 196. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, M.L.; Ma, B.; Harbaugh, R.E. Quantified aneurysm shape and rupture risk. J. Neurosurg. 2005, 102, 355–362. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.; Liang, S.; Yan, P.; Liang, F.; Guo, F.; Jiang, C. Morphologic feature elongation can predict occlusion status following pipeline embolization of intracranial aneurysms. World Neurosurg. 2018, 119, e934–e940. [Google Scholar] [CrossRef]
- Baharoglu, M.I.; Lauric, A.; Gao, B.L.; Malek, A.M. Identification of a dichotomy in morphological predictors of rupture status between sidewall- and bifurcation-type intracranial aneurysms. J. Neurosurg. 2012, 116, 871–881. [Google Scholar] [CrossRef]
- Xiang, J.; Natarajan, S.K.; Tremmel, M.; Ma, D.; Mocco, J.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I.; Meng, H. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke 2011, 42, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Barkeij Wolf, J.J.H.; Foster-Dingley, J.C.; Moonen, J.E.F.; van Osch, M.J.P.; de Craen, A.J.M.; de Ruijter, W.; van der Mast, R.C.; van der Grond, J. Unilateral fetal-type circle of Willis anatomy causes right-left asymmetry in cerebral blood flow with pseudo-continuous arterial spin labeling: A limitation of arterial spin labeling-based cerebral blood flow measurements? J. Cereb. Blood Flow Metab. 2016, 36, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.E.; Koivisto, T.; Björkman, J.; von Und Zu Fraunberg, M.; Helin, K.; Jääskeläinen, J.E.; Frösen, J. Irregular shape of intracranial aneurysm indicates rupture risk irrespective of size in a population-based cohort. Stroke 2016, 47, 1219–1226. [Google Scholar] [CrossRef]
- Duan, Z.; Li, Y.; Guan, S.; Ma, C.; Han, Y.; Ren, X.; Wei, L.; Li, W.; Lou, J.; Yang, Z. Morphological parameters and anatomical locations associated with rupture status of small intracranial aneurysms. Sci. Rep. 2018, 8, 6440. [Google Scholar] [CrossRef]
- Benemerito, I.; Ewbank, F.; Narracott, A.; Villa-Uriol, M.C.; Narata, A.P.; Patel, U.; Bulters, D.; Marzo, A. Computational fluid dynamics and shape analysis enhance aneurysm rupture risk stratification. Int. J. Comput. Assist. Radiol. Surg. 2024, 20, 31–41. [Google Scholar] [CrossRef]
| Parameters | Ruptured (n = 39) | Unruptured (n = 48) | p-Value | AUC | Univariate Analysis |
|---|---|---|---|---|---|
| Sex (Male) | 6 (15.4%) | 7 (14.6%) | 0.917 | 0.496 | 0.917 |
| Age (years, mean ± SD) | 63.18 ± 11.77 | 65.56 ± 11.1 | 0.168 | 0.436 | 0.283 |
| BMI (kg/m2) | 22.44 ± 3.05 | 23.16 ± 3.74 | 0.159 | 0.458 | 0.546 |
| Hypertension | 23 (59.0%) | 32 (66.7%) | 0.459 | 0.462 | 0.459 |
| Diabetes mellitus | 2 (5.1%) | 12 (25.0%) | 0.012 | 0.401 | 0.012 |
| Hyperlipidemia | 10 (25.6%) | 16 (33.3%) | 0.436 | 0.462 | 0.436 |
| Smoking | 9 (23.1%) | 8 (16.7%) | 0.413 | 0.534 | 0.413 |
| Parameters | Ruptured (n = 39) | Unruptured (n = 48) | p-Value | Univariate Analysis |
|---|---|---|---|---|
| Neck (mm) | 4.47 ± 1.89 | 4.09 ± 1.39 | 0.156 | 0.450 |
| Height (mm) | 6.62 ± 3.37 | 5.24 ± 2.62 | 0.020 | 0.480 |
| Width (mm) | 5.86 ± 2.98 | 4.71 ± 2.22 | 0.024 | 0.480 |
| Size maximum (mm) | 8.13 ± 3.96 | 6.7 ± 3.11 | 0.035 | 0.419 |
| Area (mm2) | 171.58 ± 151.47 | 114.16 ± 100.43 | 0.023 | 0.450 |
| Volume (mm3) | 191.58 ± 249.19 | 103.41 ± 130.92 | 0.025 | 0.450 |
| Height/Width | 1.14 ± 0.21 | 1.12 ± 0.28 | 0.371 | 0.537 |
| Flow angle (°) | 116.94 ± 25.38 | 107.77 ± 28.3 | 0.058 | 0.390 |
| Inclination angle (°) | 97.78 ± 24.93 | 98.9 ± 18.97 | 0.409 | 0.513 |
| Patent artery angle (°) | 111.98 ± 26.7 | 111.33 ± 25.42 | 0.454 | 0.513 |
| Vessel angle (°) | 50.42 ± 33.39 | 37.94 ± 29.45 | 0.036 | 0.481 |
| Proximal diameter of parent artery (mm) | 4.65 ± 0.92 | 4.68 ± 0.72 | 0.446 | 0.390 |
| Distal diameter of parent artery (mm) | 3.47 ± 0.74 | 3.47 ± 0.71 | 0.494 | 0.419 |
| Aspect ratio | 1.52 ± 0.65 | 1.28 ± 0.53 | 0.036 | 0.527 |
| Size ratio | 2.86 ± 6.12 | 1.59 ± 2.92 | 0.119 | 0.633 |
| Bottleneck factor | 1.34 ± 0.53 | 1.15 ± 0.4 | 0.034 | 0.303 |
| Fetal PCA | 29 (74.4%) | 14 (29.2%) | <0.001 | <0.001 |
| Parameters | Ruptured (n = 39) | Unruptured (n = 48) | p-Value | Univariate Analysis |
|---|---|---|---|---|
| Convex Hull Volume (Vch) (mm3) | 213.11 ± 274.69 | 116.57 ± 157.31 | 0.028 | 0.450 |
| Convex Hull Surface Area (Sch) (mm2) | 157.1 ± 142.41 | 106.5 ± 96.23 | 0.031 | 0.450 |
| Non-sphericity Index | 0.37 ± 0.4 | 0.33 ± 0.7 | 0.003 | 0.216 |
| Ellipticity Index | 0.26 ± 0.02 | 0.26 ± 0.03 | 0.462 | 0.205 |
| Undulation Index | 0.08 ± 0.07 | 0.05 ± 0.07 | 0.031 | 0.253 |
| Elongation | 0.72 ± 0.15 | 0.75 ± 0.14 | 0.128 | 0.334 |
| Flatness | 0.6 ± 0.14 | 0.62 ± 0.13 | 0.227 | 0.655 |
| LeastAxisLength (mm) | 4.54 ± 2.22 | 3.7 ± 1.59 | 0.228 | 0.450 |
| MajorAxisLength (mm) | 7.67 ± 3.19 | 6.17 ± 2.84 | 0.022 | 0.450 |
| Maximum2DDiameterColumn (mm) | 7.6 ± 3.43 | 6.5 ± 2.64 | 0.026 | 0.439 |
| Maximum2DDiameterRow (mm) | 8.18 ± 3.99 | 6.42 ± 3.01 | 0.012 | 0.461 |
| Maximum2DDiameterSlice (mm) | 7.83 ± 3.52 | 6.34 ± 2.89 | 0.013 | 0.525 |
| Maximum3DDiameter (mm) | 10.3 ± 5.54 | 7.34 ± 3.11 | 0.047 | 0.480 |
| MeshVolume | 191.84 ± 249 | 103.47 ± 130.89 | 0.052 | 0.450 |
| MinorAxisLength (mm) | 5.35 ± 2.43 | 4.44 ± 1.81 | 0.010 | 0.450 |
| Sphericity | 0.77 ± 0.05 | 0.8 ± 0.05 | 0.013 | 0.234 |
| Surfacearea1 (mm2) | 171.66 ± 151.4 | 114.57 ± 100.11 | 0.016 | 0.450 |
| SurfaceVolumeRatio | 1.62 ± 0.91 | 1.76 ± 0.69 | 0.018 | 0.388 |
| Non-Fetal PCA (n = 49) | Fetal PCA (n = 38) | |||||
|---|---|---|---|---|---|---|
| Parameters | Ruptured (n = 15) | Unruptured (n = 34) | p-Value | Ruptured (n = 24) | Unruptured (n = 14) | p-Value |
| Neck (mm) | 4.22 ± 1.92 | 4.01 ± 1.58 | 0.356 | 4.62 ± 1.9 | 4.31 ± 0.8 | 0.243 |
| Height (mm) | 6.83 ± 3.54 | 4.88 ± 2.57 | 0.034 | 6.48 ± 3.33 | 6.11 ± 2.63 | 0.355 |
| Width (mm) | 6.03 ± 3.2 | 4.62 ± 2.39 | 0.070 | 5.76 ± 2.9 | 4.93 ± 1.8 | 0.142 |
| Size maximum (mm) | 8.48 ± 4.29 | 6.47 ± 3.24 | 0.059 | 7.91 ± 3.82 | 7.25 ± 2.78 | 0.274 |
| Area (mm2) | 190.84 ± 167.08 | 107.94 ± 103.1 | 0.046 | 159.55 ± 143.25 | 129.26 ± 95.59 | 0.220 |
| Volume (mm3) | 223.19 ± 272.07 | 95.91 ± 126.53 | 0.051 | 171.82 ± 237.64 | 121.65 ± 144.31 | 0.212 |
| Height/Width | 1.14 ± 0.18 | 1.08 ± 0.28 | 0.175 | 1.14 ± 0.23 | 1.23 ± 0.27 | 0.146 |
| Flow angle (°) | 116.97 ± 23.27 | 104.7 ± 29.66 | 0.064 | 116.93 ± 27.11 | 115.24 ± 24.04 | 0.422 |
| Inclination angle (°) | 100.27 ± 22.46 | 96.53 ± 18.03 | 0.288 | 96.22 ± 26.71 | 104.66 ± 20.65 | 0.142 |
| Patent artery angle (°) | 122.7 ± 21.13 | 113.06 ± 23.83 | 0.084 | 105.28 ± 28 | 107.14 ± 29.45 | 0.425 |
| Vessel angle (°) | 41.83 ± 35.75 | 37.7 ± 31.54 | 0.351 | 55.78 ± 31.39 | 38.51 ± 24.71 | 0.035 |
| Proximal diameter of parent artery (mm) | 4.4 ± 0.85 | 4.58 ± 0.67 | 0.234 | 4.81 ± 0.95 | 4.9 ± 0.8 | 0.374 |
| Distal diameter of parent artery (mm) | 3.49 ± 0.7 | 3.52 ± 0.68 | 0.457 | 3.45 ± 0.78 | 3.34 ± 0.81 | 0.344 |
| Aspect ratio | 1.68 ± 0.8 | 1.22 ± 0.49 | 0.027 | 1.42 ± 0.53 | 1.43 ± 0.62 | 0.475 |
| Size ratio | 2.63 ± 5.06 | 1.85 ± 3.43 | 0.295 | 3.01 ± 6.8 | 0.96 ± 0.64 | 0.078 |
| Bottleneck factor | 1.46 ± 0.61 | 1.14 ± 0.39 | 0.040 | 1.26 ± 0.47 | 1.16 ± 0.43 | 0.255 |
| Non-Fetal PCA (n = 49) | Fetal PCA (n = 38) | |||||
|---|---|---|---|---|---|---|
| Parameters | Ruptured (n = 15) | Unruptured (n = 34) | p-Value | Ruptured (n = 24) | Unruptured (n = 14) | p-Value |
| Convex Hull Volume (Vch) (mm3) | 253.89 ± 307.17 | 107.85 ± 148.58 | 0.049 | 187.62 ± 255.87 | 137.75 ± 180.96 | 0.244 |
| Convex Hull Surface Area (Sch) (mm2) | 176.5 ± 159.17 | 99.48 ± 94.74 | 0.049 | 144.97 ± 132.99 | 123.55 ± 101.26 | 0.290 |
| Non-sphericity Index | 3.8 ± 0.5 | 3.3 ± 0.7 | 0.003 | 03.7 ± 0.4 | 3.3 ± 0.6 | 0.019 |
| Ellipticity Index | 0.26 ± 0.02 | 0.26 ± 0.03 | 0.482 | 0.26 ± 0.02 | 0.26 ± 0.02 | 0.443 |
| Undulation Index | 0.11 ± 0.09 | 0.05 ± 0.07 | 0.011 | 0.06 ± 0.05 | 0.06 ± 0.06 | 0.491 |
| Elongation | 0.72 ± 0.13 | 0.75 ± 0.15 | 0.236 | 0.71 ± 0.16 | 0.76 ± 0.14 | 0.174 |
| Flatness | 0.61 ± 0.12 | 0.62 ± 0.14 | 0.383 | 0.6 ± 0.15 | 0.64 ± 0.1 | 0.193 |
| LeastAxisLength (mm) | 4.73 ± 2.56 | 3.53 ± 1.64 | 0.056 | 4.43 ± 2.03 | 4.13 ± 1.45 | 0.297 |
| MajorAxisLength (mm) | 7.7 ± 3.45 | 5.97 ± 2.94 | 0.053 | 7.65 ± 3.08 | 6.66 ± 2.61 | 0.151 |
| Maximum2DDiameterColumn (mm) | 7.7 ± 3.8 | 6.26 ± 2.75 | 0.101 | 7.54 ± 3.26 | 7.07 ± 2.35 | 0.305 |
| Maximum2DDiameterRow (mm) | 8.2 ± 4.05 | 6.3 ± 3.13 | 0.060 | 8.17 ± 4.04 | 6.7 ± 2.78 | 0.096 |
| Maximum2DDiameterSlice (mm) | 7.84 ± 3.67 | 5.97 ± 2.86 | 0.047 | 7.82 ± 3.5 | 7.24 ± 2.85 | 0.289 |
| Maximum3DDiameter (mm) | 9.26 ± 4.41 | 7.18 ± 3.25 | 0.058 | 10.96 ± 6.14 | 7.75 ± 2.81 | 0.037 |
| MeshVolume (mm3) | 223.27 ± 272.01 | 95.98 ± 126.49 | 0.051 | 172.2 ± 237.39 | 121.66 ± 144.31 | 0.210 |
| MinorAxisLength (mm) | 5.46 ± 2.71 | 4.27 ± 1.9 | 0.069 | 5.28 ± 2.29 | 4.87 ± 1.53 | 0.258 |
| Sphericity | 0.77 ± 0.06 | 0.8 ± 0.06 | 0.077 | 0.78 ± 0.04 | 0.81 ± 0.04 | 0.025 |
| Surface Area (mm2) | 190.84 ± 167.08 | 108.53 ± 102.68 | 0.047 | 159.67 ± 143.14 | 129.26 ± 95.59 | 0.219 |
| SurfaceVolumeRatio | 1.69 ± 1.16 | 1.86 ± 0.71 | 0.296 | 1.57 ± 0.73 | 1.51 ± 0.56 | 0.383 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, K.; Nahm, M.; Ko, S.-W.; Yi, H.-J.; Chun, H.-J.; Lee, Y.-J.; Lee, S.H.; Ryu, J.; Song, S.; Choi, K.-S. Influence of Fetal-Type Posterior Cerebral Artery on Morphological Characteristics and Rupture Risk of Posterior Communicating Artery Aneurysms: A Radiomics Approach. J. Clin. Med. 2025, 14, 3682. https://doi.org/10.3390/jcm14113682
Han K, Nahm M, Ko S-W, Yi H-J, Chun H-J, Lee Y-J, Lee SH, Ryu J, Song S, Choi K-S. Influence of Fetal-Type Posterior Cerebral Artery on Morphological Characteristics and Rupture Risk of Posterior Communicating Artery Aneurysms: A Radiomics Approach. Journal of Clinical Medicine. 2025; 14(11):3682. https://doi.org/10.3390/jcm14113682
Chicago/Turabian StyleHan, Kunhee, Minu Nahm, Shin-Woong Ko, Hyeong-Joong Yi, Hyoung-Joon Chun, Young-Jun Lee, Sang Hyung Lee, Jaiyoung Ryu, Simon Song, and Kyu-Sun Choi. 2025. "Influence of Fetal-Type Posterior Cerebral Artery on Morphological Characteristics and Rupture Risk of Posterior Communicating Artery Aneurysms: A Radiomics Approach" Journal of Clinical Medicine 14, no. 11: 3682. https://doi.org/10.3390/jcm14113682
APA StyleHan, K., Nahm, M., Ko, S.-W., Yi, H.-J., Chun, H.-J., Lee, Y.-J., Lee, S. H., Ryu, J., Song, S., & Choi, K.-S. (2025). Influence of Fetal-Type Posterior Cerebral Artery on Morphological Characteristics and Rupture Risk of Posterior Communicating Artery Aneurysms: A Radiomics Approach. Journal of Clinical Medicine, 14(11), 3682. https://doi.org/10.3390/jcm14113682






