Cracking the LUTS Code: A Pre-Urodynamic Tool for DU vs. BOO Diagnosis in Female Patients with Non-Neurogenic LUTS
Abstract
:1. Introduction
2. Materials and Methods
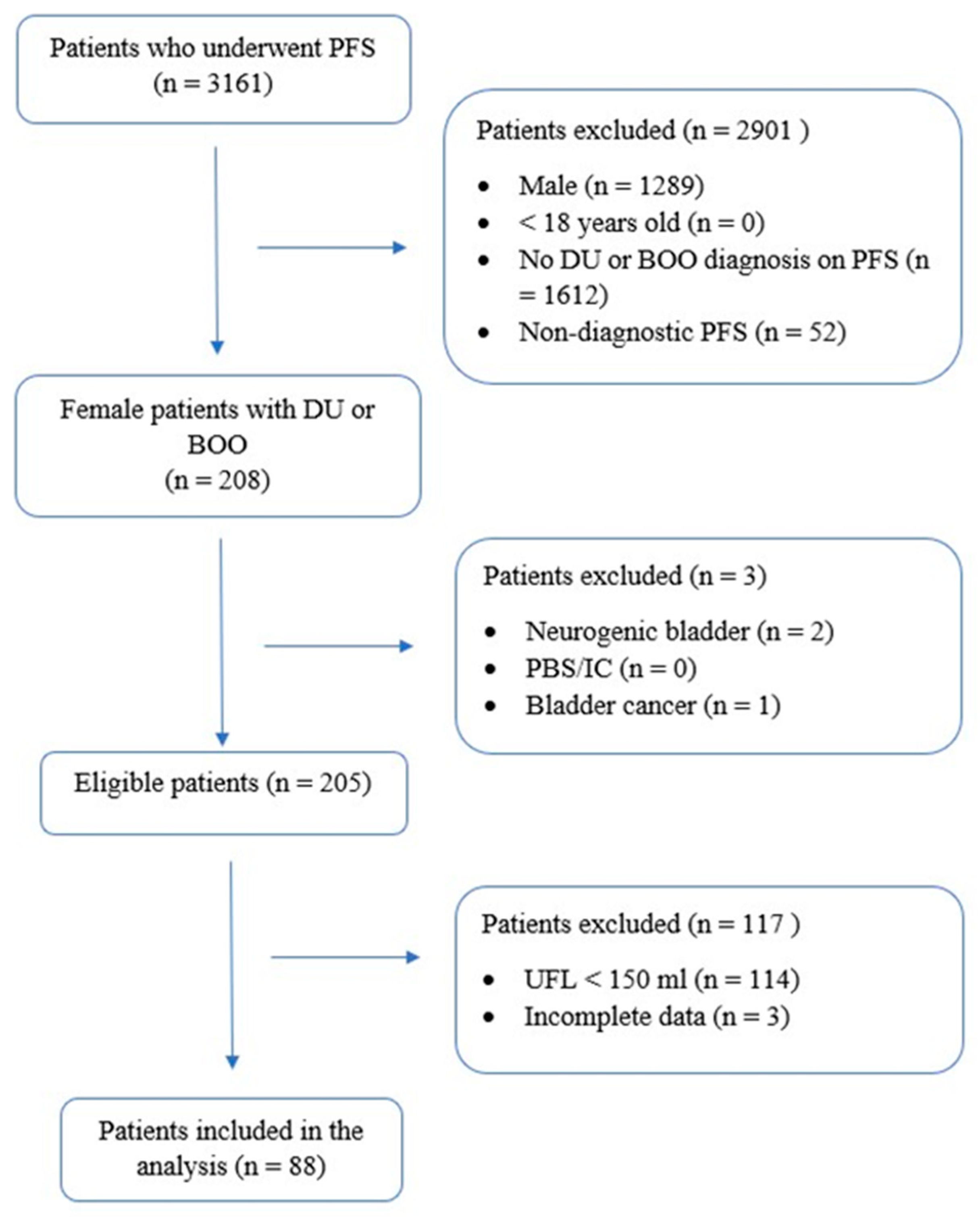
2.1. Patient Selection and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Uni- and Multivariate Logistic Regression Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DU | Detrusor Underactivity |
| VD | Voiding Dysfunction |
| LUTS | Lower Urinary Tract Symptoms |
| BOO | Bladder Outlet Obstruction |
| UDS | Urodynamic Studies |
| ICS | International Continence Society |
| SUFU | Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction |
| BCI | Bladder Contractility Index |
| ICS-DCI | ICS-Detrusor Contractility Index |
| DVC | Detrusor Voiding Contraction |
| D[B]CI | Detrusor/Bladder Contractility Index |
| PIP1 | Projected Isovolumetric Pressure 1 |
| BOOIf | Female-Specific Bladder Outlet Obstruction Index |
| BOOI | Bladder Outlet Obstruction Index |
| ICS-PFS | Provisional ICS Pressure-Flow Study (Nomogram) |
| CLSS | Core Lower Urinary Tract Symptoms (questionnaire) |
| POP-Q | Pelvic Organ Prolapse Quantification |
| PFS | Pressure-Flow Studies |
| UFL | Urine Flow Test (Uroflowmetry) |
| PVR | Post-Void Residual |
| PVR-R | Post-Void Residual Ratio |
| IQR | Interquartile Range |
| OR | Odds Ratio |
| CI | Confidence Interval |
| PPV | Positive Predictive Value |
| NPV | Negative Predictive Value |
References
- Wu, C.J.; Hsiao, S.M.; Wu, P.C.; Chang, T.C.; Chen, C.H.; Sheu, B.C.; Lin, H.H. Prevalence and predictors of detrusor underactivity and bladder outlet obstruction in women with lower urinary tract symptoms. Sci. Rep. 2024, 14, 25141. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Hsiao, S.; Lin, H. Age-specific prevalence, clinical and urodynamic findings of detrusor underactivity and bladder outlet obstruction in female voiding dysfunction. Int. J. Gynecol. Obstet. 2024, 167, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Kitta, T.; Wada, N.; Shinohara, S.; Hayashi, N.; Yamamura, H.; Yamamoto, T.; Takagi, H.; Hatakeyama, T.; Nagabuchi, M.; Morishita, S.; et al. Validation of the area under the Watts factor curve during the voiding cycle as a novel parameter for diagnosing detrusor underactivity in females. Int. J. Urol. 2024, 31, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- ICS Standards 2024. Available online: https://www.ics.org/Publications/ICS%20Standards%202024.pdf (accessed on 20 October 2024).
- Rosier, P.F.W.M.; Valdevenito, J.P.; Smith, P.; Sinha, S.; Speich, J.; Gammie, A.; Members of the ICS Working Group PFS23. ICS-SUFU standard: Theory, terms, and recommendations for pressure-flow studies performance, analysis, and reporting. Part 1: Background theory and practice. Neurourol. Urodyn. 2023, 42, 1590–1602. [Google Scholar] [CrossRef]
- Rosier, P.F.W.M.; Gammie, A.; Valdevenito, J.P.; Speich, J.; Smith, P.; Sinha, S. ICS-SUFU standard: Theory, terms, and recommendations for pressure-flow studies performance, analysis, and reporting, Part 2: Analysis of PFS, reporting, and diagnosis. Continence 2023, 7, 100709. [Google Scholar] [CrossRef]
- Valentini, F.A.; Marti, B.G.; Robain, G.; Zimern, P.E.; Nelson, P.P. Comparison of indices allowing an evaluation of detrusor contractility in women. Progrès Urol. 2020, 30, 396–401. [Google Scholar] [CrossRef]
- Lindsay, J.; Solomon, E.; Nadeem, M.; Pakzad, M.; Hamid, R.; Ockrim, J.; Greenwell, T. Treatment validation of the Solomon-Greenwell nomogram for female bladder outlet obstruction. Neurourol. Urodyn. 2020, 39, 1371–1377. [Google Scholar] [CrossRef]
- Homma, Y.; Yoshida, M.; Yamanishi, T.; Gotoh, M. Core Lower Urinary Tract Symptom Score (CLSS) questionnaire: A reliable tool in the overall assessment of lower urinary tract symptoms. Int. J. Urol. 2008, 15, 816–820. [Google Scholar] [CrossRef]
- Cornu (Chair), J.N.; Gacci, M.; Hashim, H.; Herrmann, T.R.W.; Malde, S.; Netsch, C.; De Nunzio, C.; Rieken, M.; Sakalis, V.; Tutolo, M.; et al. Eau Guidelines on the Management of Non-Neurogenic Male LUTS; EAU: Arnhem, The Netherlands, 2024; ISBN 978-94-92671-23-3. [Google Scholar]
- Garbas, K.; Zapała, Ł.; Ślusarczyk, A.; Piecha, T.; Gwara, P.; Żuk-Łapan, A.; Piekarczyk, H.; Zapała, P.; Radziszewski, P. (A)voiding misdiagnosis: Prediction of detrusor underactivity vs. bladder outlet obstruction using pre-urodynamic nomogram in male patients with LUTS. Int. Urol. Nephrol. 2024, 56, 3485–3494. [Google Scholar] [CrossRef]
- Bump, R.C. The POP-Q system: Two decades of progress and debate. Int. Urogynecol. J. 2014, 25, 441–443. [Google Scholar] [CrossRef]
- Schäfer, W.; Abrams, P.; Liao, L.; Mattiasson, A.; Pesce, F.; Spangberg, A.; Sterling, A.M.; Zinner, N.R.; Kerrebroeck, P.V. Good urodynamic practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol. Urodyn. 2002, 21, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Kalil, J.; D’Ancona, C.A.L. Detrusor underactivity versus bladder outlet obstruction clinical and urodynamic factors. Int. Braz. J. Urol. 2020, 46, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Qu, L.G.; Gani, J. Urodynamic findings in patients with nocturia and their associations with patient characteristics. Can. Urol. Assoc. J. 2022, 16, E455. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Palmieri, S.; Cola, A.; Barba, M.; Manodoro, S.; Frigerio, M. Detrusor underactivity prevalence and risk factors according to different definitions in women attending urogynecology clinic. Int. Urogynecol. J. 2022, 33, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Gammie, A.; Kaper, M.; Dorrepaal, C.; Kos, T.; Abrams, P. Signs and Symptoms of Detrusor Underactivity: An Analysis of Clinical Presentation and Urodynamic Tests From a Large Group of Patients Undergoing Pressure Flow Studies. Eur. Urol. 2016, 69, 361–369. [Google Scholar] [CrossRef]
- Rubilotta, E.; Gubbiotti, M.; Herms, A.; Goldman, H.; Antonelli, A.; Balzarro, M. Detrusor underactivity in symptomatic anterior pelvic organ prolapse. Cent Eur. J. Urol. 2024, 77, 77–81. [Google Scholar]
- Cheng, Y.; Li, T.; Wu, X.; Du, G.; Xu, S. A novel predictive model for noninvasively diagnosing bladder outlet obstruction in female patients based on clinical features and uroflowmetry parameters. Int. J. Gynecol. Obstet. 2024, 166, 655–662. [Google Scholar] [CrossRef]
- Szmydki, D.; Burzy, B.; Sołtysiak-Gibała, Z.; Przymuszała, P.; Trzewik, M.; Chudek, J.; Owczarek, A.J. Prediction of detrusor underactivity based on non-invasive functional tests and clinical data in patients with symptoms of bladder outlet obstruction. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10992–10998. [Google Scholar]
- Apostolidis, A. Can We Predict a Diagnosis of Detrusor Underactivity (DUA) or Bladder Outlet Obstruction in Women by Non-invasive Parameters? 2017. Available online: https://www.morressier.com/article/59b63e40d462b80292389481 (accessed on 4 March 2025).
- Kocadag, H.; Toia, B.; Axell, R.; Yasmin, H.; Pakzad, M.H.; Hamid, R.; Greenwell, T.J.; Ockrim, J.L. A comparison of flow rate curve shape and video-urodynamic findings in women with lower urinary tract symptoms: Can flow rate curve shape predict female bladder outflow obstruction or detrusor underactivity? World J. Urol. 2021, 39, 1591–1595. [Google Scholar] [CrossRef]
- Çetinel, B.; Önal, B.; Selçuk, B.; Can, G.; Aferin, U.; Yıldırım, Ö. Urodynamic Curve Patterns may Predict Female Bladder Outlet Obstruction and Detrusor Underactivity. Urology 2022, 165, 150–156. [Google Scholar] [CrossRef]
- Soedarman, S.; Rahardjo, H.E. Potential predictors of detrusor underactivity in a urology outpatient clinic: A 5-year single center experience study. Med. J. Indones. 2021, 30, 207–210. [Google Scholar] [CrossRef]
| BOO (n = 50) | DU (n = 38) | ||||||
|---|---|---|---|---|---|---|---|
| No. of Patients/Median | % of Patients/IQR | No. of Patients/Median | % of Patients/IQR | p-Value | |||
| Age | Years | 56 | 42–64 | 59.5 | 44–69 | 0.22 | |
| Symptoms | Urgency | 37 | 42.05 | 27 | 30.68 | 0.76 | |
| Frequency | 33 | 37.50 | 24 | 27.27 | 0.78 | ||
| Nocturia | 37 | 42.05 | 24 | 27.27 | 0.27 | ||
| Weak stream | 21 | 23.86 | 19 | 21.59 | 0.46 | ||
| Hesitancy | 11 | 12.50 | 14 | 15.91 | 0.13 | ||
| Intermittency | 11 | 12.50 | 7 | 7.95 | 0.68 | ||
| Straining | 5 | 5.68 | 7 | 7.95 | 0.25 | ||
| Incomplete emptying | 17 | 19.32 | 20 | 22.73 | 0.08 | ||
| Dribble | 0 | 0 | 1 | 1.14 | 0.25 | ||
| UUI | 23 | 26.14 | 21 | 23.86 | 0.39 | ||
| SUI | 25 | 28.41 | 16 | 18.18 | 0.46 | ||
| Pads (daytime) | 0 | 0–3 | 0 | 0–3 | 0.36 | ||
| Pads (nighttime) | 0 | 0–0 | 0 | 0–0 | 0.62 | ||
| CLSS questionnaire | Frequency | points | 2 | 0–2 | 2 | 0–2 | 0.74 |
| Nocturia | points | 2 | 1–2 | 2 | 1–2 | 0.62 | |
| Urgency | points | 2 | 0–2 | 2 | 1–3 | 0.32 | |
| Slow stream | points | 3 | 0–3 | 0 | 0–3 | 0.35 | |
| Straining | points | 0 | 0–0 | 0 | 0–0 | 0.16 | |
| POP assessment | POP-Q < 3 | 37 | 42.05 | 35 | 39.77 | 0.03 | |
| Delivery method | Vaginal birth | 2 | 1–2 | 2 | 0–2 | 0.10 | |
| Cesarean section | 0 | 0–0 | 0 | 0–0 | 0.44 | ||
| BOO (n = 50) | DU (n = 38) | ||||||
|---|---|---|---|---|---|---|---|
| UFL Parameters | Qmax | mL/s | No. of Patients/Median | % of Patients/IQR | No. of Patients/Median | % of Patients/IQR | p-Value |
| Voided volume | mL | 16.6 | 11.6–20.5 | 15.5 | 10.9–20.1 | 0.65 | |
| Qav | mL/s | 279 | 202–406 | 274.5 | 207–446 | 0.97 | |
| Qmax–Qav difference | mL/s | 6.8 | 5.3–9.8 | 6.3 | 5.5–9.3 | 0.65 | |
| Voiding time | s | 8 | 5.7–11.4 | 7.5 | 5.9–10.4 | 0.7 | |
| Time to Qmax | s | 47.5 | 29–74 | 44 | 35–64 | 0.99 | |
| PVR | mL | 9 | 6–13 | 15 | 6–26 | 0.06 | |
| PVR ratio | 17.5 | 10–100 | 45 | 10–200 | 0.25 | ||
| UFL curve shapes | Bell-shape | 0.07 | 0.03–0.26 | 0.09 | 0.04–0.34 | 0.17 | |
| Fluctuating | 8 | 9.09 | 6 | 6.82 | 0.98 | ||
| Intermittent | 21 | 23.86 | 18 | 20.45 | 0.62 | ||
| Fluctuating-Intermittent | 0 | 0 | 0 | 0 | |||
| Plateau | 17 | 19.32 | 13 | 14.77 | 0.98 | ||
| Pressure-flow study parameters | Qmax | mL/s | 13 | 14.77 | 8 | 9.09 | 0.59 |
| Pdetmax | cm H2O | 9.7 | 7.4–12.5 | 3.45 | 0–7.9 | <0.0001 | |
| Pdet@Qmax | cm H2O | 64 | 51–113 | 12.5 | 7–21 | <0.0001 | |
| PIP1 | 54.5 | 40–80 | 9 | 7–18 | <0.0001 | ||
| BOOIf | 62.9 | 49.7–89.1 | 14.25 | 7.4–21.9 | <0.0001 | ||
| 34.8 | 20.4–60 | 3.2 | 0–7 | <0.0001 | |||
| Detrusor Underactivity | ||||||
|---|---|---|---|---|---|---|
| Variable | OR | 0.99–1.04 | 0.32 | |||
| Age | Urgency | yes vs. no | 1.01 | 0.34–2.21 | 0.76 | |
| Symptoms | Frequency | Urgency | yes vs. no | 0.86 | 0.37–2.13 | 0.78 |
| Nocturia | Frequency | yes vs. no | 0.88 | 0.24–1.50 | 0.28 | |
| Weak stream | Nocturia | yes vs. no | 0.60 | 0.59–3.23 | 0.46 | |
| Hesitancy | Weak stream | yes vs. no | 1.38 | 0.81–5.29 | 0.13 | |
| Intermittency | Hesitancy | yes vs. no | 2.07 | 0.28–2.31 | 0.68 | |
| Straining | Intermittency | yes vs. no | 0.80 | 0.59–6.99 | 0.26 | |
| Incomplete emptying | Straining | yes vs. no | 2.03 | 0.91–5.12 | 0.08 | |
| Dribble | Incomplete emptying | yes vs. no | 2.16 | 0.001–999.99 | 0.99 | |
| UUI | Dribble | yes vs. no | >999.99 | 0.62–3.38 | 0.39 | |
| SUI | UUI | yes vs. no | 1.45 | 0.31–1.70 | 0.46 | |
| Pads (daytime) | SUI | points | 0.73 | 0.66–1.43 | 0.87 | |
| CLSS questionnaire | Pads (nighttime) | Frequency | points | 0.97 | 0.74–1.67 | 0.62 |
| Frequency | Nocturia | points | 1.11 | 0.78–1.76 | 0.44 | |
| Nocturia | Urgency | points | 1.17 | 0.71–1.73 | 0.64 | |
| Urgency | UUI | points | 1.11 | 0.78–1.60 | 0.56 | |
| Slow stream | SUI | points | 1.11 | 0.65–1.16 | 0.34 | |
| Straining | Slow stream | points | 0.87 | 0.88–1.99 | 0.18 | |
| POP-Q < 3 | Straining | points | 1.32 | 0.81–1.45 | 0.60 | |
| Incomplete emptying | continuous | 1.08 | 0.95–1.05 | 0.90 | ||
| UFL | Qmax | continuous | 0.99 | 0.99–1.00 | 0.77 | |
| Voided volume | continuous | 1.0 | 0.87–1.08 | 0.58 | ||
| Qav | continuous | 0.97 | 0.94–1.09 | 0.84 | ||
| Qmax–Qav difference | continuous | 1.01 | 0.99–1.01 | 0.63 | ||
| Voiding time | continuous | 0.99 | 1.00–1.06 | 0.04 | ||
| Time to Qmax | continuous | 1.03 | 0.99–1.00 | 0.49 | ||
| PVR | continuous | 1.00 | 0.37–24.82 | 0.30 | ||
| PVR ratio | yes vs. no | 3.02 | 0.31–3.12 | 0.99 | ||
| UFL curve shapes | Bell-shape | yes vs. no | 0.98 | 0.53–2.91 | 0.62 | |
| Fluctuating | yes vs. no | 1.24 | 0.42–2.46 | 0.98 | ||
| Fluctuating-intermittent | yes vs. no | 1.01 | 0.28–2.07 | 0.59 | ||
| Vaginal birth | Plateau | yes vs. no | 0.76 | 1.08–15.62 | 0.04 | |
| POP-Q scale | Cesarean section | POP-Q < 3 | yes vs. no | 4.10 | 0.21–4.69 | 0.99 |
| DM | yes vs. no | 0.99 | 0.14–5.49 | 0.88 | ||
| Chronic diseases | Recurring UTIs | yes vs. no | 0.87 | 0.24–31.19 | 0.42 | |
| Hashimoto | yes vs. no | 2.72 | 0.41–4.66 | 0.61 | ||
| Hypothyroidism | yes vs. no | 1.38 | 95% CI | p-value | ||
| Detrusor Underactivity | |||||
|---|---|---|---|---|---|
| Variable | OR | 95% CI | p-Value | ||
| Symptoms | Hesitancy | 0 | ref | - | |
| 1 | 2.06 | 0.71–5.98 | 0.18 | ||
| Incomplete emptying | 0 | ref | - | ||
| 1 | 3.52 | 1.27–9.79 | 0.02 | ||
| POP-Q scale | POPQ < 3 | 0 | ref | - | |
| 1 | 0.15 | 0.03–0.75 | 0.02 | ||
| UFL parameters | Time to Qmax | 1.05 | 1.02–1.09 | 0.004 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbas, K.; Zapała, Ł.; Ślusarczyk, A.; Piecha, T.; Radziszewski, P. Cracking the LUTS Code: A Pre-Urodynamic Tool for DU vs. BOO Diagnosis in Female Patients with Non-Neurogenic LUTS. J. Clin. Med. 2025, 14, 3674. https://doi.org/10.3390/jcm14113674
Garbas K, Zapała Ł, Ślusarczyk A, Piecha T, Radziszewski P. Cracking the LUTS Code: A Pre-Urodynamic Tool for DU vs. BOO Diagnosis in Female Patients with Non-Neurogenic LUTS. Journal of Clinical Medicine. 2025; 14(11):3674. https://doi.org/10.3390/jcm14113674
Chicago/Turabian StyleGarbas, Karolina, Łukasz Zapała, Aleksander Ślusarczyk, Tomasz Piecha, and Piotr Radziszewski. 2025. "Cracking the LUTS Code: A Pre-Urodynamic Tool for DU vs. BOO Diagnosis in Female Patients with Non-Neurogenic LUTS" Journal of Clinical Medicine 14, no. 11: 3674. https://doi.org/10.3390/jcm14113674
APA StyleGarbas, K., Zapała, Ł., Ślusarczyk, A., Piecha, T., & Radziszewski, P. (2025). Cracking the LUTS Code: A Pre-Urodynamic Tool for DU vs. BOO Diagnosis in Female Patients with Non-Neurogenic LUTS. Journal of Clinical Medicine, 14(11), 3674. https://doi.org/10.3390/jcm14113674






