A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity
Abstract
1. Introduction
2. Methods
3. Pre-, Pro-, Syn-, and Postbiotics
3.1. Prebiotics
3.2. Probiotics
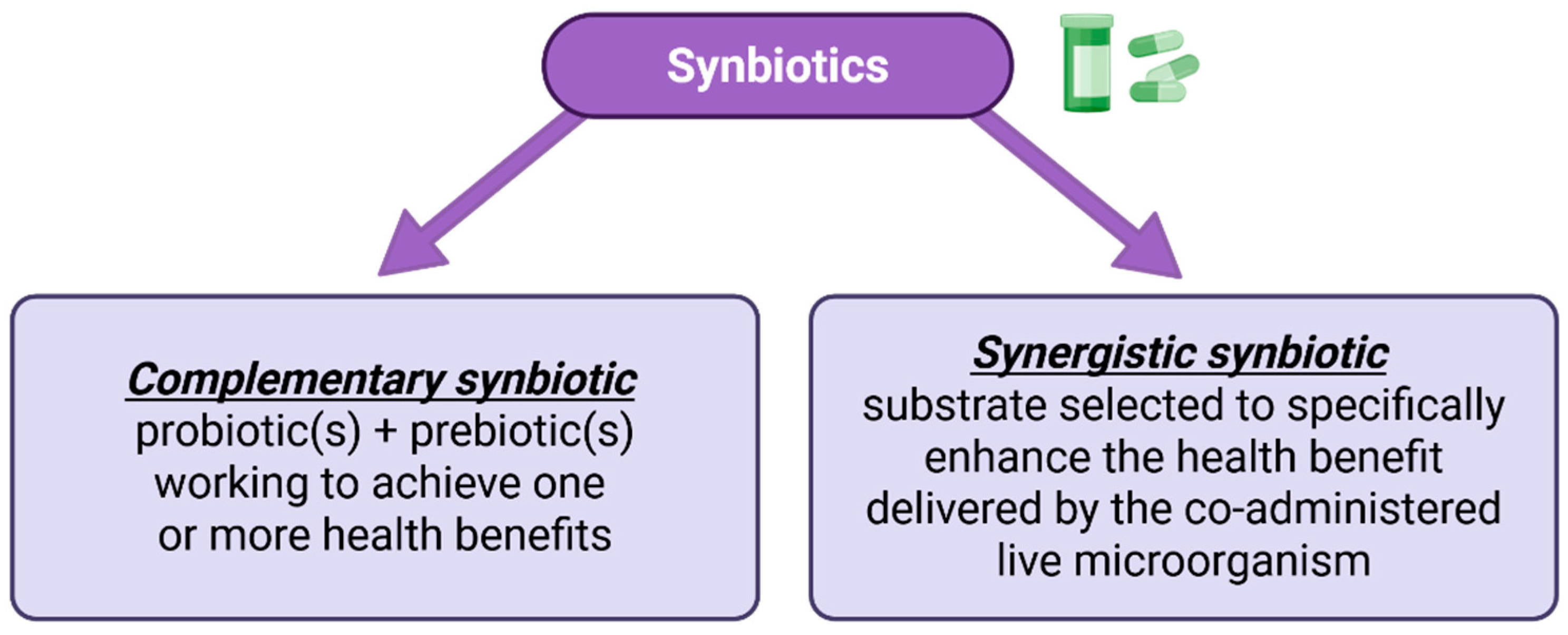
3.3. Synbiotics
3.4. Postbiotics
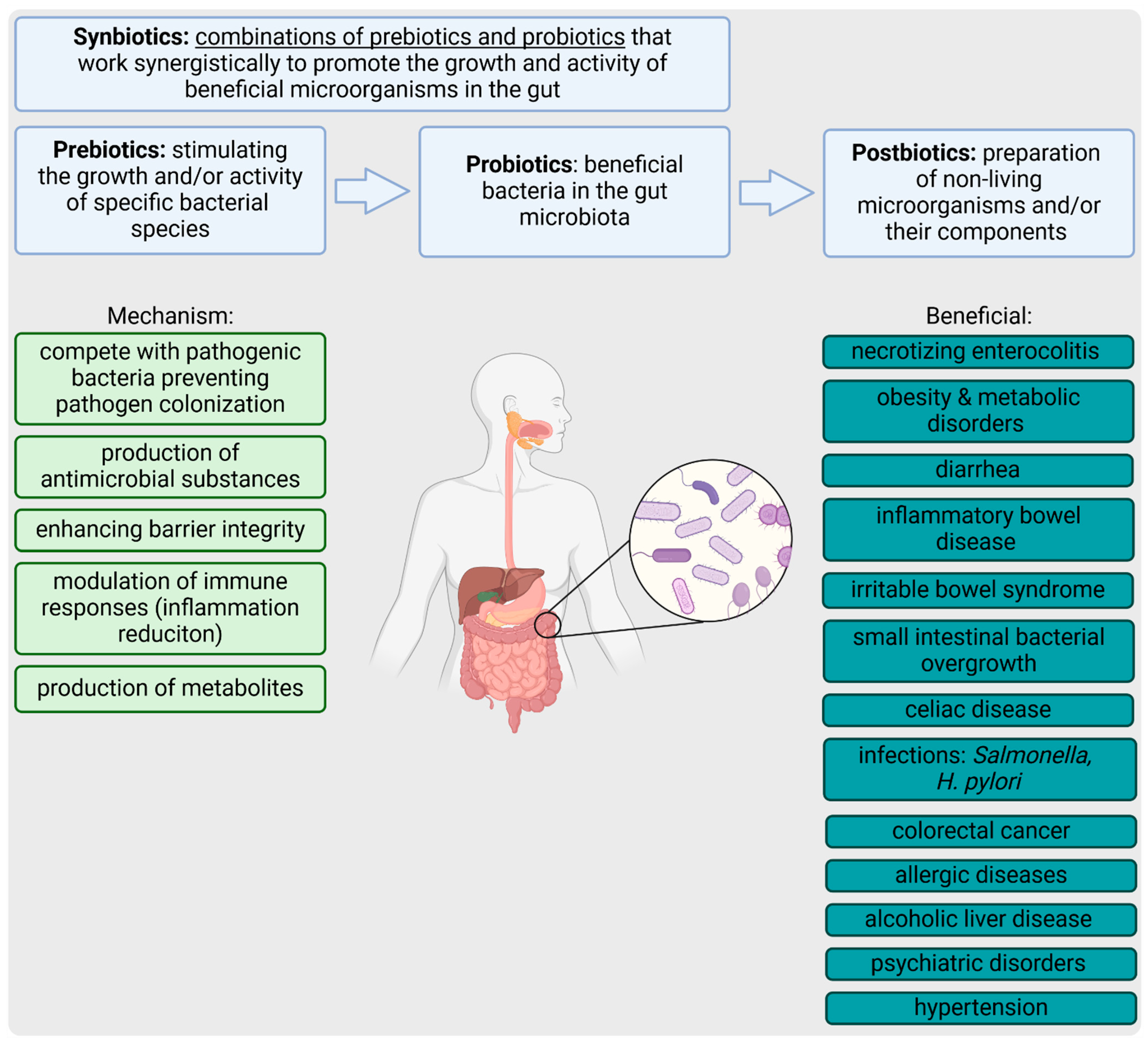
4. Gut Health and Disease Management by Pre-, Pro-, Syn-, and Postbiotics
5. Biotics and Intestinal Epithelial Integrity
6. Limitations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAD | antibiotic-associated diarrhea |
| BB-12 | bifidobacterium animalis subsp. lactis strain bb-12 |
| EPS | exopolysaccharide |
| FDA | food and drug administration |
| FFAR2/3 | free fatty acid receptor 2/3 |
| FFAs | free fatty acids |
| FOSs | fructooligosaccharides |
| GALT | gut-associated lymphoid tissue |
| GLP-1 | glucagon-like peptide-1 |
| GOSs | Galactooligosaccharides |
| GPCRs | g-protein coupled receptors |
| GPR109A | hydroxycarboxylic acid receptor hcar2 |
| GPR41/43 | g protein-coupled receptor 41/43 |
| HDACs | histone deacetylases |
| HIF | hypoxia-inducible factor |
| IBD | inflammatory bowel disease |
| ICs | intestinal cells |
| IEB | intestinal epithelial barier |
| IL | Interleukin |
| ILC3 | innate lymphoid cells type 3 |
| INF | intestinal inflammation |
| ISAPPs | international scientific association for probiotics and prebiotics |
| ITFs | inulin-type fructans |
| LTA | lipoteichoic acid |
| MAPKs | mitogen-activated protein kinases |
| MUC2 | Mucin-2 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated b cells |
| NGPs | next-generation probiotics |
| PDX | Polydextrose |
| PP | plasma protein |
| PS | Postbiotics |
| PYY | peptide YY |
| SCFAs | short-chain fatty acids |
| SIBO | small intestinal bacterial overgrowth |
| TJ | tight junction |
| TNF-α | tumor necrosis factor alpha |
| ZO-1 | zonula occludens-1 |
References
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Odriozola, A.; González, A.; Odriozola, I.; Álvarez-Herms, J.; Corbi, F. Microbiome-based precision nutrition: Prebiotics, probiotics and postbiotics. Adv. Genet. 2024, 111, 237–310. [Google Scholar] [PubMed]
- Wang, G.; Ding, T.; Ai, L. Editorial: Effects and mechanisms of probiotics, prebiotics, synbiotics and postbiotics on intestinal health and disease. Front. Cell. Infect. Microbiol. 2024, 14, 1430312. [Google Scholar] [CrossRef]
- Precision Business Insights. Probiotics, Prebiotics, and Postbiotics Market. Available online: www.precisionbusinessinsights.com/market-reports/probiotics-prebiotics-and-postbiotics-market (accessed on 5 August 2024).
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Cunningham, M.; Hill, C. Frequently asked questions about the ISAPP postbiotic definition. Front. Microbiol. 2023, 14, 1324565. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Kocot, A.M.; Jarocka-Cyrta, E.; Drabińska, N. Overview of the Importance of Biotics in Gut Barrier Integrity. Int. J. Mol. Sci. 2022, 23, 2896. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Kandasamy, S.; Chattha, K.S.; Rajashekara, G.; Saif, L.J. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet. Immunol. Immunopathol. 2016, 172, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Turroni, S.; Centanni, M.; Fiori, J.; Bergmann, S.; Hammerschmidt, S.; Brigidi, P. Relevance of Bifidobacterium animalis subsp. lactis plasminogen binding activity in the human gastrointestinal microenvironment. Appl. Environ. Microbiol. 2011, 77, 7072–7076. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Palaria, A.; Johnson-Kanda, I.; O’Sullivan, D.J. Effect of a synbiotic yogurt on levels of fecal bifidobacteria, clostridia, and enterobacteria. Appl. Environ. Microbiol. 2012, 78, 933–940. [Google Scholar] [CrossRef]
- Son, S.; Koh, J.; Park, M.; Ryu, S.; Lee, W.; Yun, B.; Lee, J.-H.; Oh, S.; Kim, Y. Effect of the Lactobacillus rhamnosus strain GG and tagatose as a synbiotic combination in a dextran sulfate sodium-induced colitis murine model. J. Dairy Sci. 2019, 102, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Xiao, S.D.; De Zhang, Z.; Lu, H.; Jiang, S.H.; Liu, H.Y.; Wang, G.S.; Xu, G.M.; Zhang, Z.B.; Lin, G.L.; Wang, G.L. Multicenter, randomized, controlled trial of heat-killed Lactobacillus acidophilus LB in patients with chronic diarrhea. Adv Ther. 2003, 20, 253–260. [Google Scholar] [CrossRef]
- Andresen, V.; Gschossmann, J.; Layer, P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: A multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol. Hepatol. 2020, 5, 658–666. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- da Silva, T.F.; Casarotti, S.N.; de Oliveira, G.L.V.; Penna, A.L.B. The impact of probiotics, prebiotics, and synbiotics on the biochemical, clinical, and immunological markers, as well as on the gut microbiota of obese hosts. Crit. Rev. Food Sci. Nutr. 2021, 61, 337–355. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-biotics, and Post-biotics. Front. Nutr. 2021, 8, 634897. [Google Scholar] [CrossRef] [PubMed]
- Basnet, J.; Eissa, M.A.; Cardozo, L.L.Y.; Romero, D.G.; Rezq, S. Impact of Probiotics and Prebiotics on Gut Microbiome and Hormonal Regulation. Gastrointest. Disord. 2024, 6, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Yu, X.; Luo, Y.; Chen, B.; Ma, D.; Zhu, J. Effect of Fructooligosaccharides Supplementation on the Gut Microbiota in Human: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3298. [Google Scholar] [CrossRef]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X.-H. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Do Carmo, M.M.R.; Walker, J.C.L.; Novello, D.; Caselato, V.M.; Sgarbieri, V.C.; Ouwehand, A.C.; Andreollo, N.A.; Hiane, P.A.; Dos Santos, E.F. Polydextrose: Physiological Function, and Effects on Health. Nutrients 2016, 8, 553. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Śliżewska, K.; Barczyńska, R.; Kapuśniak, J. Effects of Resistant Dextrin from Potato Starch on the Growth Dynamics of Selected Co-Cultured Strains of Gastrointestinal Bacteria and the Activity of Fecal Enzymes. Nutrients 2022, 14, 2158. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, T.; Shi, L.; Wang, Y.; Deng, X.; Wang, J.; Zhou, Y. The synbiotic combination of probiotics and inulin improves NAFLD though modulating gut microbiota. J. Nutr. Biochem. 2024, 125, 109546. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Hong, Q.; Yu, X.; Wang, H.; Shi, X.; Liu, W.; Yuan, T.; Tu, Z. Dynamic changes in volatile profiles and bacterial communities during natural fermentation of Mei yu, traditional Chinese fermented fish pieces. Food Res. Int. 2024, 194, 114882. [Google Scholar] [CrossRef] [PubMed]
- Tawfick, M.M.; Xie, H.; Zhao, C.; Shao, P.; Farag, M.A. Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. Int. J. Biol. Macromol. 2022, 208, 948–961. [Google Scholar] [CrossRef]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; A Story, J.; MacDonald-Clarke, C.J.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M. Soluble Corn Fiber Increases Calcium Absorption Associated with Shifts in the Gut Microbiome: A Randomized Dose-Response Trial in Free-Living Pubertal Females. J. Nutr. 2016, 146, 1298–1306. [Google Scholar] [CrossRef]
- Tekin, T.; Dincer, E. Effect of resistant starch types as a prebiotic. Appl. Microbiol. Biotechnol. 2022, 107, 491–515. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.; Romeiro, C.; Petriz, B.; Cavichiolli, N.; Almeida, J.A.; Castro, A.; Franco, O.L. Endurance exercise associated with a fructooligosaccharide diet modulates gut microbiota and increases colon absorptive area. J. Gastroenterol. Hepatol. 2024, 39, 1145–1154. [Google Scholar] [CrossRef]
- Parhi, P.; Song, K.P.; Choo, W.S. Growth and survival of Bifidobacterium breve and Bifidobacterium longum in various sugar systems with fructooligosaccharide supplementation. J. Food Sci. Technol. 2022, 59, 3775–3786. [Google Scholar] [CrossRef]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kosikowski, W.; Szczerbiński, M.; Gantzel, J.; Cukrowska, B. The Effectiveness of Synbiotic Preparation Containing Lactobacillus and Bifidobacterium Probiotic Strains and Short Chain Fructooligosaccharides in Patients with Diarrhea Predominant Irritable Bowel Syndrome—A Randomized Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, 1999. [Google Scholar] [CrossRef]
- Hu, Y.; Aljumaah, M.R.; Azcarate-Peril, M.A. Galacto-Oligosaccharides and the Elderly Gut: Implications for Immune Restoration and Health. Adv. Nutr. Int. Rev. J. 2024, 15, 100263. [Google Scholar] [CrossRef]
- Chen, T.; Wang, C.; Nie, C.; Yuan, X.; Tu, A.; Li, J. Galactooligosaccharide or 2′-Fucosyllactose Modulates Gut Microbiota and Inhibits LPS/TLR4/NF-kappaB Signaling Pathway to Prevent DSS-Induced Colitis Aggravated by a High-Fructose Diet in Mice. J. Agric. Food Chem. 2023, 71, 9349–9360. [Google Scholar] [CrossRef]
- Kjølbæk, L.; Benítez-Páez, A.; del Pulgar, E.M.G.; Brahe, L.K.; Liebisch, G.; Matysik, S.; Rampelli, S.; Vermeiren, J.; Brigidi, P.; Larsen, L.H.; et al. Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial. Clin. Nutr. 2020, 39, 67–79. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Śliżewska, K. Efficiency of Resistant Starch and Dextrins as Prebiotics: A Review of the Existing Evidence and Clinical Trials. Nutrients 2021, 13, 3808. [Google Scholar] [CrossRef]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef]
- Zaman, S.A.; Sarbini, S.R. The potential of resistant starch as a prebiotic. Crit. Rev. Biotechnol. 2016, 36, 578–584. [Google Scholar] [CrossRef]
- Ansari, F.; Pimentel, T.C.; Pourjafar, H.; Ibrahim, S.A.; Jafari, S.M. The Influence of Prebiotics on Wheat Flour, Dough, and Bread Properties; Resistant Starch, Polydextrose, and Inulin. Foods 2022, 11, 3366. [Google Scholar] [CrossRef]
- Park, M.; Lee, H.-B.; Kim, H.R.; Kang, M.-C.; Jeong, D.; Choi, H.-D.; Hong, J.S.; Park, H.-Y. Resistant starch-enriched brown rice exhibits prebiotic properties and enhances gut health in obese mice. Food Res. Int. 2024, 187, 114417. [Google Scholar] [CrossRef]
- Hijová, E.; Bertková, I.; Štofilová, J. Dietary fibre as prebiotics in nutrition. Central Eur. J. Public Health 2019, 27, 251–255. [Google Scholar] [CrossRef]
- Devi, R.; Sharma, E.; Thakur, R.; Lal, P.; Kumar, A.; Altaf, M.A.; Singh, B.; Tiwari, R.K.; Lal, M.K.; Kumar, R. Non-dairy prebiotics: Conceptual relevance with nutrigenomics and mechanistic understanding of the effects on human health. Food Res. Int. 2023, 170, 112980. [Google Scholar] [CrossRef]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Dreher, M.L. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Naseer, M.; Poola, S.; Uraz, S.; Tahan, V. Therapeutic Effects of Prebiotics on Constipation: A Schematic Review. Curr. Clin. Pharmacol. 2020, 15, 207–215. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Vaughan, E.E. Probiotic and gut lactobacilli and bifidobacteria: Molecular approaches to study diversity and activity. Annu. Rev. Microbiol. 2009, 63, 269–290. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Sinigaglia, M.; Corbo, M.R. An Update on Prebiotics and on Their Health Effects. Foods 2024, 13, 446. [Google Scholar] [CrossRef]
- Filidou, E.; Kandilogiannakis, L.; Shrewsbury, A.; Kolios, G.; Kotzampassi, K. Probiotics: Shaping the gut immunological responses. World J. Gastroenterol. 2024, 30, 2096–2108. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, C.; Li, R.; Zheng, M.; Feng, B.; Gao, J.; Long, X.; Li, L.; Li, S.; Zuo, X.; et al. Lactobacillus-derived indole-3-lactic acid ameliorates colitis in cesarean-born offspring via activation of aryl hydrocarbon receptor. iScience 2023, 26, 108279. [Google Scholar] [CrossRef]
- Hrdý, J.; Couturier-Maillard, A.; Boutillier, D.; Lapadatescu, C.; Blanc, P.; Procházka, J.; Pot, B.; Ryffel, B.; Grangette, C.; Chamaillard, M. Oral supplementation with selected Lac-tobacillus acidophilus triggers IL-17-dependent innate defense response, activation of innate lymphoid cells type 3 and improves colitis. Sci. Rep. 2022, 12, 17591. [Google Scholar] [CrossRef]
- Chen, Z.; Yi, L.; Pan, Y.; Long, X.; Mu, J.; Yi, R.; Zhao, X. Lactobacillus fermentum ZS40 Ameliorates Inflammation in Mice with Ulcerative Colitis Induced by Dextran Sulfate Sodium. Front. Pharmacol. 2021, 12, 700217. [Google Scholar] [CrossRef]
- De Gregorio, A.; Serafino, A.; Krasnowska, E.K.; Superti, F.; Di Fazio, M.R.; Fuggetta, M.P.; Ferri, I.H.; Fiorentini, C. Protective Effect of Limosilactobacillus fermentum ME-3 against the Increase in Paracellular Permeability Induced by Chemotherapy or Inflammatory Conditions in Caco-2 Cell Models. Int. J. Mol. Sci. 2023, 24, 6225. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Belguesmia, Y.; Bendali, F.; Spano, G.; Seal, B.S.; Drider, D. Lactobacillus fermentum: A bacterial species with potential for food preservation and biomedical applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Mirpuri, J.; Sotnikov, I.; Myers, L.; Denning, T.L.; Yarovinsky, F.; Parkos, C.A.; Denning, P.W.; Louis, N.A. Lactobacillus rhamnosus (LGG) regulates IL-10 signaling in the developing murine colon through upregulation of the IL-10R2 receptor subunit. PLoS ONE 2012, 7, e51955. [Google Scholar] [CrossRef]
- Zheng, J.; Ahmad, A.A.; Yang, Y.; Liang, Z.; Shen, W.; Feng, M.; Shen, J.; Lan, X.; Ding, X. Lactobacillus rhamnosus CY12 Enhances Intestinal Barrier Function by Regulating Tight Junction Protein Expression, Oxidative Stress, and Inflammation Response in Lipopolysaccharide-Induced Caco-2 Cells. Int. J. Mol. Sci. 2022, 23, 11162. [Google Scholar] [CrossRef]
- Carbonne, C.; Chadi, S.; Kropp, C.; Molimard, L.; Chain, F.; Langella, P.; Martin, R. Ligilactobacillus salivarius CNCM I-4866, a potential probiotic candidate, shows anti-inflammatory properties in vitro and in vivo. Front. Microbiol. 2023, 14, 1270974. [Google Scholar] [CrossRef]
- Abramov, V.M.; Kosarev, I.V.; Machulin, A.V.; Deryusheva, E.I.; Priputnevich, T.V.; Panin, A.N.; Chikileva, I.O.; Abashina, T.N.; Manoyan, A.M.; Ahmetzyanoya, A.A.; et al. Ligilactobacillus salivarius 7247 Strain: Probiotic Properties and Anti-Salmonella Effect with Prebiotics. Antibiotics 2023, 12, 1535. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Jiang, L.; Han, D.; Si, D.; Sun, Z.; Wu, Z.; Dai, Z. Limosilactobacillus mucosae and Lactobacillus amylovorus Protect Against Experimental Colitis via Upregulation of Colonic 5-Hydroxytryptamine Receptor 4 and Transforming Growth Factor-β2. J. Nutr. 2023, 153, 2512–2522. [Google Scholar] [CrossRef]
- Li, W.; Kai, L.; Jiang, Z.; He, H.; Yang, M.; Su, W.; Wang, Y.; Jin, M.; Lu, Z. Bifidobacterium longum, Lactobacillus plantarum and Pediococcus acidilactici Reversed ETEC-Inducing Intestinal Inflammation in Mice. Microorganisms 2022, 10, 2350. [Google Scholar] [CrossRef] [PubMed]
- Fitri, L.E.; Sardjono, T.W.; Winaris, N.; Pawestri, A.R.; Endharti, A.T.; Norahmawati, E.; Handayani, D.; Kurniawan, S.N.; Azizah, S.; Alifia, L.I.; et al. Bifidobacterium longum Administration Diminishes Parasitemia and Inflammation During Plasmodium berghei Infection in Mice. J. Inflamm. Res. 2023, 16, 1393–1404. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Zhou, L.; Zhang, Y.; Yu, W.; Li, S.; Gao, J. Bifidobacterium breve Protects the Intestinal Epithelium and Mitigates Inflammation in Colitis via Regulating the Gut Microbiota–Cholic Acid Pathway. J. Agric. Food Chem. 2024, 72, 3572–3583. [Google Scholar] [CrossRef]
- Park, I.S.; Kim, J.H.; Yu, J.; Shin, Y.; Kim, K.; Kim, T.I.; Kim, S.W.; Cheon, J.H. Bifidobacterium breve CBT BR3 is effective at relieving intestinal inflammation by augmenting goblet cell regeneration. J. Gastroenterol. Hepatol. 2023, 38, 1346–1354. [Google Scholar] [CrossRef]
- Qu, D.; Yu, L.; Tian, F.; Zhang, H.; Chen, W.; Gu, Z.; Zhai, Q. Bifidobacterium bifidum FJSWX19M5 alleviated 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced chronic colitis by mitigating gut barrier injury and increasing regulatory T cells. Food Funct. 2023, 14, 181–194. [Google Scholar] [CrossRef]
- Nian, F.; Wu, L.; Xia, Q.; Tian, P.; Ding, C.; Lu, X. Akkermansia muciniphila and Bifidobacterium bifidum Prevent NAFLD by Regulating FXR Expression and Gut Microbiota. J. Clin. Transl. Hepatol. 2023, 11, 763–776. [Google Scholar] [CrossRef]
- Kil, B.J.; Pyung, Y.J.; Park, H.; Kang, J.-W.; Yun, C.-H.; Huh, C.S. Probiotic potential of Saccharomyces cerevisiae GILA with alleviating intestinal inflammation in a dextran sulfate sodium induced colitis mouse model. Sci. Rep. 2023, 13, 6687. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, X.; Liang, L.; Wang, X.; Bai, X.; Zhu, L.; He, Q.; Liang, H.; Xin, X.; Wang, L.; et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021, 12, 777665. [Google Scholar] [CrossRef]
- Wang, M.; Gao, C.; Lessing, D.J.; Chu, W. Saccharomyces cerevisiae SC-2201 Attenuates AOM/DSS-Induced Colorectal Cancer by Modulating the Gut Microbiome and Blocking Proinflammatory Mediators. Probiotics Antimicrob. Proteins 2024, 17, 1523–1535. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liang, X.; Lei, J.; Shen, F.; Yang, F.; Tang, C. Enterococcus faecium inhibits NF-kappaB/NLRP3/IL-1beta signaling pathway and antagonizes Salmonella-mediated inflammatory response. Future Microbiol. 2024, 19, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Pu, S.; Liu, J.; Yang, F.; Chen, D. Enterococcus faecium inhibits NF-kappaB/NLRP3/Caspase-1 signaling pathway to an-tagonize enterotoxigenic Escherichia coli-mediated inflammatory response. Can. J. Microbiol. 2024, 70, 109–118. [Google Scholar] [CrossRef]
- Ferro, L.E.; Crowley, L.N.; Bittinger, K.; Friedman, E.S.; Decker, J.E.; Russel, K.; Katz, S.; Kim, J.K.; Trabulsi, J.C. Effects of prebiotics, probiotics, and synbiotics on the infant gut microbiota and other health outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5620–5642. [Google Scholar] [CrossRef]
- Wieers, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.M.; Dequenne, I.; de Timary, T.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell Infect. Microbiol. 2019, 9, 454. [Google Scholar]
- Rau, S.; Gregg, A.; Yaceczko, S.; Limketkai, B. Prebiotics and Probiotics for Gastrointestinal Disorders. Nutrients 2024, 16, 778. [Google Scholar] [CrossRef] [PubMed]
- Al-Fakhrany, O.M.; Elekhnawy, E. Next-generation probiotics: The upcoming biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Nguyen, P.-T.; Pham, M.-N.; Razafindralambo, H.; Hoang, Q.-K.; Nguyen, H.-T. Synbiotics: A New Route of Self-production and Applications to Human and Animal Health. Probiotics Antimicrob. Proteins 2022, 14, 980–993. [Google Scholar] [CrossRef] [PubMed]
- Gomez Quintero, D.F.; Kok, C.R.; Hutkins, R. The Future of Synbiotics: Rational Formulation and Design. Front. Microbiol. 2022, 13, 919725. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.-P.; Choi, H.-J.; Jeong, H.; Park, Y.-S. A comprehensive review of synbiotics: An emerging paradigm in health promotion and disease management. World J. Microbiol. Biotechnol. 2024, 40, 280. [Google Scholar] [CrossRef]
- Kuru-Yasar, R.; Ustun-Aytekin, O. The Crucial Roles of Diet, Microbiota, and Postbiotics in Colorectal Cancer. Curr. Nutr. Rep. 2024, 13, 126–151. [Google Scholar] [CrossRef] [PubMed]
- Kavita Om, H.; Chand, U.; Kushawaha, P.K. Postbiotics: An alternative and innovative intervention for the therapy of inflam-matory bowel disease. Microbiol. Res. 2024, 279, 127550. [Google Scholar] [CrossRef]
- Xie, W.; Zhong, Y.-S.; Li, X.-J.; Kang, Y.-K.; Peng, Q.-Y.; Ying, H.-Z. Postbiotics in colorectal cancer: Intervention mechanisms and perspectives. Front. Microbiol. 2024, 15, 1360225. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Kango, N.; Nath, S. Prebiotics, Probiotics and Postbiotics: The Changing Paradigm of Functional Foods. J. Diet. Suppl. 2024, 21, 709–735. [Google Scholar] [CrossRef]
- Calvo, L.N.; Greenberg, R.G.; Gray, K.D. Safety and Effectiveness of Probiotics in Preterm Infants with Necrotizing Enterocolitis. Neoreviews 2024, 25, e193–e206. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Denning, P.W. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: What is the current evidence? Clin. Perinatol. 2013, 40, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Underwood, M.A. Probiotics and necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 39–46. [Google Scholar] [CrossRef]
- Aguilera, X.E.L.; Manzano, A.; Pirela, D.; Bermúdez, V. Probiotics and Gut Microbiota in Obesity: Myths and Realities of a New Health Revolution. J. Pers. Med. 2022, 12, 1282. [Google Scholar] [CrossRef] [PubMed]
- Kober, A.K.M.H.; Saha, S.; Ayyash, M.; Namai, F.; Nishiyama, K.; Yoda, K.; Villena, J.; Kitazawa, H. Insights into the Anti-Adipogenic and Anti-Inflammatory Potentialities of Probiotics against Obesity. Nutrients 2024, 16, 1373. [Google Scholar] [CrossRef] [PubMed]
- Houttu, N.; Vahlberg, T.; Miles, E.A.; Calder, P.C.; Laitinen, K. The impact of fish oil and/or probiotics on serum fatty acids and the interaction with low-grade inflammation in pregnant women with overweight and obesity: Secondary analysis of a randomised controlled trial. Br. J. Nutr. 2024, 131, 296–311. [Google Scholar] [CrossRef]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med. Infect. Dis. 2007, 5, 97–105. [Google Scholar] [CrossRef]
- Yang, B.; Lu, P.; Li, M.-X.; Cai, X.-L.; Xiong, W.-Y.; Hou, H.-J.; Ha, X.-Q. A meta-analysis of the effects of probiotics and synbiotics in children with acute diarrhea. Medicine 2019, 98, e16618. [Google Scholar] [CrossRef]
- Martins, E.; da Silva, L.N.; Carmo, M. Probiotics, prebiotics, and synbiotics in childhood diarrhea. Braz. J. Med. Biol. Res. 2024, 57, e13205. [Google Scholar] [CrossRef]
- Liu, G.; Kragh, M.L.; Aabo, S.; Jensen, A.N.; Olsen, J.E. Inhibition of Virulence Gene Expression in Salmonella Dublin, Escherichia coli F5 and Clostridium perfringens Associated with Neonatal Calf Diarrhea by Factors Produced by Lactic Acid Bacteria During Fermentation of Cow Milk. Front. Microbiol. 2022, 13, 828013. [Google Scholar] [CrossRef]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of anti-biotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [PubMed]
- Saviano, A.; Petruzziello, C.; Cancro, C.; Macerola, N.; Petti, A.; Nuzzo, E.; Migneco, A.; Ojetti, V. The Efficacy of a Mix of Probiotics (Limosilactobacillus reuteri LMG P-27481 and Lacticaseibacillus rhamnosus GG ATCC 53103) in Preventing Antibiotic-Associated Diarrhea and Clostridium difficile Infection in Hospitalized Patients: Single-Center, Open-Label, Randomized Trial. Microorganisms 2024, 12, 198. [Google Scholar] [CrossRef]
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Tan, T.; Herbin Smith, K. Exploratory Pilot Studies to Demonstrate Mechanisms of Preventing Antibiotic-Associated Diarrhea and the Role for Probiotics. Ann. Fam. Med. 2024, 21, 4766. [Google Scholar]
- Lin, S.; Shen, Y. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: A systematic review and meta-analysis based on 23 randomized studies. Int. J. Surg. 2020, 84, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Thet, D.; Areepium, N.; Siritientong, T. Effects of Probiotics on Chemotherapy-induced Diarrhea. Nutr. Cancer 2023, 75, 1811–1821. [Google Scholar] [CrossRef]
- López-Gómez, L.; Alcorta, A.; Abalo, R. Probiotics and Probiotic-like Agents against Chemotherapy-Induced Intestinal Mucositis: A Narrative Review. J. Pers. Med. 2023, 13, 1487. [Google Scholar] [CrossRef]
- Du, Z.; Li, J.; Li, W.; Fu, H.; Ding, J.; Ren, G.; Zhou, L.; Pi, X.; Ye, X. Effects of prebiotics on the gut microbiota in vitro associated with functional diarrhea in children. Front. Microbiol. 2023, 14, 1233840. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Godoy-Brewer, G.; Shah, N.D.; Maas, L.; White, J.; Parian, A.M.; E Mullin, G. Prebiotics for Induction and Maintenance of Remission in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2024, 31, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Bertani, L.; Balestrini, L.; Chico, L.; DELLA Scala, G.; Geri, F.; Tornar, A.; Belcari, C. Specific probiotics and prebiotics to improve the quality of life of patients with chronic irritable bowel syndrome. Minerva Gastroenterol. 2024, 70, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.; Gordon, M.; Sinopoulou, V.; Akobeng, A.K. Probiotics for management of functional abdominal pain disorders in children. Cochrane Database Syst. Rev. 2023, 2, CD012849. [Google Scholar]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef]
- Gasiorowska, A.; Romanowski, M.; Walecka-Kapica, E.; Kaczka, A.; Chojnacki, C.; Padysz, M.; Siedlecka, M.; Bierta, J.B.; Steinert, R.E.; Cukrowska, B. Effects of Microencapsulated Sodium Butyrate, Probiotics and Short Chain Fructooligosaccharides in Patients with Irritable Bowel Syndrome: A Study Pro-tocol of a Randomized Double-Blind Placebo-Controlled Trial. J. Clin. Med. 2022, 11, 6587. [Google Scholar] [CrossRef]
- Schoemaker, M.H.; Hageman, J.H.J.; Haaf, D.T.; Hartog, A.; Scholtens, P.A.M.J.; Boekhorst, J.; Nauta, A.; Bos, R. Prebiotic Galacto-Oligosaccharides Impact Stool Frequency and Fecal Microbiota in Self-Reported Constipated Adults: A Randomized Clinical Trial. Nutrients 2022, 14, 309. [Google Scholar] [CrossRef]
- Marteau, P.; Jacobs, H.; Cazaubiel, M.; Signoret, C.; Prevel, J.-M.; Housez, B. Effects of chicory inulin in constipated elderly people: A double-blind controlled trial. Int. J. Food Sci. Nutr. 2011, 62, 164–170. [Google Scholar] [CrossRef]
- Erhardt, R.; E Harnett, J.; Steels, E.; Steadman, K.J. Functional constipation and the effect of prebiotics on the gut microbiota: A review. Br. J. Nutr. 2022, 130, 1015–1023. [Google Scholar] [CrossRef]
- Li, T.; Lu, X.; Yang, X. Evaluation of clinical safety and beneficial effects of stachyose-enriched α-galacto-oligosaccharides on gut microbiota and bowel function in humans. Food Funct. 2017, 8, 262–269. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef]
- Guo, Y.; Song, L.; Huang, Y.; Li, X.; Xiao, Y.; Wang, Z.; Ren, Z. Latilactobacillus sakei Furu2019 and stachyose as probiotics, prebiotics, and synbiotics alleviate constipation in mice. Front. Nutr. 2022, 9, 1039403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, R.; Li, D.; Zhao, L.; Zhu, L. Role of gut microbiota in functional constipation. Gastroenterol. Rep. 2021, 9, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Cha, J.M.; Oh, J.K.; Tan, P.L.; Kim, S.H.; Kwak, M.S.; Jeon, J.W.; Shin, H.P. Probiotics Ameliorate Stool Consistency in Patients with Chronic Constipation: A Randomized, Double-Blind, Placebo-Controlled Study. Dig. Dis. Sci. 2018, 63, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Li, Y.; He, Y.; Chen, F.; Mi, B.; Li, J.; Xie, J.; Ma, G.; Yang, J.; Xu, K.; et al. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: A double-blinded randomized placebo trial. Gut Microbes 2023, 15, 2197837. [Google Scholar] [CrossRef]
- Araújo, M.M.; Botelho, P.B. Probiotics, prebiotics, and synbiotics in chronic constipation: Outstanding aspects to be considered for the current evidence. Front. Nutr. 2022, 9, 935830. [Google Scholar] [CrossRef]
- Efremova, I.; Maslennikov, R.; Zharkova, M.; Poluektova, E.; Benuni, N.; Kotusov, A.; Demina, T.; Ivleva, A.; Adzhieva, F.; Krylova, T.; et al. Efficacy and Safety of a Probiotic Containing Saccharomyces boulardii CNCM I-745 in the Treatment of Small Intestinal Bacterial Overgrowth in Decompensated Cirrhosis: Randomized, Placebo-Controlled Study. J. Clin. Med. 2024, 13, 919. [Google Scholar] [CrossRef]
- Redondo-Cuevas, L.; Belloch, L.; Martín-Carbonell, V.; Nicolás, A.; Alexandra, I.; Sanchis, L.; Ynfante, M.; Colmenares, M.; Mora, M.; Liebana, A.R.; et al. Do Herbal Supplements and Probiotics Complement Antibiotics and Diet in the Management of SIBO? A Randomized Clinical Trial. Nutrients 2024, 16, 1083. [Google Scholar] [CrossRef]
- Marasco, G.; Cirota, G.G.; Rossini, B.; Lungaro, L.; Di Biase, A.R.; Colecchia, A.; Volta, U.; De Giorgio, R.; Festi, D.; Caio, G. Probiotics, Prebiotics and Other Dietary Supplements for Gut Microbiota Modulation in Celiac Disease Patients. Nutrients 2020, 12, 2674. [Google Scholar] [CrossRef]
- Wagh, S.K.; Lammers, K.M.; Padul, M.V.; Rodriguez-Herrera, A.; Dodero, V.I. Celiac Disease and Possible Dietary Interventions: From Enzymes and Probiotics to Postbiotics and Viruses. Int. J. Mol. Sci. 2022, 23, 11748. [Google Scholar] [CrossRef]
- Dias, T.G.; Rodrigues, L.D.S.; Farias, J.R.; Pereira, A.L.F.; Ferreira, A.G.N.; Neto, M.S.; Dutra, R.P.; Reis, A.S.; Guerra, R.N.M.; Monteiro-Neto, V.; et al. Immunomodulatory Activity of Probiotics in Models of Bacterial Infections. Probiotics Antimicrob. Proteins 2024, 16, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Salmonella infection—Prevention and treatment by antibiotics and probiotic yeasts: A review. Microbiology 2018, 164, 1327–1344. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Ferreira, R.; Azevedo, N.F.; Oleastro, M.; Azeredo, J.; Figueiredo, C.; Melo, L.D.R. Helicobacter pylori infection: From standard to alternative treatment strategies. Crit. Rev. Microbiol. 2022, 48, 376–396. [Google Scholar] [CrossRef]
- Bai, X.; Zhu, M.; He, Y.; Wang, T.; Tian, D.; Shu, J. The impacts of probiotics in eradication therapy of Helicobacter pylori. Arch. Microbiol. 2022, 204, 692. [Google Scholar] [CrossRef]
- Homan, M.; Orel, R. Are probiotics useful in Helicobacter pylori eradication? World J. Gastroenterol. 2015, 21, 10644–10653. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. The Gut Microbiota Influenced by the Intake of Probiotics and Functional Foods with Prebiotics Can Sustain Wellness and Alleviate Certain Ailments like Gut-Inflammation and Colon-Cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Alam, Z.; Shang, X.; Effat, K.; Kanwal, F.; He, X.; Li, Y.; Xu, C.; Niu, W.; War, A.R.; Zhang, Y. The potential role of prebiotics, probiotics, and synbiotics in adjuvant cancer therapy especially colorectal cancer. J. Food Biochem. 2022, 46, e14302. [Google Scholar] [CrossRef]
- Xiong, S.-Y.; Wu, G.-S.; Li, C.; Ma, W.; Luo, H.-R. Clinical efficacy of probiotics in the treatment of alcoholic liver disease: A systematic review and meta-analysis. Front. Cell. Infect. Microbiol. 2024, 14, 1358063. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Psallida, S.; Vythoulkas-Biotis, N.; Adamou, A.; Zachariadou, T.; Kargioti, S.; Karampela, I.; Dalamaga, M. NAFLD/MASLD and the Gut–Liver Axis: From Pathogenesis to Treatment Options. Metabolites 2024, 14, 366. [Google Scholar] [CrossRef]
- Steiner, N.C.; Lorentz, A. Probiotic Potential of Lactobacillus Species in Allergic Rhinitis. Int. Arch. Allergy Immunol. 2021, 182, 807–818. [Google Scholar] [CrossRef]
- Han, H.; Chen, G.; Zhang, B.; Zhang, X.; He, J.; Du, W.; Li, M.D. Probiotic Lactobacillus plantarum GUANKE effectively alleviates allergic rhinitis symptoms by modulating functions of various cytokines and chemokines. Front. Nutr. 2023, 10, 1291100. [Google Scholar] [CrossRef]
- Li, L.; Wen, X.; Gong, Y.; Chen, Y.; Xu, J.; Sun, J.; Deng, H.; Guan, K. HMGN2 and Histone H1.2: Potential targets of a novel probiotic mixture for seasonal allergic rhinitis. Front. Microbiol. 2023, 14, 1202858. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, H.; Awan, M.S.; Mustafa, K.; Das, J.K.; Ahmed, S.K. Role of Probiotics in Patients with Allergic Rhinitis: A Systematic Review of Systematic Reviews. Int. Arch. Otorhinolaryngol. 2022, 26, e744–e752. [Google Scholar] [CrossRef]
- Carucci, L.; Coppola, S.; Carandente, R.; Canani, R.B. Targeting Food Allergy with Probiotics. Adv. Exp. Med. Biol. 2024, 1449, 79–93. [Google Scholar]
- Lei, W.; Cheng, Y.; Gao, J.; Liu, X.; Shao, L.; Kong, Q.; Zheng, N.; Ling, Z.; Hu, W. Akkermansia muciniphila in neuropsychiatric disorders: Friend or foe? Front. Cell Infect. Microbiol. 2023, 13, 1224155. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, W.; Liang, J.; Dou, J.; Guo, F.; Zhang, D.; Xu, Z.; Wang, T. Probiotics: Functional food ingredients with the potential to reduce hypertension. Front. Cell. Infect. Microbiol. 2023, 13, 1220877. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; Macdonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Li, S.; Jiang, W.; Wang, J.; Xiao, J.; Chen, T.; Ma, J.; Khan, M.Z.; Wang, W.; et al. Unlocking the power of postbiotics: A revolutionary approach to nutrition for humans and animals. Cell Metab. 2024, 36, 725–744. [Google Scholar] [CrossRef]
- Zoghi, S.; Abbasi, A.; Heravi, F.S.; Somi, M.H.; Nikniaz, Z.; Moaddab, S.Y.; Leylabadlo, H.E. The gut microbiota and celiac disease: Pathophysiology, current perspective and new therapeutic approaches. Crit. Rev. Food Sci. Nutr. 2024, 64, 2176–2196. [Google Scholar] [CrossRef]
- Lê, A.; Mantel, M.; Marchix, J.; Bodinier, M.; Jan, G.; Rolli-Derkinderen, M. Inflammatory bowel disease therapeutic strategies by modulation of the microbiota: How and when to introduce pre-, pro-, syn-, or postbiotics? Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 323, G523–G553. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Cheng, L.; Wang, J.; Raghavan, V. Gut microbiome modulation by probiotics, prebiotics, synbiotics and postbiotics: A novel strategy in food allergy prevention and treatment. Crit. Rev. Food Sci. Nutr. 2024, 64, 5984–6000. [Google Scholar] [CrossRef] [PubMed]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Biliński, J.; Roviello, G.; Iannone, L.F.; Atzeni, A.; Sobocki, B.K.; Połom, K. Gut Microbiome Modulation and Faecal Microbiota Transplantation Following Allogenic Hematopoietic Stem Cell Transplantation. Cancers 2021, 13, 4665. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.F.; Kondrashina, A.; Walsh, C.; Busca, K.; Karawugodage, A.; Park, J.; Sirisena, S.; Martin, F.-P.; Felice, V.D.; Lane, J.A. Biotics as novel therapeutics in targeting signs of skin ageing via the gut-skin axis. Ageing Res. Rev. 2024, 102, 102518. [Google Scholar] [CrossRef]
- Balendra, V.; Rosenfeld, R.; Amoroso, C.; Castagnone, C.; Rossino, M.G.; Garrone, O.; Ghidini, M. Postbiotics as Adjuvant Therapy in Cancer Care. Nutrients 2024, 16, 2400. [Google Scholar] [CrossRef]
- Kumar, D.; Bishnoi, M.; Kondepudi, K.K.; Sharma, S.S. Gut Microbiota-Based Interventions for Parkinson’s Disease: Neuroprotective Mechanisms and Current Perspective. Probiotics Antimicrob. Proteins 2025, 2025, 1–23. [Google Scholar] [CrossRef]
- Lian, P.; Henricks, P.A.J.; Wichers, H.J.; Folkerts, G.; Braber, S. Differential Effects of Oligosaccharides, Antioxidants, Amino Acids and PUFAs on Heat/Hypoxia-Induced Epithelial Injury in a Caco-2/HT-29 Co-Culture Model. Int. J. Mol. Sci. 2023, 24, 1111. [Google Scholar] [CrossRef]
- Daguet, D.; Pinheiro, I.; Verhelst, A.; Possemiers, S.; Marzorati, M. Arabinogalactan and fructooligosaccharides improve the gut barrier function in distinct areas of the colon in the Simulator of the Human Intestinal Microbial Ecosystem. J. Funct. Foods 2016, 20, 369–379. [Google Scholar] [CrossRef]
- Wongkrasant, P.; Pongkorpsakol, P.; Ariyadamrongkwan, J.; Meesomboon, R.; Satitsri, S.; Pichyangkura, R.; Barrett, K.E.; Muanprasat, C. A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed. Pharmacother. 2020, 129, 110415. [Google Scholar] [CrossRef]
- Fernandez-Lainez, C.; Logtenberg, M.J.; Tang, X.; Schols, H.A.; Lopez-Velazquez, G.; de Vos, P. β(2-->1) chicory and β(2-->1)-β(2-->6) agave fructans protect the human intestinal barrier function in vitro in a stressor-dependent fashion. Food Funct. 2022, 13, 6737–6748. [Google Scholar] [CrossRef]
- Pham, V.T.; Calatayud, M.; Rotsaert, C.; Seifert, N.; Richard, N.; Abbeele, P.V.D.; Marzorati, M.; Steinert, R.E. Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro. Nutrients 2021, 13, 1125. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Chiang, H.-H.; Liu, C.-Y.; Li, Y.-J.; Lu, C.-L.; Lee, Y.-P.; Huang, C.-J.; Lai, C.-L. Intestinal Mucosal Barrier Improvement with Prebiotics: Histological Evaluation of Longish Glucomannan Hydrolysates-Induced Innate T Lymphocyte Activities in Mice. Nutrients 2022, 14, 2220. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Cui, H.; Zhang, J.; Wang, Q.; Duan, Z. Probiotic potential of Lacticaseibacillus rhamnosus VHProbi M15 on sucralfate-induced constipation in mice. Sci. Rep. 2024, 14, 1131. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Nie, S.-P.; Zhu, K.-X.; Xiong, T.; Li, C.; Gong, J.; Xie, M.-Y. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int. J. Food Sci. Nutr. 2015, 66, 533–538. [Google Scholar] [CrossRef]
- Araki, Y.; Fujiyama, Y.; Andoh, A.; Koyama, S.; Kanauchi, O.; Bamba, T. The dietary combination of germinated barley foodstuff plus Clostridium butyricum suppresses the dextran sulfate sodium-induced experimental colitis in rats. Scand. J. Gastroenterol. 2000, 35, 1060–1067. [Google Scholar]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- DiMattia, Z.; Damani, J.J.; Van Syoc, E.; Rogers, C.J. Effect of Probiotic Supplementation on Intestinal Permeability in Overweight and Obesity: A Systematic Review of Randomized Controlled Trials and Animal Studies. Adv. Nutr. Int. Rev. J. 2023, 15, 100162. [Google Scholar] [CrossRef]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Shastri, S.; Southam, B.; Eri, R.; Stanley, R. Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD. Nutrients 2019, 11, 818. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, S.; Xu, Z.; Su, H.; Tian, X.; Han, B.; Shen, B.; Liao, Q.; Xie, Z.; Hong, Y. Dietary synbiotic ameliorates constipation through the modulation of gut mi-crobiota and its metabolic function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef]
- Thanh, N.T.; Loh, T.C.; Foo, H.L.; Hair-Bejo, M.; Azhar, B.K. Effects of feeding metabolite combinations produced by Lactobacillus plantarum on growth performance, faecal microbial population, small intestine villus height and faecal volatile fatty acids in broilers. Br. Poult. Sci. 2009, 50, 298–306. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Converti, A.; Cotter, P.D.; de Souza Oliveira, R.P. Bacteriocin production by Bifidobacterium spp. A review. Biotechnol. Adv. 2013, 31, 482–488. [Google Scholar] [CrossRef]
- Cotter, P.; Hill, C.; Ross, R. Bacterial lantibiotics: Strategies to improve therapeutic potential. Curr. Protein Pept. Sci. 2005, 6, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, A.; Abriouel, H.; López, R.L.; Ben Omar, N. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Torres, C. Bacteriocins: Potentials and prospects in health and agrifood systems. Arch. Microbiol. 2024, 206, 233. [Google Scholar] [CrossRef]
- Arbulu, S.; Kjos, M. Revisiting the Multifaceted Roles of Bacteriocins: The Multifaceted Roles of Bacteriocins. Microb. Ecol. 2024, 87, 41. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef]
- Thu, T.V.; Loh, T.C.; Foo, H.L.; Yaakub, H.; Bejo, M.H. Effects of liquid metabolite combinations produced by Lactobacillus plantarum on growth performance, faeces characteristics, intestinal morphology and diarrhoea incidence in postweaning piglets. Trop. Anim. Health Prod. 2011, 43, 69–75. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Ari, M.M.; Ohadi, E.; Talebi, M.; Zadeh, M.H.; Emamie, A.D.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2021, 36, e24093. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, P.; Peng, J.; Zhao, B.; Cai, J. Postbiotic properties of exopolysaccharide produced by Levilactobacillus brevis M-10 isolated from natural fermented sour porridge through in vitro simulated digestion and fermentation. J. Food Sci. 2024, 89, 3110–3128. [Google Scholar] [CrossRef]
- Lebeer, S.; Claes, I.; Tytgat, H.L.P.; Verhoeven, T.L.A.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W.M.; De Keersmaecker, S.C.J.; et al. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef]
- Mehling, H.; Busjahn, A. Non-viable Lactobacillus reuteri DSMZ 17648 (Pylopass™) as a new approach to Helicobacter pylori control in humans. Nutrients 2013, 5, 3062–3073. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Asasaka, T.; Sato, E.; Mori, K.; Matsumoto, M.; Ohori, H. Inhibition of binding of Helicobacter pylori to the glycolipid re-ceptors by probiotic Lactobacillus reuteri. FEMS Immunol. Med. Microbiol. 2002, 32, 105–110. [Google Scholar] [CrossRef]
- Aiba, Y.; Ishikawa, H.; Tokunaga, M.; Komatsu, Y. Anti-Helicobacter pylori activity of non-living, heat-killed form of lactobacilli including Lactobacillus johnsonii No.1088. FEMS Microbiol. Lett. 2017, 364, fnx102. [Google Scholar] [CrossRef]
- Feng, C.; Peng, C.; Zhang, W.; Zhang, T.; He, Q.; Kwok, L.-Y.; Zhang, H. Postbiotic Administration Ameliorates Colitis and Inflammation in Rats Possibly through Gut Microbiota Modulation. J. Agric. Food Chem. 2024, 72, 9054–9066. [Google Scholar] [CrossRef] [PubMed]
- Ménard, S.; Laharie, D.; Asensio, C.; Vidal-Martinez, T.; Candalh, C.; Rullier, A.; Zerbib, F.; Mégraud, F.; Matysiak-Budnik, T.; Heyman, M. Bifidobacterium breve and Streptococcus thermophilus secretion products enhance T helper 1 immune response and intestinal barrier in mice. Exp. Biol. Med. 2005, 230, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huang, N.; Ye, X.; Liu, M.; Wei, M.; Huang, Y. The postbiotic of hawthorn-probiotic ameliorating constipation caused by loperamide in elderly mice by regulating intestinal microecology. Front. Nutr. 2023, 10, 1103463. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Thomsson, K.A.; Hansson, G.C. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J. Proteome Res. 2009, 8, 3549–3557. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and molecules of Lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef]
- Izuddin, W.I.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Humam, A.M. Postbiotic L. plantarum RG14 improves ruminal epithelium growth, immune status and upregulates the intestinal barrier function in post-weaning lambs. Sci. Rep. 2019, 9, 9938. [Google Scholar] [CrossRef]
- Bendinelli, P.; De Noni, I.; Cattaneo, S.; Silvetti, T.; Brasca, M.; Piazzalunga, F.; Donetti, E.; Ferraretto, A. Surface layer proteins from Lactobacillus helveticus ATCC® 15009™ affect the gut barrier morphology and function. Tissue Barriers 2024, 12, 2289838. [Google Scholar] [CrossRef]
- Brecht, M.; Garg, A.; Longstaff, K.; Cooper, C.; Andersen, C. Lactobacillus Sepsis following a Laparotomy in a Preterm Infant: A Note of Caution. Neonatology 2016, 109, 186–189. [Google Scholar] [CrossRef]
- Dani, C.; Coviello, C.C.; Corsini, I.I.; Arena, F.; Antonelli, A.; Rossolini, G.M. Lactobacillus Sepsis and Probiotic Therapy in Newborns: Two New Cases and Literature Review. AJP Rep. 2016, 6, e25–e29. [Google Scholar] [PubMed]
- Zhou, X.; Zhang, D.; Qi, W.; Hong, T.; Xiong, T.; Wu, T.; Geng, F.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus plantarum NCU116 Facilitate Intestinal Homeostasis by Modulating Intestinal Epithelial Regeneration and Microbiota. J. Agric. Food Chem. 2021, 69, 7863–7873. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-S.; Shin, J.-S.; Lee, J.-H.; Park, S.-E.; Han, H.-S.; Rhee, Y.K.; Cho, C.-W.; Hong, H.-D.; Lee, K.-T. Protective effect of exopolysaccharide fraction from Bacillus subtilis against dextran sulfate sodium-induced colitis through maintenance of intestinal barrier and suppression of inflammatory responses. Int. J. Biol. Macromol. 2021, 178, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Ren, F. A Role of Exopolysaccharide Produced by Streptococcus thermophilus in the Intestinal Inflammation and Mucosal Barrier in Caco-2 Monolayer and Dextran Sulphate Sodium-Induced Experimental Murine Colitis. Molecules 2019, 24, 513. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Iacob, S.; Iacob, D.G. Infectious Threats, the Intestinal Barrier, and Its Trojan Horse: Dysbiosis. Front. Microbiol. 2019, 10, 1676. [Google Scholar] [CrossRef]
- Carretta, M.D.; Quiroga, J.; López, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.l.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Gulati, G.; Avadhani, R.; Rashmi, H.M.; Soumya, K.; Kumari, A.; Gupta, A.; Dwivedi, D.; Kaushik, J.K.; Grover, S. Postbiotic Lipoteichoic acid of probiotic Lactobacillus origin ameliorates inflammation in HT-29 cells and colitis mice. Int. J. Biol. Macromol. 2023, 236, 123962. [Google Scholar] [CrossRef]
- Bäuerl, C.; Abitayeva, G.; Sosa-Carrillo, S.; Mencher-Beltrán, A.; Navarro-Lleó, N.; Coll-Marqués, J.M.; Zúñiga-Cabrera, M.; Shaikhin, S.; Pérez-Martinez, G. P40 and P75 Are Singular Functional Muramidases Present in the Lactobacillus casei/paracasei/rhamnosus Taxon. Front. Microbiol. 2019, 10, 1420. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Compare, D.; Rocco, A.; Coccoli, P.; Angrisani, D.; Sgamato, C.; Iovine, B.; Salvatore, U.; Nardone, G. Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: An ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 2017, 17, 53. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Sidhu, A.; Ma, Z.; McClain, C.; Feng, W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G32–G41. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.C.; Xu, L.M.; Du, S.J.; Huang, S.S.; Wu, H.; Dong, J.J.; Huang, J.R.; Wang, X.D.; Feng, W.K.; Chen, Y.P. Lactobacillus rhamnosus GG supernatant promotes intestinal barrier function, balances Treg and TH17 cells and ameliorates hepatic injury in a mouse model of chronic-binge alcohol feeding. Toxicol. Lett. 2016, 241, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; He, F.; Yoda, K.; Miyazawa, K.; Hosoda, M.; Hiramatsu, M. Lactobacillus GG-fermented milk prevents DSS-induced colitis and regulates intestinal epithelial homeostasis through activation of epidermal growth factor receptor. Eur. J. Nutr. 2014, 53, 105–115. [Google Scholar] [CrossRef]
- Seth, A.; Yan, F.; Polk, D.B.; Rao, R.K. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1060–G1069. [Google Scholar] [CrossRef]
- He, X.; Zeng, Q.; Puthiyakunnon, S.; Zeng, Z.; Yang, W.; Qiu, J.; Du, L.; Boddu, S.; Wu, T.; Cai, D.; et al. Lactobacillus rhamnosus GG supernatant enhance neonatal resistance to systemic Escherichia coli K1 infection by accelerating development of intestinal defense. Sci. Rep. 2017, 7, srep43305. [Google Scholar] [CrossRef]
- Izuddin, W.I.; Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A. Dietary Postbiotic Lactobacillus plantarum Improves Serum and Ruminal Antioxidant Activity and Upregulates Hepatic Antioxidant Enzymes and Ruminal Barrier Function in Post-Weaning Lambs. Antioxidants 2020, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.-S.; Rafaels, N.M.; Mu, D.; Hand, T.; Murray, T.; Boguniewicz, M.; Hata, T.; Schneider, L.; Hanifin, J.M.; Gallo, R.L.; et al. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J. Allergy Clin. Immunol. 2010, 125, 1403–1407.e4. [Google Scholar] [CrossRef]
- Choksi, Y.A.; Reddy, V.K.; Singh, K.; Barrett, C.W.; Short, S.P.; Parang, B.; Keating, C.E.; Thompson, J.J.; Verriere, T.G.; Brown, R.E.; et al. BVES is required for maintenance of colonic epithelial integrity in experimental colitis by modifying intestinal permeability. Mucosal Immunol. 2018, 11, 1363–1374. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic from Lactobacillus rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Gut Microbes 2012, 3, 25–28. [Google Scholar] [CrossRef]
- Yan, F.; Liu, L.; Dempsey, P.J.; Tsai, Y.-H.; Raines, E.W.; Wilson, C.L.; Cao, H.; Cao, Z.; Liu, L.; Polk, D.B. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J. Biol. Chem. 2013, 288, 30742–30751. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, H.; Liu, L.; Wang, B.; Walker, W.; Acra, S.A.; Yan, F. Activation of epidermal growth factor receptor mediates mucin production stimulated by p40, a Lactobacillus rhamnosus GG-derived protein. J. Biol. Chem. 2014, 289, 20234–20244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Moore, D.; Shen, X.; Peek, R.; Acra, S.; Li, H.; Ren, X.; Polk, D.; Yan, F. An LGG-derived protein promotes IgA production through upregulation of APRIL expression in intestinal epithelial cells. Mucosal Immunol. 2017, 10, 373–384. [Google Scholar] [CrossRef]
- Sánchez, B.; Schmitter, J.-M.; Urdaci, M. Identification of novel proteins secreted by Lactobacillus rhamnosus GG grown in de Mann-Rogosa-Sharpe broth. Lett. Appl. Microbiol. 2009, 48, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Koskenniemi, K.; Koponen, J.; Kankainen, M.; Savijoki, K.; Tynkkynen, S.; de Vos, W.M.; Kalkkinen, N.; Varmanen, P. Proteome analysis of Lactobacillus rhamnosus GG using 2-D DIGE and mass spectrometry shows differential protein production in laboratory and industrial-type growth media. J. Proteome Res. 2009, 8, 4993–5007. [Google Scholar] [CrossRef]
- Chakravarty, K.; Gaur, S.; Kumar, R.; Jha, N.K.; Gupta, P.K. Exploring the Multifaceted Therapeutic Potential of Probiotics: A Review of Current Insights and Applications. Probiotics Antimicrob. Proteins 2025, 17, 341–363. [Google Scholar] [CrossRef]
| Category | Source | Function | Stability | Safety |
|---|---|---|---|---|
| Prebiotics | Non-digestible dietary fibers (e.g., inulin, FOSs, GOSs) | Stimulate growth/activity of beneficial gut bacteria; SCFA production | High thermal and shelf stability | Generally recognized as safe and well tolerated with minimal side effects |
| Probiotics | Live microorganisms (e.g., Lactobacillus, Bifidobacterium, Saccharomyces) | Modulate gut microbiota; enhance barrier function and immunity; antimicrobial production | Sensitive to heat, pH, oxygen; viability must be preserved | Safety well-studied in healthy populations; caution in immunocompromised |
| Synbiotics | Combination of live microbes and substrate (e.g., B. lactis + inulin) | Enhance probiotic survival and activity; improved microbial balance and function | Stability depends on formulation; better in encapsulated forms | Safety similar to probiotics; combination must be assessed for interactions |
| Postbiotics | Inactivated microbial cells and/or their metabolites | Modulate immunity; reinforce barrier integrity; deliver microbial benefits without live organisms | Very stable under heat and storage; do not require cold chain | High safety profile; no risk of translocation or infection |
| Prebiotic Type | Sources | Effects | Ref. |
|---|---|---|---|
| Polydextrose (PDX) | Polysaccharide, randomly bonded glucose polymers | Modulates gut microbiota, reduces inflammation | [8,30] |
| Dextrins | Hydrolized starch and glycogen | Enhances short-chain fatty acid (SCFA) production, increases satiety, promotes beneficial gut bacteria, decreases Clostridium spp., reduces β-glucosidase and β-glucuronidase activities | [4,9,31,32] |
| Inulin (oligofructose-enriched inulin) | Chicory root, garlic, leeks, artichokes | Increases Bifidobacterium, improves bowel movements, improves lipid metabolism antioxidant and anti-inflammatory | [33,34,35] |
| Soluble corn fiber | Corn | Improves digestive health, alters gut microbiota composition | [36] |
| Resistant starch type 4 | Chemically modified starches | Potential use in metabolic health management, increases resistance to enzymatic digestion | [37] |
| Fructooligosaccharide (FOS) | Chicory roots, onions, garlic, asparagus | Promotes growth of Bifidobacterium and Lactobacillus spp., increases colonic crypt size | [38,39,40] |
| Galactooligosaccharide (GOS) | Human milk, soybeans | Enhances immune function, reduces pathogen colonization | [41,42] |
| Arabinoxylan-oligosaccharides | Cereal grains | Improves gut health | [43] |
| Resistant starch type 1 | Grains, seeds, legumes, pastas | Improves insulin sensitivity, physically inaccessible starch; passes through the small intestine undigested | [44,45,46,47,48] |
| Resistant starch type 2 | Green bananas, raw potatoes, high amylose corn, specific legumes | Increases production of SCFAs, contains raw starch granules; resistant to enzymatic digestion | |
| Resistant starch type 3 | Cooked and cooled starchy foods like bread, cakes, cornflakes | Enhances satiety, reduces fat storage | |
| Resistant starch type 4 | Chemically modified starches | Potential use in metabolic health management, increases resistance to enzymatic digestion | |
| Resistant starch type 5 | Starch–lipid complexes formed during food processing | Resistant to amylolytic hydrolysis; improves gut health by passing undigested | |
| Wheat, oat, corn, barley, rye bran | Cereal brans, whole grains | Enhances stool bulk, supports gut microbiota diversity | [49,50,51] |
| Fruit/vegetable fibre | e.g., lupin kernel, sugar cane, bean, citrus, various fruit | Supports gut microbiota | [52,53] |
| Probiotic Species | Major Findings | Ref. |
|---|---|---|
| Lactobacillus acidophilus | Exhibits anti-inflammatory effects, enhances IL-17 and IL-22 production, improves colitis symptoms when used with Veillonella ratti, and upregulates protective cytokines | [58,59] |
| Lactobacillus fermentum | Reduces chronic gut inflammation by increasing IL-6 and IL-10, inhibits harmful bacteria, protects against gut permeability from chemotherapy, and reduces inflammation through the NF-κB pathway | [60,61,62] |
| Lacticaseibacillus rhamnosus | Lowers IL-18 levels, boosts IL-10, helps recover body weight and colon length in colitis, improves disease markers, strengthens the epithelial barrier, and promotes regeneration of intestinal stem cells | [63,64] |
| Ligilactobacillus salivarius | Reduces pro-inflammatory markers, enhances epithelial barrier function, and prevents intestinal pathogens from adhering | [65,66] |
| Limosilactobacillus mucosae | Protects against experimental colitis by upregulating colonic 5-HT4 and TGF-β2, as well as alleviates colitis symptoms | [67] |
| Bifidobacterium longum | Promotes healing of wounds, lowers IL-6 and TNF-α levels, improves colitis, enhances immune response when combined with B. bifidum, and fortifies the epithelial barrier | [68,69] |
| Bifidobacterium breve | Reduces colitis symptoms, increases goblet cell count, strengthens epithelial barrier, and decreases oxidative stress | [70,71] |
| Bifidobacterium bifidum | Ameliorates colitis symptoms, restores body weight and colon length, strengthens epithelial barrier, and increases anti-inflammatory factors; protective against non-alcoholic fatty liver disease | [72,73] |
| Saccharomyces cerevisiae (yeast) | Reduces TNF-α, increases IL-10, protects against colitis, and suppresses macrophages pyroptosis | [74,75,76] |
| Faecalibacterium prausnitzii | Decreases disease scores and significantly reduces inflammation in IBD | [77] |
| Enterococcus faecium | Enhances epithelial barrier, lowers pro-inflammatory cytokines, reduces inflammation in obesity, and reduces inflammation through the NF-κB pathway | [78,79] |
| Study Population | Intervention | Outcome | Clinical Relevance | Ref. |
|---|---|---|---|---|
| Preterm infants (NEC) | Probiotics (Lactobacillus, Bifidobacteria) | Improved epithelial barrier, reduced inflammation | Supports prevention of NEC in neonates | [92,93,94] |
| Obese individuals | Prebiotics | Improved gut barrier, reduced inflammation, enhanced insulin sensitivity | Potential therapeutic use for obesity and metabolic disorders | [95,96,97] |
| Adults with infectious diarrhea | Probiotics (S. boulardii, L. reuteri, B. lactis) | Reduced duration and incidence of diarrhea | Effective for traveler’s and acute diarrhea treatment | [98,100] |
| Patients with antibiotic-associated diarrhea (AAD) | Probiotics (S. boulardii, L. rhamnosus GG, multi-strain) | Reduced AAD occurrence, restored gut microbiota | Prevents common AAD complications | [102,104] |
| Cancer patients (chemo-/radiation-induced diarrhea) | Probiotics (L. acidophilus, B. bifidum, Saccharomyces) | Reduced diarrhea severity and frequency | Supports gut integrity during cancer treatment | [107,108] |
| Children with functional diarrhea | Prebiotics (FOS) | Improved microbiota balance, symptom relief | Alternative to antibiotics in pediatric diarrhea | [109] |
| Adults with metabolic syndrome | Synbiotic supplementation | Improved gut microbiota composition; reduced inflammation markers | Potential adjunct therapy for metabolic disorders | [2] |
| Patients undergoing stem cell transplantation | Probiotic and prebiotic administration | Modulated gut microbiota; reduced transplant-related complications | Supportive care during transplantation | [154] |
| Individuals with skin aging concerns | Probiotic and prebiotic supplementation | Improved skin health; gut–skin axis enhancement | Dermatological benefits of gut modulation | [155] |
| Cancer patients undergoing chemotherapy | Postbiotic supplementation | Reduced gastrointestinal side effects | Improved tolerability of chemotherapy | [156] |
| Patients with Parkinson’s disease | Probiotic and prebiotic interventions | Alleviated GI symptoms; possible neurological improvement | Supportive therapy in neurodegenerative disease | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolinska, S.; Popescu, F.-D.; Zemelka-Wiacek, M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J. Clin. Med. 2025, 14, 3673. https://doi.org/10.3390/jcm14113673
Smolinska S, Popescu F-D, Zemelka-Wiacek M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. Journal of Clinical Medicine. 2025; 14(11):3673. https://doi.org/10.3390/jcm14113673
Chicago/Turabian StyleSmolinska, Sylwia, Florin-Dan Popescu, and Magdalena Zemelka-Wiacek. 2025. "A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity" Journal of Clinical Medicine 14, no. 11: 3673. https://doi.org/10.3390/jcm14113673
APA StyleSmolinska, S., Popescu, F.-D., & Zemelka-Wiacek, M. (2025). A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. Journal of Clinical Medicine, 14(11), 3673. https://doi.org/10.3390/jcm14113673








