Usability Evaluation of Urinary HAI-1, STMN-1 and TN-C in the Diagnosis of Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Collection of Data
2.2. Material and Methods
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Results of HAI-1, STMN-1 and TN-C in Urine of BC Patients and Control Group
3.3. Correlations
3.4. Diagnostic Value of HAI-1, STM-1 and TN-C in BC and Subgroups NMIBC, MIBC, LG and HG
3.5. Diagnostic Value of Combinations of Parameters in BC and Subgroups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, W.W.L.; Leung, C.; Wong, M.C.S.; Fung, F.D.H.; Ng, C.F.; Goggins, W.B. The global epidemiology of bladder cancer: A joinpoint regression analysis of its incidence and mortality trends and projection. Sci. Rep. 2018, 8, 1129. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Letašiová, S.; Medve’ová, A.; Šovčíková, A.; Dušinská, M.; Volkovová, K.; Mosoiu, C.; Bartonová, A. Bladder cancer, a review of the environmental risk factors. Environ. Health 2012, 11 (Suppl. S1), S11. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, M.S.; Zaghloul, T.M.; Bishr, M.K.; Baumann, B.C. Urinary schistosomiasis and the associated bladder cancer: Update. J. Egypt. Natl. Cancer Inst. 2020, 32, 44. [Google Scholar] [CrossRef]
- Hughes, F.M.; Corn, A.G., Jr.; Nimmich, A.R.; Pratt-Thomas, J.D.; Purves, J.T. Cyclophosphamide Induces an Early Wave of Acrolein-Independent Apoptosis in the Urothelium. Adv. Biosci. Biotechnol. 2013, 4, 9–14. [Google Scholar] [CrossRef]
- Yee, D.S.; Shariat, S.F.; Lowrance, W.T.; Sterbis, J.R.; Vora, K.C.; Bochner, B.H.; Donat, S.M.; Herr, H.W.; Dalbagni, G.; Sandhu, J.S. Impact of previous radiotherapy for prostate cancer on clinical outcomes of patients with bladder cancer. J. Urol. 2010, 183, 1751–1756. [Google Scholar] [CrossRef]
- Barone, B.; Finati, M.; Cinelli, F.; Fanelli, A.; Del Giudice, F.; De Berardinis, E.; Sciarra, A.; Russo, G.; Mancini, V.; D’Altilia, N.; et al. Bladder Cancer and Risk Factors: Data from a Multi-Institutional Long-Term Analysis on Cardiovascular Disease and Cancer Incidence. J. Pers. Med. 2023, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Grubmüller, B.; Roupret, M.; Briganti, A.; Shariat, S.F. The Use of Biomarkers for Bladder Cancer Diagnosis and Surveillance. Mini Rev. Med. Chem. 2016, 16, 1444–1449. [Google Scholar] [CrossRef]
- Leiblich, A. Recent Developments in the Search for Urinary Biomarkers in Bladder Cancer. Curr. Urol. Rep. 2017, 18, 100. [Google Scholar] [CrossRef]
- Hajdinjak, T. UroVysion FISH test for detecting urothelial cancers: Meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol. Oncol. 2008, 26, 646–651. [Google Scholar] [CrossRef]
- D’Costa, J.J.; Ward, D.G.; Bryan, R.T. Urinary biomarkers for the diagnosis of urothelial bladder cancer. New Horiz. Transl. Med. 2017, 3, 221–223. [Google Scholar]
- Kawaguchi, M.; Kataoka, H. Mechanisms of Hepatocyte Growth Factor Activation in Cancer Tissues. Cancers 2014, 6, 1890–1904. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Kawaguchi, M.; Yamasaki, M.; Tanaka, H.; Yorita, K.; Kataoka, H. Hepatocyte growth factor activator inhibitor type 1 suppresses metastatic pulmonary colonization of pancreatic carcinoma cells. Cancer Sci. 2011, 102, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.M.; Babicky, M.L.; Lowy, A.M. The RON receptor tyrosine kinase in pancreatic cancer pathogenesis and its potential implications for future targeted therapies. Pancreas 2014, 43, 183–189. [Google Scholar] [CrossRef]
- Baba, T.; Kawaguchi, M.; Fukushima, T.; Sato, Y.; Orikawa, H.; Yorita, K.; Tanaka, H.; Lin, C.Y.; Sakoda, S.; Kataoka, H. Loss of membrane-bound serine protease inhibitor HAI-1 induces oral squamous cell carcinoma cells’ invasiveness. J. Pathol. 2012, 228, 181–192. [Google Scholar] [CrossRef]
- Nemunaitis, J. Stathmin 1: A protein with many tasks. New biomarker and potential target in cancer. Expert Opin. Ther. Targets 2012, 16, 631–634. [Google Scholar] [CrossRef]
- Shimizu, K.; Ohtaki, Y.; Altan, B.; Yokobori, T.; Nagashima, T.; Arai, M.; Mogi, A.; Kuwano, H. Prognostic impact of stathmin 1 expression in patients with lung adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2017, 154, 1406–1417.e3. [Google Scholar] [CrossRef]
- Nie, W.; Xu, M.D.; Gan, L.; Huang, H.; Xiu, Q.; Li, B. Overexpression of stathmin 1 is a poor prognostic biomarker in non-small cell lung cancer. Lab. Investig. 2015, 95, 56–64. [Google Scholar] [CrossRef]
- Shimizu, K.; Goto, Y.; Kawabata-Iwakawa, R.; Ohtaki, Y.; Nakazawa, S.; Yokobori, T.; Obayashi, K.; Kawatani, N.; Yajima, T.; Kaira, K.; et al. Stathmin-1 Is a Useful Diagnostic Marker for High-Grade Lung Neuroendocrine Tumors. Ann. Thorac. Surg. 2019, 108, 235–243. [Google Scholar] [CrossRef]
- Yoshida, T.; Akatsuka, T.; Imanaka-Yoshida, K. Tenascin-C and integrins in cancer. Cell Adhes. Migr. 2015, 9, 96–104. [Google Scholar] [CrossRef]
- Lowy, C.M.; Oskarsson, T. Tenascin C in metastasis: A view from the invasive front. Cell Adhes. Migr. 2015, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Orend, G. The role of tenascin-C in tissue injury and tumorigenesis. J. Cell Commun. Signal. 2009, 3, 287–310. [Google Scholar] [CrossRef] [PubMed]
- Angel, I.; Pilo Kerman, O.; Rousso-Noori, L.; Friedmann-Morvinski, D. Tenascin C promotes cancer cell plasticity in mesenchymal glioblastoma. Oncogene 2020, 39, 6990–7004. [Google Scholar] [CrossRef]
- Gebauer, F.; Gelis, S.; Zander, H.; Meyer, K.F.; Wolters-Eisfeld, G.; Izbicki, J.R.; Bockhorn, M.; Tachezy, M. Tenascin-C serum levels and its prognostic power in non-small cell lung cancer. Oncotarget 2016, 7, 20945–20952. [Google Scholar] [CrossRef]
- Zheng, Q.; Wu, H.; Cao, J.; Ye, J. Hepatocyte growth factor activator inhibitor type 1 in cancer: Advances and perspectives (Review). Mol. Med. Rep. 2014, 10, 2779–2785. [Google Scholar] [CrossRef]
- Kataoka, H.; Miyata, S.; Uchinokura, S.; Itoh, H. Roles of hepatocyte growth factor (HGF) activator and HGF activator inhibitor in the pericellular activation of HGF/scatter factor. Cancer Metastasis Rev. 2003, 22, 223–236. [Google Scholar] [CrossRef]
- Sakugawa, C.; Haruyama, Y.; Tanaka, H.; Fukushima, T.; Kawaguchi, M.; Kataoka, H. Prognostic significance of hepatocyte growth factor activator inhibitor type 1 (HAI-1) immunoreactivity in pancreatic ductal adenocarcinoma. BMC Res. Notes 2017, 10, 674. [Google Scholar] [CrossRef]
- Tanabe, L.M.; List, K. The role of type II transmembrane serine protease-mediated signaling in cancer. FEBS J. 2017, 284, 1421–1436. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.D.; Kulp, K.S.; Loots, G.G. Prostate cancer invasion and metastasis: Insights from mining genomic data. Brief Funct. Genom. 2013, 12, 397–410. [Google Scholar] [CrossRef]
- Steinmetz, M.O. Structure and thermodynamics of the tubulin-stathmin interaction. J. Struct. Biol. 2007, 158, 137–147. [Google Scholar] [CrossRef]
- Belmont, L.D.; Mitchison, T.J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell 1996, 84, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Cassimeris, L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 2002, 14, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Howell, B.; Larsson, N.; Gullberg, M.; Cassimeris, L. Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin. Mol. Biol. Cell 1999, 10, 105–118. [Google Scholar] [CrossRef]
- Manna, T.; Thrower, D.; Miller, H.P.; Curmi, P.; Wilson, L. Stathmin strongly increases the minus end catastrophe frequency and induces rapid treadmilling of bovine brain microtubules at steady state in vitro. J. Biol. Chem. 2006, 281, 2071–2078. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C.; Xu, Y.F.; Cheng, H.; Shi, S.; Wu, C.T.; Yu, X.J. Stathmin destabilizing microtubule dynamics promotes malignant potential in, cancer cells by epithelial-mesenchymal transition. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 386–394. [Google Scholar] [CrossRef]
- Chiquet-Ehrismann, R.; Tucker, R.P. Tenascins and the importance of adhesion modulation. Cold Spring Harb. Perspect. Biol. 2011, 3, a004960. [Google Scholar] [CrossRef]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Yoshida, T.; Miyagawa-Tomita, S. Tenascin-C in development and disease of blood vessels. Anat. Rec. 2014, 297, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Hussenet, T.; Langlois, B.; Orend, G. Advances in tenascin-C biology. Cell. Mol. Life Sci. 2011, 68, 3175–3199. [Google Scholar] [CrossRef]
- Soloway, M.S.; Sofer, M.; Vaidya, A. Contemporary management of stage T1 transitional cell carcinoma of the bladder. J. Urol. 2002, 167, 1573–1583. [Google Scholar] [CrossRef]
- Botteman, M.F.; Pashos, C.L.; Redaelli, A.; Laskin, B.; Hauser, R. The health economics of bladder cancer: A comprehensive review of the published literature. Pharmacoeconomics 2003, 21, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, V.B.; Soloway, M.S. Current bladder tumor tests: Does their projected utility fulfill clinical necessity? J. Urol. 2001, 165, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, B.; Długosz, A. The role of the BLCA-4 nuclear matrix protein in bladder cancer. Postep. Hig. I Med. Doswiadczalnej 2017, 71, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, B.; Pawlik, K.J.; Sawicka, E.; Dembowski, J.; Kowal, P.; Zdrojowy, R.; Długosz, A. Evaluation of NMP22 in bladder cancer patients sensitive to environmental toxins. Adv. Clin. Exp. Med. 2017, 26, 1069–1075. [Google Scholar] [CrossRef]
- Szymańska, B.; Sawicka, E.; Guzik, A.; Zdrojowy, R.; Długosz, A. The Diagnostic Value of Nuclear Matrix Proteins in Bladder Cancer in the Aspect of Environmental Risk from Carcinogens. BioMed Res. Int. 2017, 2017, 9643139. [Google Scholar] [CrossRef]
- Ferro, M.; Falagario, U.G.; Barone, B.; Maggi, M.; Crocetto, F.; Busetto, G.M.; Giudice, F.; Terracciano, D.; Lucarelli, G.; Lasorsa, F.; et al. Artificial Intelligence in the Advanced Diagnosis of Bladder Cancer-Comprehensive Literature Review and Future Advancement. Diagnostics 2023, 13, 2308. [Google Scholar] [CrossRef]
- Snell, K.I.E.; Ward, D.G.; Gordon, N.S.; Goldsmith, J.C.; Sutton, A.J.; Patel, P.; James, N.D.; Zeegers, M.P.; Cheng, K.K.; Bryan, R.T. Exploring the roles of urinary HAI-1, EpCAM & EGFR in bladder cancer prognosis & risk stratification. Oncotarget 2018, 9, 25244–25253. [Google Scholar]
- Yamasaki, K.; Mukai, S.; Nagai, T.; Nakahara, K.; Fujii, M.; Terada, N.; Ohno, A.; Sato, Y.; Toda, Y.; Kataoka, H.; et al. Matriptase-Induced Phosphorylation of MET is Significantly Associated with Poor Prognosis in Invasive Bladder Cancer; an Immunohistochemical Analysis. Int. J. Mol. Sci. 2018, 19, 3708. [Google Scholar] [CrossRef]
- Mukai, S.; Yamasaki, K.; Fujii, M.; Nagai, T.; Terada, N.; Kataoka, H.; Kamoto, T. Dysregulation of Type II Transmembrane Serine Proteases and Ligand-Dependent Activation of MET in Urological Cancers. Int. J. Mol. Sci. 2020, 21, 2663. [Google Scholar] [CrossRef]
- McNeil, B.K.; Sorbellini, M.; Grubb, R.L.; Apolo, A.; Cecchi, F.; Athauda, G.; Cohen, B.; Giubellino, A.; Simpson, H.; Agarwal, P.K. Preliminary evaluation of urinary soluble Met as a biomarker for urothelial carcinoma of the bladder. J. Transl. Med. 2014, 21, 199. [Google Scholar] [CrossRef]
- Matsumoto, K.; Umitsu, M.; De Silva, D.M.; Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Shimwell, N.J.; Bryan, R.T.; Wei, W.; James, N.D.; Cheng, K.K.; Zeegers, M.P.; Johnson, P.J.; Martin, A.; Ward, D.G. Combined proteome and transcriptome analyses for the discovery of urinary biomarkers for urothelial carcinoma. Br. J. Cancer 2013, 108, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, T.; Lindén, M.; Lind, S.B.; Namuduri, A.V.; Sjöstedt, E.; de Ståhl, T.D.; Asplund, A.; Malmström, P.U.; Segersten, U. The prognostic value and therapeutic target role of stathmin-1 in urinary bladder cancer. Br. J. Cancer 2014, 111, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Dubosq, F.; Ploussard, G.; Soliman, H.; Turpin, E.; Latil, A.; Desgrandchamps, F.; Mongiat-Artus, P. Identification of a three-gene expression signature of early recurrence in non-muscle-invasive urothelial cell carcinoma of the bladder. Urol. Oncol. 2011, 30, 833–840. [Google Scholar] [CrossRef]
- Brunner, A.; Mayerl, C.; Tzankov, A.; Verdorfer, I.; Tschörner, I.; Rogatsch, H.; Mikuz, G. Prognostic significance of tenascin-C expression in superficial and invasive bladder cancer. J. Clin. Pathol. 2004, 57, 927–931. [Google Scholar] [CrossRef]

| Population Characteristic | BC | C |
|---|---|---|
| N(%) | (100) 56 | (100) 32 |
| age range (median years) | 41–88 (69) | 50–81 (67) |
| smoking | 43 (77) | 26 (81) |
| non-smoking | 13 (23) | 10 (19) |
| Men | 48 (86) | 27 (84) |
| Women | 8 (14) | 5 (16) |
| Clinical grading | ||
| LG | 30 (54) | |
| HG | 26 (46) | |
| Clinical subgroups | ||
| NMIBC | 45 (80) | |
| MIBC | 11(20) |
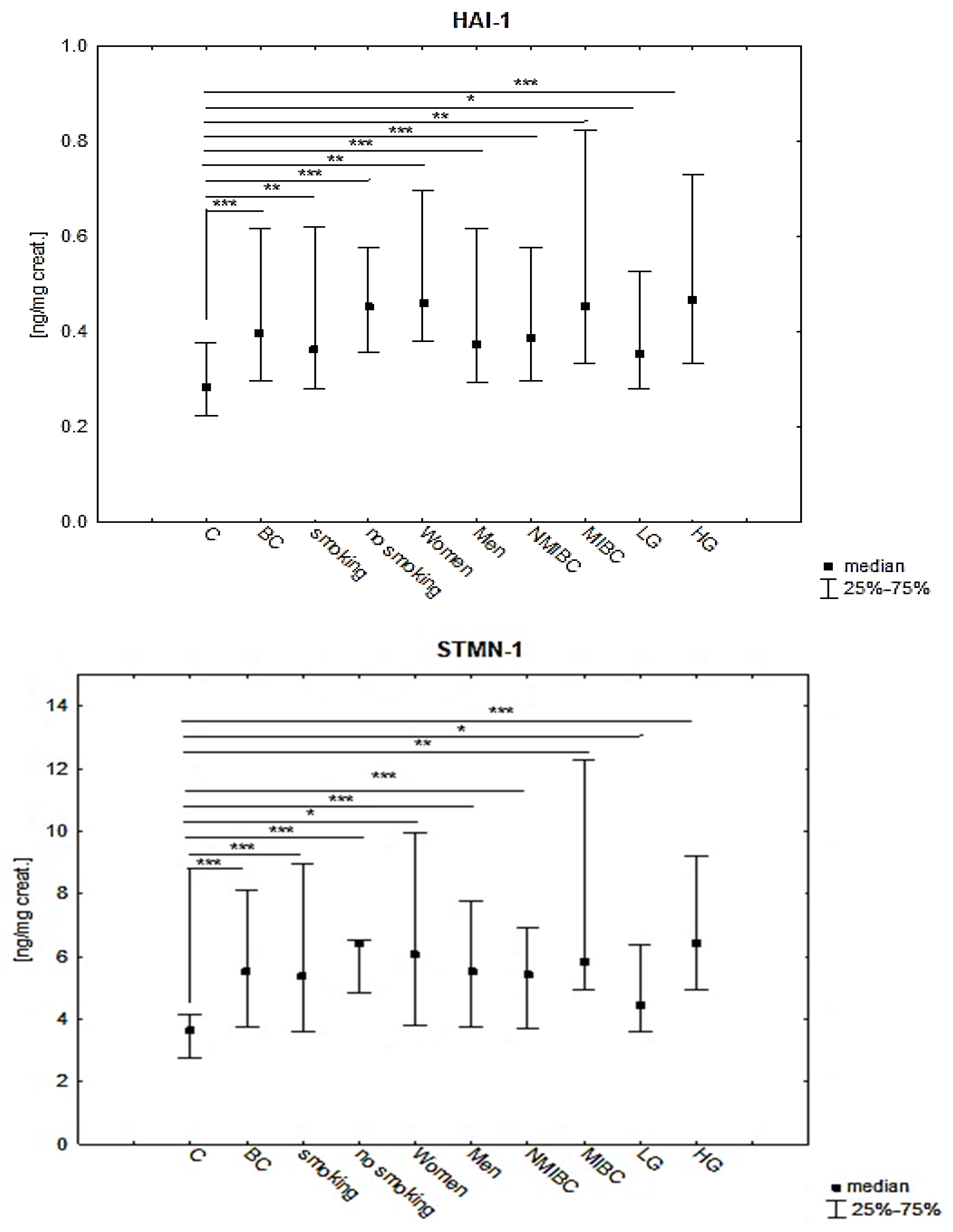
| HAI-1 [ng/mg Creat.] | Mean ± SD | Median (IQR) | p |
|---|---|---|---|
| BC | 0.56 ± 0.56 | 0.39 (0.29–0.61) | ≤0.001 * |
| C | 0.29 ± 0.88 | 0.28 (0.22–0.37) | |
| smoking patients of BC | 0.57 ± 0.63 | 0.36 (0.27–0.62) | ≤0.001 ** BC(S):C = 0.004 BC(nonS):C ≤ 0.001 BC(S):BC (nonS) = NS |
| non-smoking patients of BC | 0.49 ± 0.16 | 0.45 (0.35–0.57) | |
| Women of BC | 0.94 ± 0.12 | 0.40 (0.36–0.86) | ≤0.001 ** BC(w):C = 0.002 BC(m):C = 0.001 BC(w):BC(m) = NS |
| Men of BC | 0.50 ± 0.38 | 0.37 (0.29–0.61) | |
| NMIBC | 0.50 ± 0.52 | 0.38 (0.29–0.55) | 0.0004 ** NMIBC:C = 0.001 MIBC:C = 0.006 NMIBC:MIBC = NS |
| MIBC | 0.65 ± 0.59 | 0.45 (0.33–0.82) | |
| LG | 0.40 ± 0.17 | 0.36 (0.27–0.51) | ≤0.001 ** LG:C = 0.046 HG:C ≤ 0.001 LG:HG = NS |
| HG | 0.68 ± 0.73 | 0.46 (0.33–0.72) |
| STMN-1 [ng/mg Creat.] | Mean ± SD | Median (IQR) | p |
|---|---|---|---|
| BC | 7.65 ± 4.45 | 5.52 (3.75–8.13) | ≤0.001 * |
| C | 3.60 ± 1.20 | 3.61 (2.76–4.15) | |
| smoking patients of BC | 7.94 ± 8.41 | 5.35 (3.53–8.93) | ≤0.001 * BC(S):C ≤ 0.001 BC(nonS):C ≤ 0.001 BC(S):BC (nonS) = NS |
| non-smoking patients of BC | 6.68 ± 3.19 | 6.39 (4.85–6.54) | |
| Women of BC | 11.37 ± 14.72 | 5.32 (3.59–12.29) | ≤0.001 * BC(w):C = 0.013 BC(m):C ≤ 0.001 BC(w):BC(m) = NS |
| Men of BC | 7.11 ± 6.00 | 5.52 (3.75–7.77) | |
| NMIBC | 6.72 ± 6.38 | 5.42 (3.71–6.12) | ≤0.001 ** NMIBC:C ≤ 0.001 MIBC:C = 0.001 NMIBC:MIBC = NS |
| MIBC | 9.35 ± 8.23 | 5.83 (4.95–12.30) | |
| LG | 5.47 ± 2.87 | 4.45 (3.59–6.40) | ≤0.001 ** LG:C = 0.024 HG:C ≤ 0.001 LG:HG = NS |
| HG | 9.27 ± 9.14 | 6.43 (4.95–9.21) |
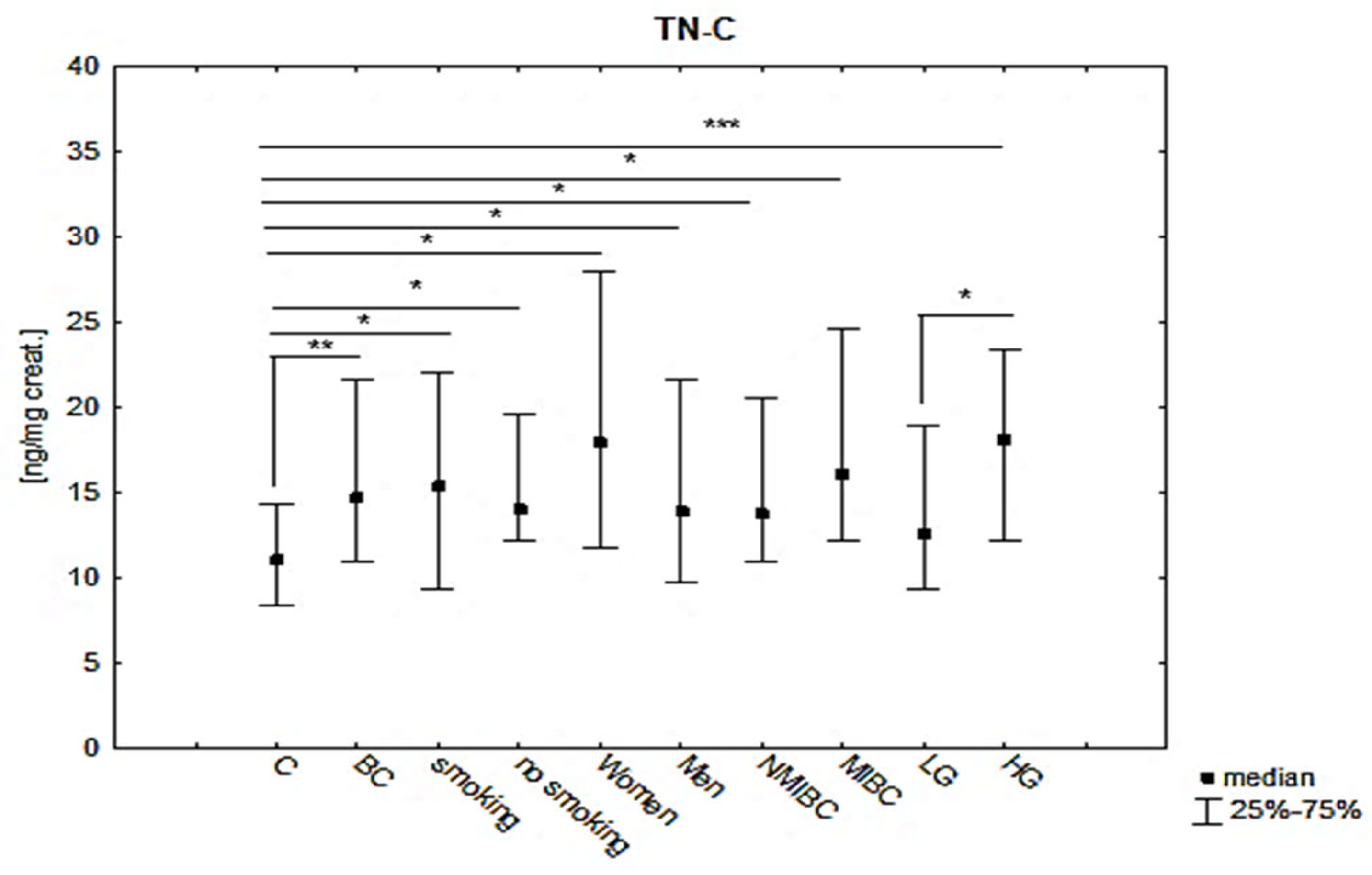
| TNC [ng/mg Creat.] | Mean ± SD | Median (IQR) | p |
|---|---|---|---|
| BC | 20.29 ± 23.24 | 14.66 (10.85–21.62) | 0.002 * |
| C | 11.25 ± 3.71 | 11.04 (8.39–14.32) | |
| smoking patients of BC | 21.49 ± 26.33 | 15.37 (9.35–21.96) | 0.005 ** BC(S):C = 0.022 BC(nonS):C = 0.017 BC(S):BC (nonS) = NS |
| non-smoking patients of BC | 16.34 ± 5.17 | 13.96 (12.10–19.55) | |
| Women of BC | 38.39 ± 54.53 | 18.14 (11.40–36.29) | 0.004 ** BC(w):C = 0.028 BC(m):C = 0.015 BC(w):BC(m) = NS |
| Men of BC | 17.71 ± 14.02 | 13.82 (9.68–21.620) | |
| NMIBC | 18.43 ± 22.6 | 13.68 (10.85–20.08) | 0.005 ** NMIBC:C = 0.018 MIBC:C = 0.022 NMIBC:MIBC = NS |
| MIBC | 22.99 ± 20.41 | 16.05 (12.10–24.56) | |
| LG | 14.03 ± 6.69 | 12.52 (9.35–18.96) | ≤0.001 ** LG:C = NS HG:C ≤ 0.001 LG:HG = NS |
| HG | 25.44 ± 30.80 | 18.04 (12.19–23.30) |
| Parameters | R | p | |
|---|---|---|---|
| HAI-1 (MIBC) vs. STMN-1 (MIBC) | 0.972 | ≤0.001 | almost complete dependence |
| HAI-1 (MIBC) vs. TN-C (MIBC) | 0.956 | ≤0.001 | |
| STMN-1 (MIBC) vs. TN-C (MIBC) | 0.955 | ≤0.001 | |
| HAI-1 (HG) vs. STMN-1 (HG) | 0.943 | ≤0.001 | |
| HAI-1 vs. TN-C | 0.942 | ≤0.001 | |
| HAI-1 vs. STMN-1 | 0.941 | ≤0.001 | |
| HAI-1 (LG) vs. TN-C(LG) | 0.938 | ≤0.001 | |
| HAI-1 (NMIBC) vs. TN-C (NMIBC) | 0.930 | ≤0.001 | |
| HAI-1 (HG) vs. TN-C (HG) | 0.912 | ≤0.001 | |
| HAI-1 (NMIBC) vs. STMN-1 (NMIBC) | 0.911 | ≤0.001 | |
| STMN-1 vs. TN-C | 0.890 | ≤0.001 | very high dependence |
| STMN-1(HG) vs. TN-C (HG) | 0.887 | ≤0.001 | |
| STMN-1 (NMIBC) vs. TN-c (NMIBC) | 0.857 | ≤0.001 | |
| HAI-l (LG) vs. STMN-1 (LG) | 0.883 | ≤0.001 | |
| STMN-1 (LG) vs. TN-C (LG) | 0.842 | ≤0.001 |
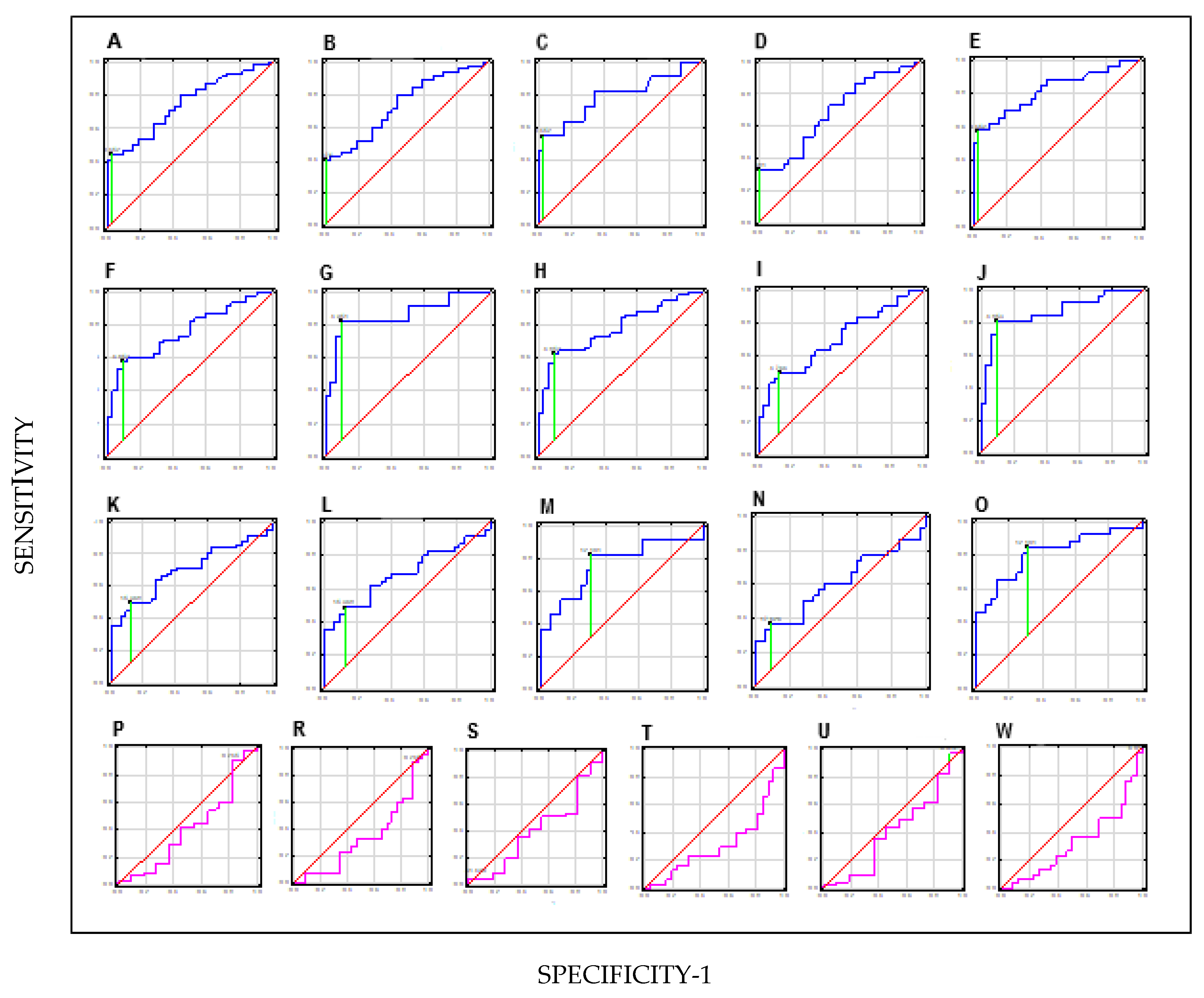
| Sensitivity | Specificity | PPV | NPV | AUC | p | |
|---|---|---|---|---|---|---|
| HAI-1 | ||||||
| BC vs. C | 47% | 98% | 96% | 50% | 0.75 | 0.0000 |
| NMIBC vs. C | 43% | 98% | 100% | 54% | 0.74 | 0.0000 |
| MIBC vs. C | 57% | 97% | 86% | 86% | 0.79 | 0.0021 |
| LG vs. C | 35% | 99% | 100% | 62% | 0.69 | 0.0043 |
| HG vs. C | 60% | 82% | 94% | 74% | 0.82 | 0.0000 |
| NMIBC vs. MIBC | 82% | 9% | 18% | 33% | 0.41 | 0.3879 |
| LG vs. HG | 88% | 10% | 54% | 50% | 0.34 | 0.3460 |
| STMN-1 | ||||||
| BC vs. C | 65% | 91% | 92% | 58% | 0.78 | 0.0000 |
| NMIBC vs. C | 60% | 91% | 90% | 60% | 0.76 | 0.0000 |
| MIBC vs. C | 85% | 89% | 75% | 94% | 0.86 | 0.0000 |
| LG vs. C | 53% | 88% | 88% | 60% | 0.70 | 0.0023 |
| HG vs. C | 81% | 89% | 89% | 85% | 0.87 | 0.0000 |
| NMIBC vs. MIBC | 9% | 96% | 10% | 20% | 0.40 | 0.3177 |
| LG vs. HG | 0% | 100% | - | - | 0.32 | 0.0123 |
| TN-C | ||||||
| BC vs. C | 51% | 88% | 88% | 14% | 0.70 | 0.0020 |
| NMIBC vs. C | 51% | 88% | 85% | 55% | 0.68 | 0.0018 |
| MIBC vs. C | 85% | 69% | 47% | 92% | 0.78 | 0.0079 |
| LG vs. C | 38% | 88% | 24% | 60% | 0.61 | 0.1516 |
| HG vs. C | 87% | 69% | 69% | 85% | 0.81 | 0.0000 |
| NMIBC vs. MIBC | 98% | 9% | 81% | 50% | 0.41 | 0.3902 |
| LG vs. HG | 96% | 3% | 54% | 50% | 0.33 | 0.0157 |
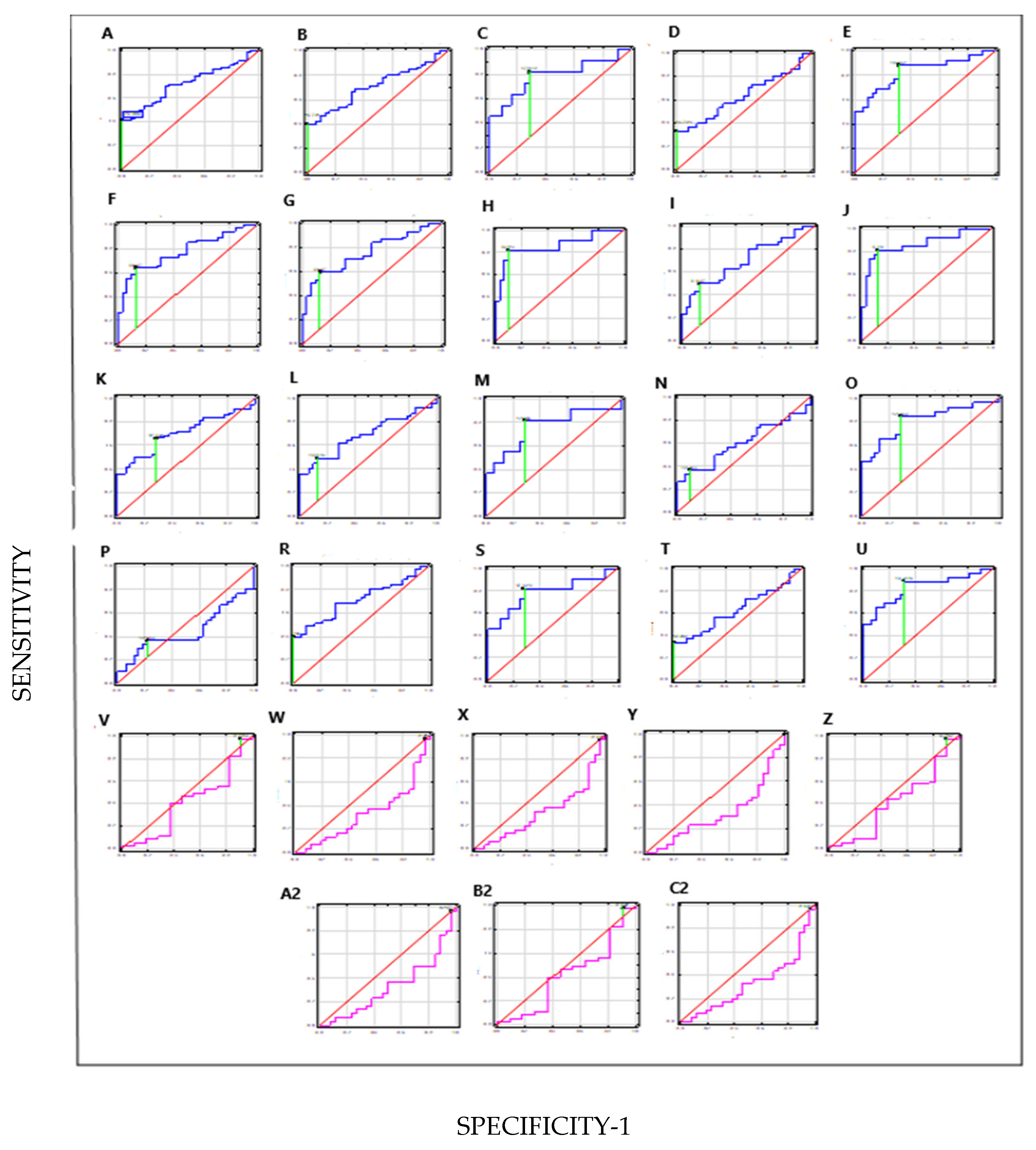
| Combinations Parameters | Sensitivity | Specificity | PPV | NPV | AUC | p |
|---|---|---|---|---|---|---|
| HAI-1+STMN-1+TN-C vs. C | 41% | 99% | 100% | 49% | 0.73 | 0.0000 |
| HAI-1(NMIBC)+STMN-1(NMIBC)+TN-C(NMIBC) vs. C | 40% | 99% | 100% | 54% | 0.71 | 0.0002 |
| HAI-1(MIBC)+STMN-1(MIBC)+TN- C(MIBC) vs. C | 82% | 72% | 50% | 92% | 0.78 | 0.0020 |
| HAI-1(LG)+STMN-1(LG)+TN-C(LG) vs. C | 33% | 99% | 100% | 62% | 0.64 | 0.0603 |
| HAI-1(HG)+STMN-1(HG)+TN-C(HG) vs. C | 88% | 69% | 70% | 88% | 0.83 | 0.0000 |
| HAI-1+STMN-1 vs. C | 64% | 87% | 90% | 58% | 0.78 | 0.0000 |
| HAI-1(NMIBC)+STMN-1(NIMBC) vs. C | 61% | 88% | 87% | 61% | 0.76 | 0.0000 |
| HAI-1(MIBC)+STMN-1(MIBC) vs. C | 82% | 91% | 75% | 94% | 0.86 | 0.0000 |
| HAI-1(LG)+STMN-1(LG) vs. C | 50% | 87% | 79% | 65% | 0.71 | 0.0021 |
| HAI-1(HG)+STMN-1(HG) vs. C | 89% | 85% | 84% | 85% | 0.87 | 0.0000 |
| HAI-1+TN-C vs. C | 66% | 72% | 80% | 55% | 0.70 | 0.0002 |
| HAI-1(NMIBC)+STMN-1(NMIBC) vs. C | 49% | 88% | 92% | 55% | 0.69 | 0.0072 |
| HAI-1(MIBC)+TN-C(MIBC) vs. C | 82% | 72% | 43% | 91% | 0.71 | 0.0072 |
| HAI-1(LG)+TN-C(LG) vs. C | 37% | 91% | 38% | 88% | 0.61 | 0.1513 |
| HAI-1(HG)+TN-C(HG) vs. C | 85% | 72% | 71% | 85% | 0.81 | 0.0000 |
| STMN-1+TN-C vs. C | 37% | 78% | 100% | 49% | 0.45 | 0.4883 |
| STMN-1(NMIBC)+TN-C(NMIBC) vs. C | 43% | 98% | 100% | 53% | 0.71 | 0.0003 |
| STMN-1(MIBC)+TN-C(MIBC) vs. C | 82% | 72% | 50% | 92% | 0.78 | 0.0020 |
| STMN-1(LG)+TN-C(LG) vs. C | 33% | 99% | 100% | 64% | 0.63 | 0.0652 |
| STMN-1(HG)+TN-C(HG) vs. C | 88% | 70% | 70% | 88% | 0.83 | 0.0000 |
| HAI-1(NMIBC)+STMN-1(NMIBC)+TN-C(NMIBC) vs. HAI-1(MIBC)+STMN-1(MIBC)+TN-C(MIBC) | 98% | 10% | 81% | 50% | 0.41 | 0.3725 |
| HAI-1(LG)+STMN-1(LG)+TN-C(LG) vs. HAI-1(HG)+STMN-1(HG)+TN-C(HG) | 97% | 4% | 54% | 50% | 0.32 | 0.0152 |
| HAI-1(NMIBC)+STMN-1(NMIBC) vs. HAI-1(MIBC)+STMN-1(MIBC) | 84% | 19% | 80% | 22% | 0.41 | 0.3375 |
| HAI-1(LG)+STMN-1(LG) vs. HAI-1(HG)+STMN-1(HG) | 100% | 0% | - | - | 0.32 | 0.0156 |
| HAI-1(NMIBC)+TN-C(NMIBC) vs. HAI-1(MIBC)+TN-C(MIBC) | 97% | 9% | 81% | 33% | 0.42 | 0.3721 |
| HAI-1(LG)+TN-C(LG) vs. HAI-1(HG)+TN-C(HG) | 97% | 4% | 54% | 50% | 0.33 | 0.0157 |
| STMN-1(NMIBC)+TN-C(NMIBC) vs. STMN1(MIBC)+TN-C(MIBC) | 98% | 10% | 81% | 50% | 0.41 | 0.3725 |
| STMN-1(LG)+TN-C(LG) vs. STMN-1(HG)+TN-C(HG) | 97% | 4% | 54% | 50% | 0.32 | 0.0152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, B.; Małkiewicz, B.; Dembowski, J.; Piwowar, A. Usability Evaluation of Urinary HAI-1, STMN-1 and TN-C in the Diagnosis of Bladder Cancer. J. Clin. Med. 2025, 14, 3664. https://doi.org/10.3390/jcm14113664
Szymańska B, Małkiewicz B, Dembowski J, Piwowar A. Usability Evaluation of Urinary HAI-1, STMN-1 and TN-C in the Diagnosis of Bladder Cancer. Journal of Clinical Medicine. 2025; 14(11):3664. https://doi.org/10.3390/jcm14113664
Chicago/Turabian StyleSzymańska, Beata, Bartosz Małkiewicz, Janusz Dembowski, and Agnieszka Piwowar. 2025. "Usability Evaluation of Urinary HAI-1, STMN-1 and TN-C in the Diagnosis of Bladder Cancer" Journal of Clinical Medicine 14, no. 11: 3664. https://doi.org/10.3390/jcm14113664
APA StyleSzymańska, B., Małkiewicz, B., Dembowski, J., & Piwowar, A. (2025). Usability Evaluation of Urinary HAI-1, STMN-1 and TN-C in the Diagnosis of Bladder Cancer. Journal of Clinical Medicine, 14(11), 3664. https://doi.org/10.3390/jcm14113664







