Cardiovascular Disease Risk in Women with Menopause
Abstract
1. Introduction
2. Methods
3. Menopause as a Risk Factor for CVD
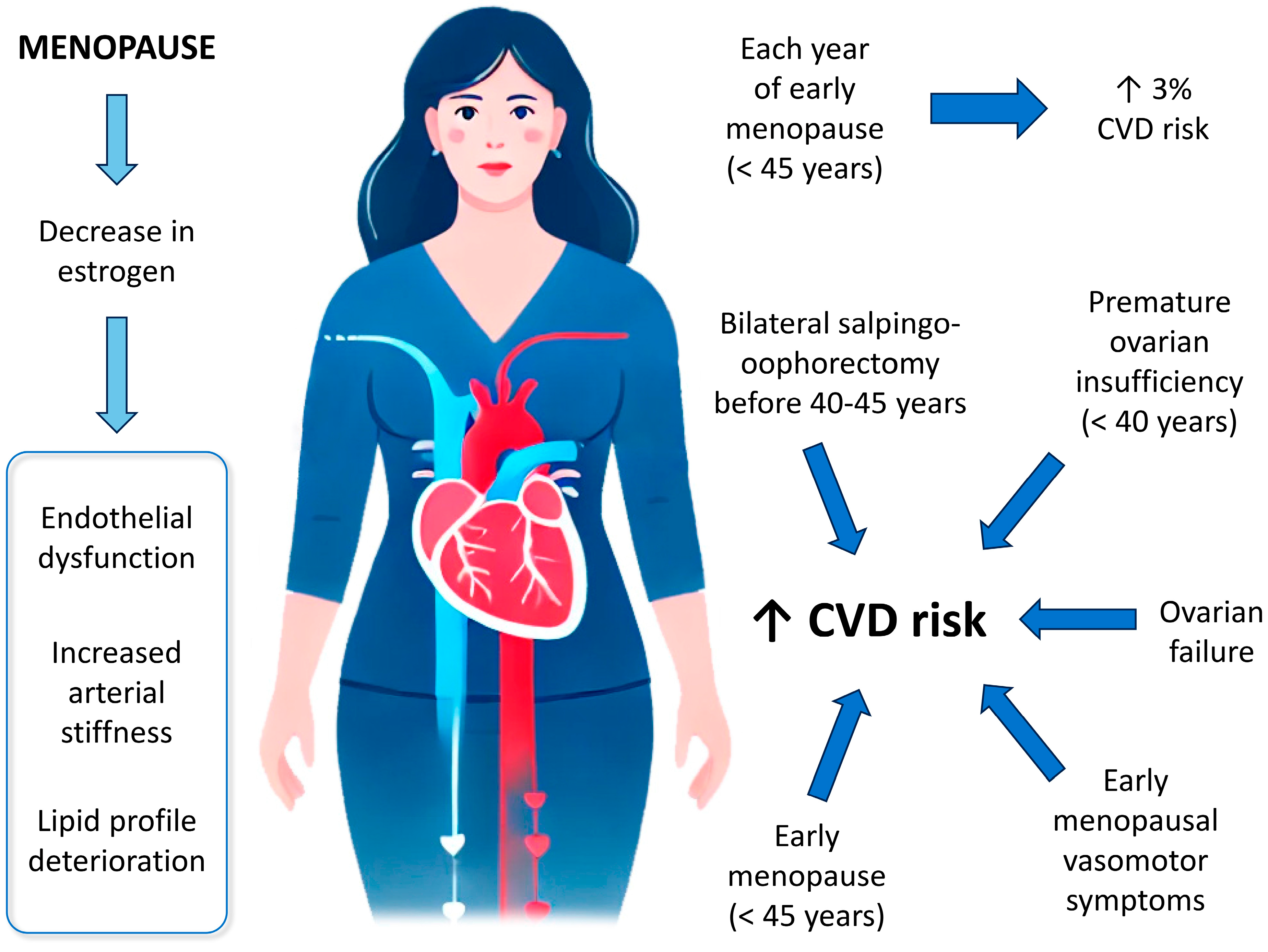
3.1. Cardiovascular Health-Related Changes During Menopause
3.2. Characteristics of Menopause About the Risk of CVD
3.2.1. Age of Onset of Menopause
3.2.2. Type or Cause of Menopause
3.2.3. Stage of Menopause
3.2.4. Endogenous Estrogens Levels
3.2.5. Vasomotor Symptoms
3.2.6. Other Symptoms of Menopause
3.2.7. Novel Biomarkers for Cardiovascular Risk Prediction in Postmenopausal Women
4. Strategies for the Prevention of CVD During Menopause
4.1. Hormone Therapy
4.2. Lifestyle Changes
4.3. Lipid-Lowering Therapy in Women
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| BSO | Bilateral salpingo-oophorectomy |
| CEE | Conjugated equine estrogens |
| CHD | Coronary heart disease |
| CVD | Cardiovascular disease |
| DASH | Dietary Approaches to Stop Hypertension |
| FDA | Food and Drug Administration |
| FSH | Follicle-stimulating hormone |
| HDL | High-density lipoprotein |
| HMG-CoA | Hydroxymethylglutaryl-CoA |
| hs-CRP | high-sensitivity C-reactive protein |
| HT | Hormone therapy |
| LDL | Low-density lipoprotein |
| LH | Luteinizing hormone |
| MeSH | Medical Subject Headings |
| MI | Myocardial infarction |
| MPA | Medroxyprogesterone acetate |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| WHI | Women’s health initiative |
| WHLP | Women’s Healthy Lifestyle Project |
| WISE | Women’s Ischemia Syndrome Evaluation |
References
- Montoy, J.C.C.; Shen, Y.C.; Hsia, R.Y. Trends in inequities in the treatment of and outcomes for women and minorities with myocardial infarction. Ann. Emerg. Med. 2022, 80, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The lancet women and cardiovascular disease commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.E.M.; Rosano, G.; Cifkova, R.; Chieffo, A.; van Dijken, D.; Hamoda, H.; Kunadian, V.; Laan, E.; Lambrinoudaki, I.; Maclaran, K.; et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: A consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur. Heart J. 2021, 42, 967–984. [Google Scholar] [CrossRef]
- Castro Conde, A.; Goya, M.; Delgado Marín, J.L.; Martínez Sánchez, N.; Pallarés Carratalá, V.; Obaya, J.C.; Díaz Sánchez, S.; Castellanos Rodríguez, A.; Mayó Amengua, A.; Seoane Vicente, M.C.; et al. Follow-up recommendations for the “fourth trimester” in women with vascular and metabolic complications during pregnancy. Consensus document of SEC, SEMERGEN, semFYC, and SEGO. Rev. Esp. Cardiol. 2020, 55, 38–46. [Google Scholar]
- Angeli, F.; Ricci, F.; Moscucci, F.; Sciomer, S.; Bucciarelli, V.; Bianco, F.; Mattioli, A.V.; Pizzi, C.; Gallina, S. Sex- and gender-related disparities in chest pain syndromes: The feminine mystique of chest pain. Curr. Probl. Cardiol. 2024, 49, 102457. [Google Scholar] [CrossRef]
- Mosca, L.; Hammond, G.; Mochari-Greenberger, H.; Towfighi, A.; Albert, M.A.; American Heart Association Cardiovascular Disease and Stroke in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular Nursing; Council on High Blood Pressure Research; Council on Nutrition Physical Activity and Metabolism. Fifteen-year trends in awareness of heart disease in women: Results of a 2012 American heart association national survey. Circulation 2013, 127, 1254–1263. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Andersen, H.; Sprague, E.; Burns, A.; Keida, M.; Walsh, M.N.; Greenberger, P.; Campbell, S.; Pollin, I.; McCullough, C.; et al. Knowledge, attitudes, and beliefs regarding cardiovascular disease in women: The women’s heart alliance. J. Am. Coll. Cardiol. 2017, 70, 123–132. [Google Scholar] [CrossRef]
- Kek, H.P.; Su, Y.T.; Tey, S.J.; Yang, M.C.; Chang, L.C.; Hung, Y.H.; Tsai, C.C. The joint effect of gestational diabetes mellitus and hypertension contribute to higher risk of diabetes mellitus after delivery: A nationwide population-based study. BMC Pregnancy Childbirth 2023, 23, 539. [Google Scholar] [CrossRef]
- Marschner, S.; Pant, A.; Henry, A.; Maple-Brown, L.J.; Moran, L.; Cheung, N.W.; Chow, C.K.; Zaman, S. Cardiovascular risk management following gestational diabetes and hypertensive disorders of pregnancy: A narrative review. Med. J. Aust. 2023, 218, 484–491. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, H.; Lin, S.; Zheng, Y. Reproductive factors and risk of cardiovascular diseases and all-cause and cardiovascular mortality in American women: NHANES 2003–2018. BMC Womens Health 2024, 24, 222. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.S.; Beckie, T.M.; DeVon, H.A.; Grines, C.L.; Krumholz, H.M.; Johnson, M.N.; Lindley, K.J.; Vaccarino, V.; Wang, T.Y.; Watson, K.E.; et al. Acute myocardial infarction in women: A scientific statement from the American heart association. Circulation 2016, 133, 916–947. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics: 2013 update: A report from the American heart association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart disease and stroke statistics-2016 update: A report from the American heart association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause transition and cardiovascular disease risk: Implications for timing of early prevention: A scientific statement from the American heart association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G.; et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.C.; McNamara, P.M.; Gordon, T. Menopause and risk of cardiovascular disease: The framingham study. Ann. Intern. Med. 1976, 85, 447–452. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Johnson, B.D.; Sharaf, B.L.; Bittner, V.; Berga, S.L.; Braunstein, G.D.; Hodgson, T.K.; Matthews, K.A.; Pepine, C.J.; Reis, S.E.; et al. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: A report from the NHLBI-sponsored wise study. J. Am. Coll. Cardiol. 2003, 41, 413–419. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J.; STRAW +10 Collaborative Group. Executive summary of the stages of reproductive aging workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab. 2012, 97, 1159–1168. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Thurston, R.C. Cardiovascular implications of the menopause transition: Endogenous sex hormones and vasomotor symptoms. Obstet. Gynecol. Clin. N. Am. 2018, 45, 641–661. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Greendale, G.; Crawford, S.L.; Avis, N.E.; Brooks, M.M.; Thurston, R.C.; Karvonen-Gutierrez, C.; Waetjen, L.E.; Matthews, K. The menopause transition and women’s health at midlife: A progress report from the study of women’s health across the nation (swan). Menopause 2019, 26, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chung, H.F.; Pandeya, N.; Dobson, A.J.; Cade, J.E.; Greenwood, D.C.; Crawford, S.L.; Avis, N.E.; Gold, E.B.; Mitchell, E.S.; et al. Relationships between intensity, duration, cumulative dose, and timing of smoking with age at menopause: A pooled analysis of individual data from 17 observational studies. PLoS Med. 2018, 15, e1002704. [Google Scholar] [CrossRef] [PubMed]
- Thurston, R.C.; Karvonen-Gutierrez, C.A.; Derby, C.A.; El Khoudary, S.R.; Kravitz, H.M.; Manson, J.E. Menopause versus chronologic aging: Their roles in women’s health. Menopause 2018, 25, 849–854. [Google Scholar] [CrossRef]
- Gurka, M.J.; Vishnu, A.; Santen, R.J.; DeBoer, M.D. Progression of metabolic syndrome severity during the menopausal transition. J. Am. Heart Assoc. 2016, 5, e003609. [Google Scholar] [CrossRef]
- Matthews, K.A.; Crawford, S.L.; Chae, C.U.; Everson-Rose, S.A.; Sowers, M.F.; Sternfeld, B.; Sutton-Tyrrell, K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J. Am. Coll. Cardiol. 2009, 54, 2366–2373. [Google Scholar] [CrossRef]
- Guthrie, J.R.; Ball, M.; Dudley, E.C.; Garamszegi, C.V.; Wahlqvist, M.L.; Dennerstein, L.; Burger, H.G. Impaired fasting glycaemia in middle-aged women: A prospective study. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 646–651. [Google Scholar] [CrossRef]
- Davis, S.R.; Castelo-Branco, C.; Chedraui, P.; Lumsden, M.A.; Nappi, R.E.; Shah, D.; Villaseca, P.; Writing Group of the International Menopause Society for World Menopause Day 2012. Understanding weight gain at menopause. Climacteric 2012, 15, 419–429. [Google Scholar] [CrossRef]
- Drozdová, D.; Donková, Z.; Čerňanová, V.; Siváková, D. Body composition of Slovak midlife women with cardiovascular complications. Anthropol. Rev. 2016, 79, 169–180. [Google Scholar] [CrossRef]
- Kok, H.S.; van Asselt, K.M.; van der Schouw, Y.T.; van der Tweel, I.; Peeters, P.H.; Wilson, P.W.; Pearson, P.L.; Grobbee, D.E. Heart disease risk determines menopausal age rather than the reverse. J. Am. Coll. Cardiol. 2006, 47, 1976–1983. [Google Scholar] [CrossRef]
- Zhu, D.; Chung, H.F.; Pandeya, N.; Dobson, A.J.; Hardy, R.; Kuh, D.; Brunner, E.J.; Bruinsma, F.; Giles, G.G.; Demakakos, P.; et al. Premenopausal cardiovascular disease and age at natural menopause: A pooled analysis of over 170,000 women. Eur. J. Epidemiol. 2019, 34, 235–246. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Nasr, A. Cardiovascular disease in women: Does menopause matter? Curr. Opin. Endocr. Metab. Res. 2022, 27, 100419. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Oliver-Williams, C.; Kunutsor, S.; Laven, J.S.; Fauser, B.C.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: A systematic review and meta-analysis. JAMA Cardiol. 2016, 1, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Appiah, D.; Schreiner, P.J.; Demerath, E.W.; Loehr, L.R.; Chang, P.P.; Folsom, A.R. Association of age at menopause with incident heart failure: A prospective cohort study and meta-analysis. J. Am. Heart Assoc. 2016, 5, e003769. [Google Scholar] [CrossRef]
- Zhu, D.; Chung, H.F.; Dobson, A.J.; Pandeya, N.; Giles, G.G.; Bruinsma, F.; Brunner, E.J.; Kuh, D.; Hardy, R.; Avis, N.E.; et al. Age at natural menopause and risk of incident cardiovascular disease: A pooled analysis of individual patient data. Lancet Public Health 2019, 4, e553–e564. [Google Scholar] [CrossRef]
- Colditz, G.A.; Willett, W.C.; Stampfer, M.J.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. Menopause and the risk of coronary heart disease in women. N. Engl. J. Med. 1987, 316, 1105–1110. [Google Scholar] [CrossRef]
- Lobo, R.A. Surgical menopause and cardiovascular risks. Menopause 2007, 14, 562–566. [Google Scholar] [CrossRef]
- Honigberg, M.C.; Zekavat, S.M.; Aragam, K.; Finneran, P.; Klarin, D.; Bhatt, D.L.; Januzzi Jr, J.L.; Scott, N.S.; Natarajan, P. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA 2019, 322, 2411–2421. [Google Scholar] [CrossRef]
- Ossewaarde, M.E.; Bots, M.L.; Verbeek, A.L.; Peeters, P.H.; van der Graaf, Y.; Grobbee, D.E.; van der Schouw, Y.T. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 2005, 16, 556–562. [Google Scholar] [CrossRef]
- Atsma, F.; Bartelink, M.L.; Grobbee, D.E.; van der Schouw, Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause 2006, 13, 265–279. [Google Scholar] [CrossRef]
- Son, M.K.; Lim, N.K.; Lim, J.Y.; Cho, J.; Chang, Y.; Ryu, S.; Cho, M.C.; Park, H.Y. Difference in blood pressure between early and late menopausal transition was significant in healthy Korean women. BMC Womens Health 2015, 15, 64. [Google Scholar] [CrossRef]
- Derby, C.A.; Crawford, S.L.; Pasternak, R.C.; Sowers, M.; Sternfeld, B.; Matthews, K.A. Lipid changes during the menopause transition in relation to age and weight: The study of women’s health across the nation. Am. J. Epidemiol. 2009, 169, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Wildman, R.P.; Matthews, K.; Thurston, R.C.; Bromberger, J.T.; Sutton-Tyrrell, K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause 2013, 20, 8–14. [Google Scholar] [CrossRef]
- Thurston, R.C.; Bhasin, S.; Chang, Y.; Barinas-Mitchell, E.; Matthews, K.A.; Jasuja, R.; Santoro, N. Reproductive hormones and subclinical cardiovascular disease in midlife women. J. Clin. Endocrinol. Metab. 2018, 103, 3070–3077. [Google Scholar] [CrossRef]
- Muka, T.; Oliver-Williams, C.; Colpani, V.; Kunutsor, S.; Chowdhury, S.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0157417. [Google Scholar] [CrossRef]
- Thurston, R.C.; Sutton-Tyrrell, K.; Everson-Rose, S.A.; Hess, R.; Matthews, K.A. Hot flashes and subclinical cardiovascular disease: Findings from the study of women’s health across the nation heart study. Circulation 2008, 118, 1234–1240. [Google Scholar] [CrossRef]
- Thurston, R.C.; Sutton-Tyrrell, K.; Everson-Rose, S.A.; Hess, R.; Powell, L.H.; Matthews, K.A. Hot flashes and carotid intima media thickness among midlife women. Menopause 2011, 18, 352–358. [Google Scholar] [CrossRef]
- Thurston, R.C.; El Khoudary, S.R.; Tepper, P.G.; Jackson, E.A.; Joffe, H.; Chen, H.Y.; Matthews, K.A. Trajectories of vasomotor symptoms and carotid intima media thickness in the study of women’s health across the nation. Stroke 2016, 47, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Sigureδardóttir, E.S.; Gislason, T.; Benediktsdottir, B.; Hustad, S.; Dadvand, P.; Demoly, P.; Franklin, K.A.; Heinrich, J.; Holm, M.; van der Plaat, D.A.; et al. Female sex hormones and symptoms of obstructive sleep apnea in European women of a population-based cohort. PLoS ONE 2022, 17, e0269569. [Google Scholar] [CrossRef]
- Hall, M.H.; Okun, M.L.; Sowers, M.; Matthews, K.A.; Kravitz, H.M.; Hardin, K.; Buysse, D.J.; Bromberger, J.T.; Owens, J.F.; Karpov, I.; et al. Sleep is associated with the metabolic syndrome in a multi-ethnic cohort of midlife women: The swan sleep study. Sleep 2012, 35, 783–790. [Google Scholar] [CrossRef]
- Thurston, R.C.; Chang, Y.; von Kanel, R.; Barinas-Mitchell, E.; Jennings, J.R.; Hall, M.H.; Santoro, N.; Buysse, D.J.; Matthews, K.A. Sleep characteristics and carotid atherosclerosis among midlife women. Sleep 2017, 40, zsw052. [Google Scholar] [CrossRef]
- Matthews, K.A.; Everson-Rose, S.A.; Kravitz, H.M.; Lee, L.; Janssen, I.; Sutton-Tyrrell, K. Do reports of sleep disturbance relate to coronary and aortic calcification in healthy middle-aged women?: Study of women’s health across the nation. Sleep Med. 2013, 14, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, R.; Li, C.; Tao, M. Sleep disorder, an independent risk associated with arterial stiffness in menopause. Sci. Rep. 2017, 7, 1904. [Google Scholar] [CrossRef]
- Janssen, I.; Powell, L.H.; Matthews, K.A.; Jasielec, M.S.; Hollenberg, S.M.; Bromberger, J.T.; Sutton-Tyrrell, K.; Everson-Rose, S.A. Relation of persistent depressive symptoms to coronary artery calcification in women aged 46 to 59 years. Am. J. Cardiol. 2016, 117, 1884–1889. [Google Scholar] [CrossRef]
- Wassertheil-Smoller, S.; Shumaker, S.; Ockene, J.; Talavera, G.A.; Greenland, P.; Cochrane, B.; Robbins, J.; Aragaki, A.; Dunbar-Jacob, J. Depression and cardiovascular sequelae in postmenopausal women: The women’s health initiative (WHI). Arch. Intern. Med. 2004, 164, 289–298. [Google Scholar] [CrossRef]
- Vorobeľová, L.; Falbová, D.; Candráková Čerňanová, V. The importance of female reproductive history on self-reported sleep quality, mood, and urogenital symptoms in midlife. Menopause 2023, 30, 1157–1166. [Google Scholar] [CrossRef]
- de Santis, I.P.; Lindenau, J.D.; Ramos, R.B.; Silva, T.R.; Casanova, G.; Oppermann, K.; Spritzer, P.M. C-reactive protein gene rs1205 polymorphism is associated with low-grade chronic inflammation in postmenopausal women. Womens Midlife Health 2020, 6, 3. [Google Scholar] [CrossRef]
- Zhang, W.; Speiser, J.L.; Ye, F.; Tsai, M.Y.; Cainzos-Achirica, M.; Nasir, K.; Herrington, D.M.; Shapiro, M.D. High-sensitivity c-reactive protein modifies the cardiovascular risk of lipoprotein(a): Multi-ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 2021, 78, 1083–1094. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, J.H. Early elevation of high-sensitivity c-reactive protein as a predictor for cardiovascular disease incidence and all-cause mortality: A landmark analysis. Sci. Rep. 2023, 13, 14118. [Google Scholar] [CrossRef]
- Vinci, P.; Di Girolamo, F.G.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Altamura, N.; Pirulli, A.; Zaccari, M.; Biasinutto, C.; et al. Lipoprotein(a) as a risk factor for cardiovascular diseases: Pathophysiology and treatment perspectives. Int. J. Environ. Res. Public Health 2023, 20, 6721. [Google Scholar] [CrossRef]
- Ridker, P.M.; Moorthy, M.V.; Cook, N.R.; Rifai, N.; Lee, I.M.; Buring, J.E. Inflammation, cholesterol, lipoprotein(a), and 30-year cardiovascular outcomes in women. N. Engl. J. Med. 2024, 391, 2087–2097. [Google Scholar] [CrossRef]
- Cybulska, A.M.; Schneider-Matyka, D.; Walaszek, I.; Panczyk, M.; Cwiek, D.; Lubkowska, A.; Grochans, E.; Rachubinska, K.; Malewicz, K.; Chabowski, M. Predictive biomarkers for cardiometabolic risk in postmenopausal women: Insights into visfatin, adropin, and adiponectin. Front. Endocrinol. 2025, 16, 1527567. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Tuz-Zahra, F.; Godbole, S.; Avitia, Y.; Bellettiere, J.; Rock, C.L.; Jankowska, M.M.; Allison, M.A.; Dunstan, D.W.; Rana, B.; et al. Endothelial-derived cardiovascular disease-related microRNAs elevated with prolonged sitting pattern among postmenopausal women. Sci. Rep. 2021, 11, 11766. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Fan, X.; Wang, J.; Yin, Y.; Zhang, Y.; Shi, K.; Yu, F. The role of gut microbiota and trimethylamine n-oxide in cardiovascular diseases. J. Cardiovasc. Transl. Res. 2023, 16, 581–589. [Google Scholar] [CrossRef]
- Pilz, N.; Heinz, V.; Ax, T.; Fesseler, L.; Patzak, A.; Bothe, T.L. Pulse wave velocity: Methodology, clinical applications, and interplay with heart rate variability. Rev. Cardiovasc. Med. 2024, 25, 266. [Google Scholar] [CrossRef]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.; Howard, B.V.; Thomson, C.A.; LaCroix, A.Z.; et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the women’s health initiative randomized trials. JAMA 2013, 310, 1353–1368. [Google Scholar] [CrossRef]
- Lokkegaard, E.; Andreasen, A.H.; Jacobsen, R.K.; Nielsen, L.H.; Agger, C.; Lidegaard, O. Hormone therapy and risk of myocardial infarction: A national register study. Eur. Heart J. 2008, 29, 2660–2668. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Manson, J.E.; Hsia, J.; Johnson, K.C.; Rossouw, J.E.; Assaf, A.R.; Lasser, N.L.; Trevisan, M.; Black, H.R.; Heckbert, S.R.; Detrano, R.; et al. Estrogen plus progestin and the risk of coronary heart disease. N. Engl. J. Med. 2003, 349, 523–534. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J. Menopausal hormone replacement therapy and reduction of all-cause mortality and cardiovascular disease: It is about time and timing. Cancer J. 2022, 28, 208–223. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; Lobo, R.A.; Shoupe, D.; Sevanian, A.; Mahrer, P.R.; Selzer, R.H.; Liu, C.R.; Liu, C.H.; Azen, S.P.; et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2001, 135, 939–953. [Google Scholar] [CrossRef]
- Grodstein, F.; Stampfer, M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Prog. Cardiovasc. Dis. 1995, 38, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F.; Stampfer, M.J. Estrogen for women at varying risk of coronary disease. Maturitas 1998, 30, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Mack, W.J. Randomized controlled trials and the effects of postmenopausal hormone therapy on cardiovascular disease: Facts, hypotheses and clinical perspective. In Treatment of the Postmenopausal Woman; Lobo, R.A., Ed.; Elsevier Academic Press: Philadelphia, PA, USA, 2007; pp. 529–564. [Google Scholar]
- Salpeter, S.R.; Walsh, J.M.E.; Greyber, E.; Ormiston, T.M.; Salpeter, E.E. Mortality associated with hormone replacement therapy in younger and older women: A meta-analysis. J. Gen. Intern. Med. 2004, 19, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Salpeter, S.R.; Walsh, J.M.E.; Greyber, E.; Salpeter, E.E. Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J. Gen. Intern. Med. 2006, 21, 363–366. [Google Scholar] [CrossRef]
- Culver, A.L.; Ockene, I.S.; Balasubramanian, R.; Olendzki, B.C.; Sepavich, D.M.; Wactawski-Wende, J.; Manson, J.E.; Qiao, Y.; Liu, S.; Merriam, P.A.; et al. Statin use and risk of diabetes mellitus in postmenopausal women in the women’s health initiative. Arch. Intern. Med. 2012, 172, 144–152. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Walsh, J.M.E.; Ormiston, T.M.; Greyber, E.; Buckley, N.S.; Salpeter, E.E. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 2006, 8, 538–554. [Google Scholar] [CrossRef]
- Aldhaleei, W.A.; Bhagavathula, A.S.; Wallace, M.B.; DeVault, K.R.; Faubion, S.S. The association between menopausal hormone therapy and gastroesophageal reflux disease: A systematic review and meta-analysis. Menopause 2023, 30, 867–872. [Google Scholar] [CrossRef]
- Harper-Harrison, G.; Carlson, K.; Shanahan, M.M. Hormone Replacement Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mikkola, T.S.; Tuomikoski, P.; Lyytinen, H.; Korhonen, P.; Hoti, F.; Vattulainen, P.; Gissler, M.; Ylikorkala, O. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J. Clin. Endocrinol. Metab. 2015, 100, 4588–4594. [Google Scholar] [CrossRef]
- Cho, L.; Kaunitz, A.M.; Faubion, S.S.; Hayes, S.N.; Lau, E.S.; Pristera, N.; Scott, N.; Shifren, J.L.; Shufelt, C.L.; Stuenkel, C.A.; et al. Rethinking menopausal hormone therapy: For whom, what, when, and how long? Circulation 2023, 147, 597–610. [Google Scholar] [CrossRef]
- British Menopause Society. Management of Menopause for Women with Cardiovascular Disease. Available online: https://thebms.org.uk/wp-content/uploads/2024/12/22-BMS-TfC-Management-of-menopause-for-women-with-CVD-DEC2024-A.pdf (accessed on 13 May 2025).
- Chen, M.N.; Lin, C.C.; Liu, C.F. Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review. Climacteric 2015, 18, 260–269. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J. The effects of a saffron extract (affron®) on menopausal symptoms in women during perimenopause: A randomised, double-blind, placebo-controlled study. J. Menopausal. Med. 2021, 27, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.H.; Simkin-Silverman, L.R.; Wing, R.R.; Meilahn, E.N.; Ives, D.G. Women’s healthy lifestyle project: A randomized clinical trial: Results at 54 months. Circulation 2001, 103, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Hu, F.B.; Manson, J.E.; Rimm, E.B.; Willett, W.C. Primary prevention of coronary heart disease in women through diet and lifestyle. N. Engl. J. Med. 2000, 343, 16–22. [Google Scholar] [CrossRef]
- He, M.; Hu, S.; Wang, J.; Wang, J.; Gaman, M.A.; Hariri, Z.; Tian, Y. Effect of resistance training on lipid profile in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 288, 18–28. [Google Scholar] [CrossRef]
- Mora, S.; Glynn, R.J.; Hsia, J.; MacFadyen, J.G.; Genest, J.; Ridker, P.M. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity c-reactive protein or dyslipidemia: Results from the justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010, 121, 1069–1077. [Google Scholar]
- Bennett, S.; Sager, P.; Lipka, L.; Melani, L.; Suresh, R.; Veltri, E.; Ezetimibe Study Group. Consistency in efficacy and safety of ezetimibe coadministered with statins for treatment of hypercholesterolemia in women and men. J. Womens Health 2004, 13, 1101–1107. [Google Scholar] [CrossRef]
- Jeenduang, N. Circulating PCSK9 concentrations are increased in postmenopausal women with the metabolic syndrome. Clin. Chim. Acta 2019, 494, 151–156. [Google Scholar] [CrossRef]
| Change in lipids | ↑ Total cholesterol ↑ LDL-cholesterol ↑ Apolipoprotein B ↓ HDL-cholesterol |
| Metabolic changes | ↑ Metabolic syndrome |
| Change in vessels | ↑ Carotid atherosclerosis ↑ Carotid adventitial diameter ↑ Carotid intima–media thickness ↑ Arterial stiffness |
| Body changes | ↑ Fat mass ↓ Lean mass ↑ Ectopic fat deposition (especially in heart and liver) |
| Pros of HT | Cons of HT |
|---|---|
| Reduction in menopausal symptoms: HT alleviates common symptoms such as hot flashes, night sweats, and vaginal dryness. | Increased risk of breast cancer: Long-term use of HT, especially combined estrogen and progestin, is associated with a higher risk of breast cancer. |
| Potential improvement in lipid profiles: HT may lead to improved cholesterol levels, such as increased HDL-cholesterol and decreased LDL-cholesterol. | Increased risk of blood clots: HT can raise the risk of deep vein thrombosis and pulmonary embolism, especially in older women or those with other risk factors. |
| Possible reduction in coronary artery disease risk: Early initiation of HT (around the time of menopause) may reduce the risk of heart disease, particularly in younger women. | Increased risk of stroke: Some forms of HT, especially oral estrogen, have been linked to an elevated risk of ischemic stroke. |
| Bone health benefits: HT helps in maintaining bone density, reducing the risk of osteoporosis and fractures. | Potential increased risk of cardiovascular events: Evidence suggests HT may not significantly reduce cardiovascular risk and could even increase the risk in some women, particularly those who start HT later after menopause. |
| Improved endothelial function: HT has been shown to improve endothelial function, which is important for cardiovascular health. | Mood swings and mental health issues: Some women experience mood swings, anxiety, or depression as side effects of HT. |
| Possible protective effect on cognitive function: Early initiation of HT may help in preserving cognitive function and reduce the risk of dementia in some women. | Not suitable for all women: HT is contraindicated in women with a history of certain cancers (e.g., breast or endometrial), liver disease, or unexplained vaginal bleeding. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasero, M.; Coronado, P.J. Cardiovascular Disease Risk in Women with Menopause. J. Clin. Med. 2025, 14, 3663. https://doi.org/10.3390/jcm14113663
Fasero M, Coronado PJ. Cardiovascular Disease Risk in Women with Menopause. Journal of Clinical Medicine. 2025; 14(11):3663. https://doi.org/10.3390/jcm14113663
Chicago/Turabian StyleFasero, María, and Pluvio J. Coronado. 2025. "Cardiovascular Disease Risk in Women with Menopause" Journal of Clinical Medicine 14, no. 11: 3663. https://doi.org/10.3390/jcm14113663
APA StyleFasero, M., & Coronado, P. J. (2025). Cardiovascular Disease Risk in Women with Menopause. Journal of Clinical Medicine, 14(11), 3663. https://doi.org/10.3390/jcm14113663






