The Gastric Vein Variants: An Evidence-Based Systematic Review of Prevalence and Clinical Considerations
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Electronic Search
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Collection Process
2.6. Assessment of the Methodological Quality of the Included Studies
2.7. Statistical Methods
2.8. Subgroup Analysis
3. Results
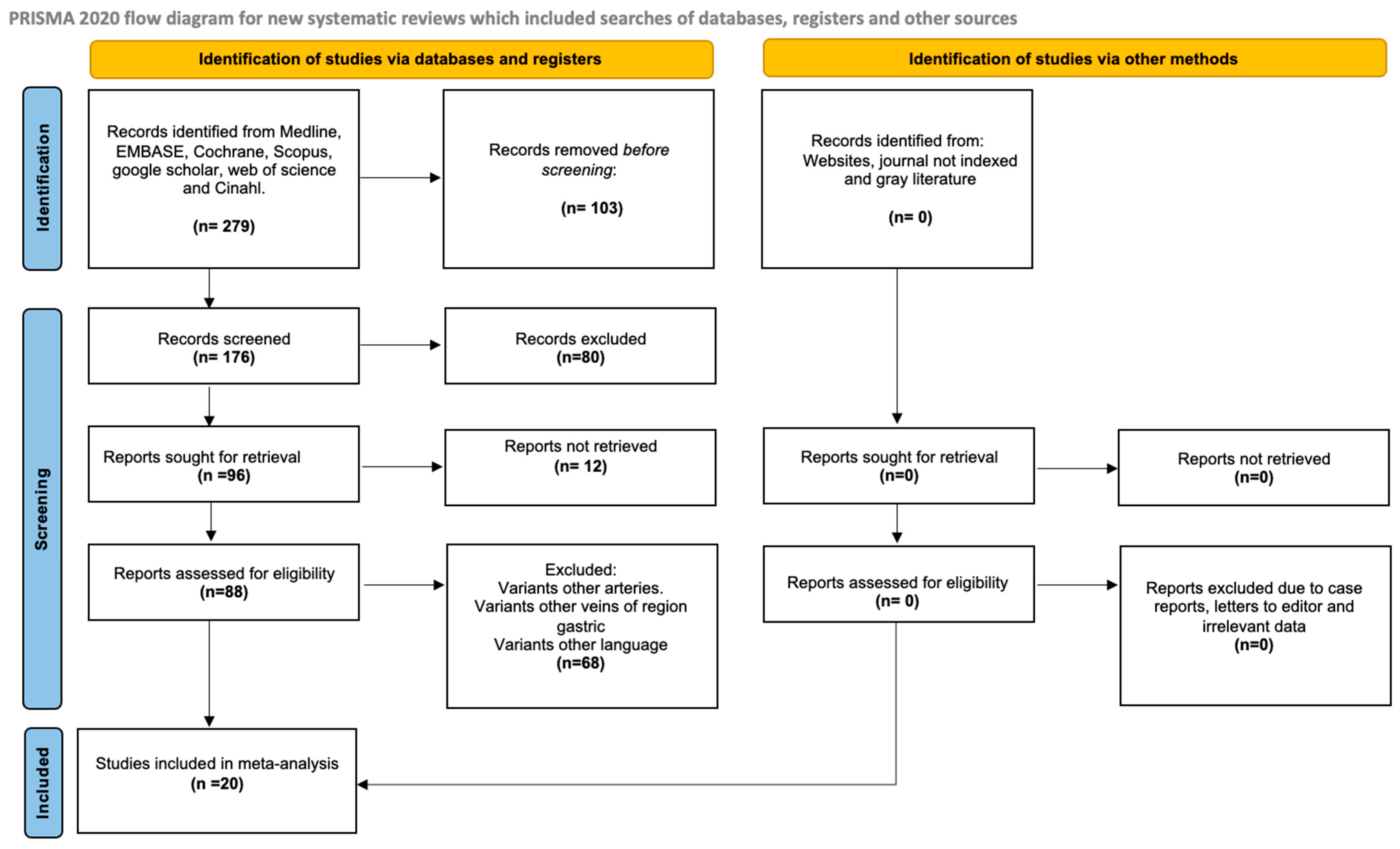
3.1. Selection of the Articles
3.2. Characteristics of the Included Studies
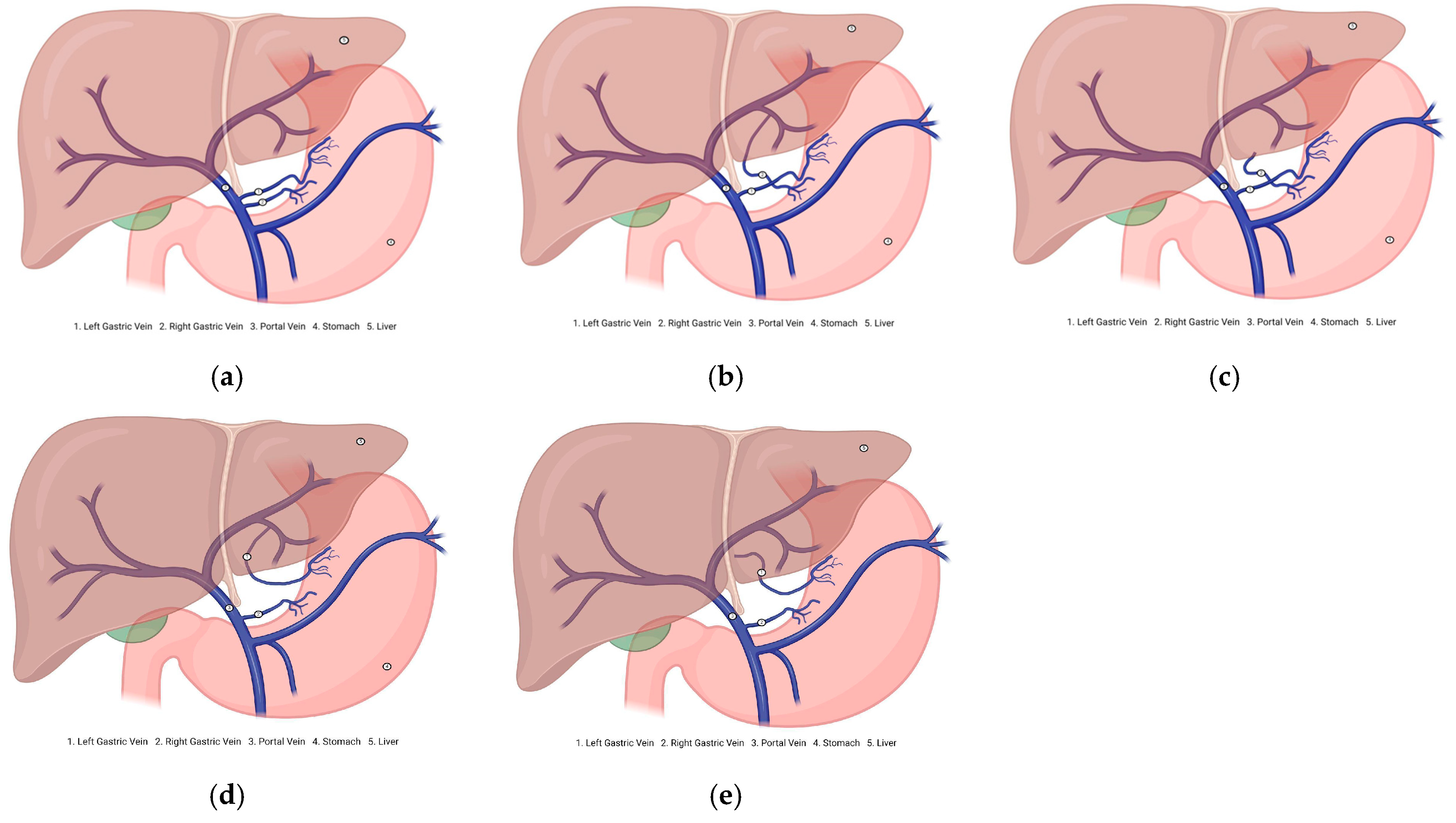
3.3. Description of the Variants
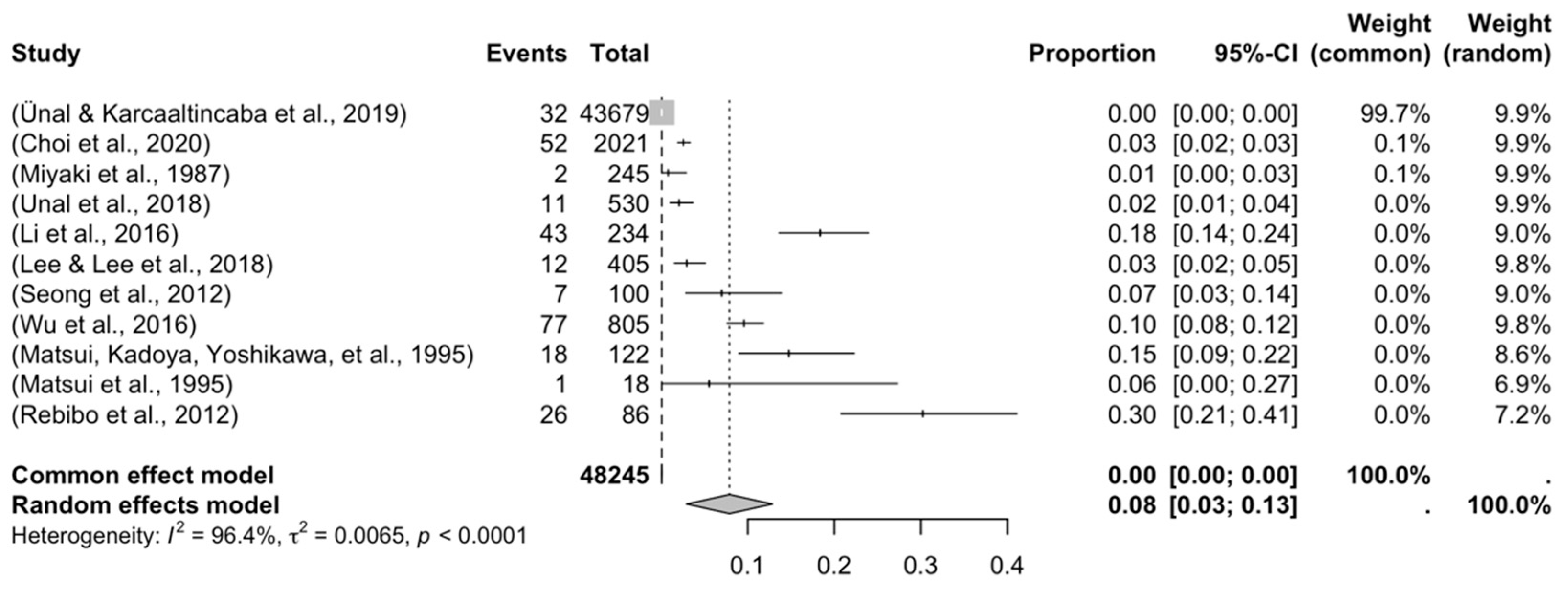
3.3.1. Prevalence and Subgroups Analyzed
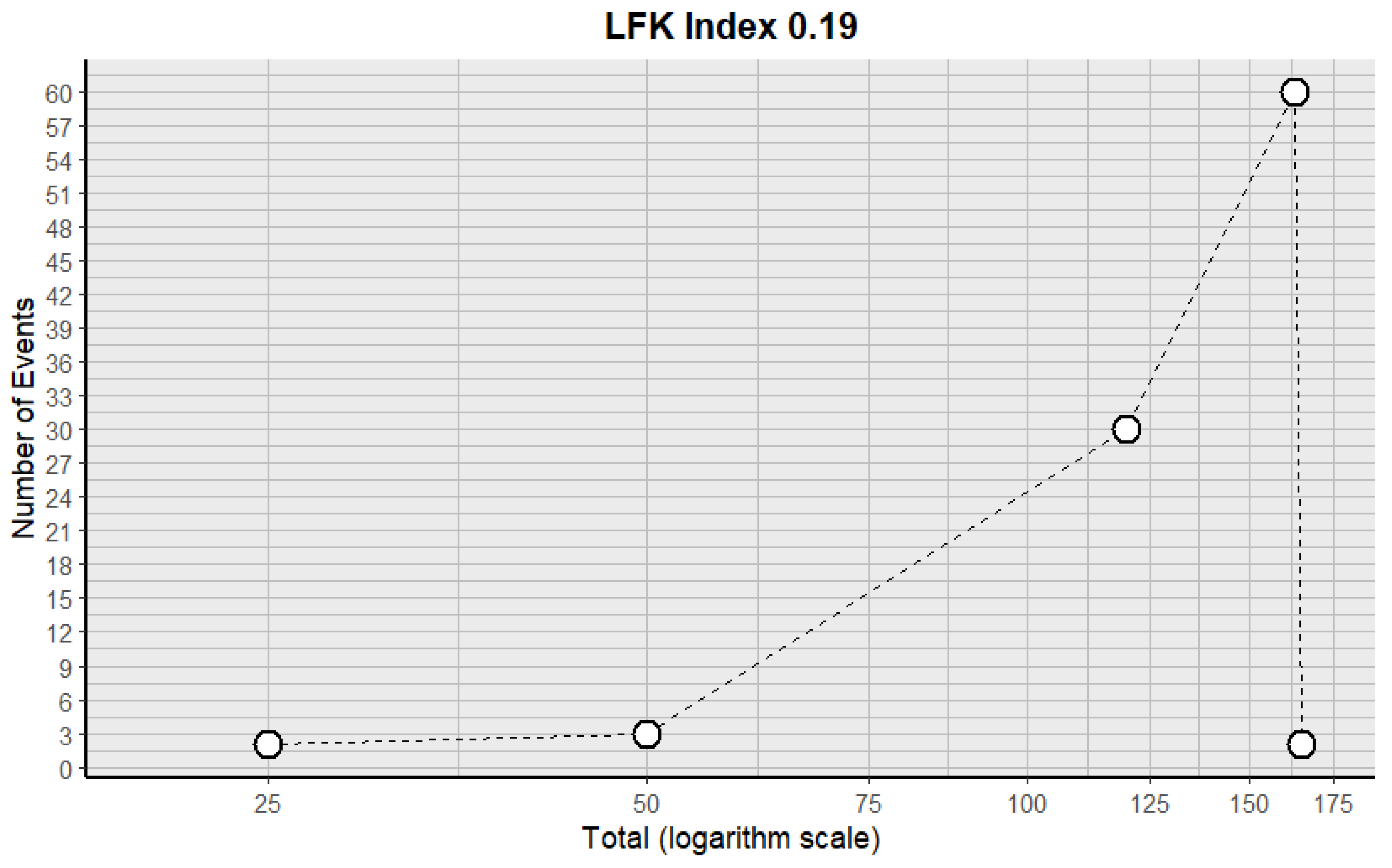
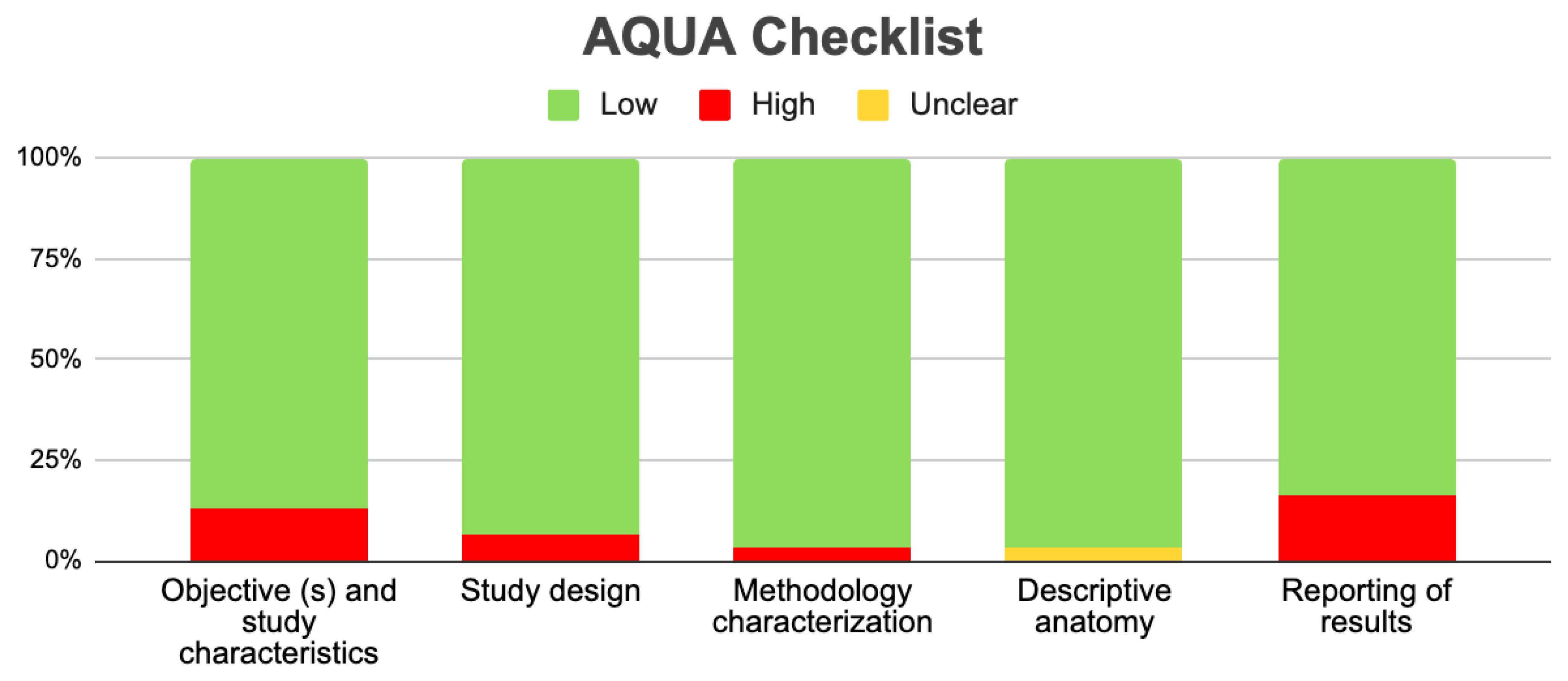
3.3.2. Risk of Bias in the Included Articles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Arviza, P.; Bombín, A.; Arrazola, J.; de Blas, C.S.; Talarico, E.F.; Bartolomé, A.M.P.; Gonzalez, A.V.; Gonzalez, L.E.; Rodriguez, C.S.; Munoz, M.D.; et al. Comparative anatomo-radiological study of intrahepatic venous vascularization in Spain. Ann. Anat. 2021, 237, 151740. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzade, M.; Akhlaghpoor, S.; Rouientan, H.; Hassanzadeh, S.; Ghorani, H.; Heidari-Foroozan, M.; Fathi, M.; Alemi, F.; Nouri, S.; Trinh, K.; et al. Splenic artery embolization for variceal bleeding in portal hypertension: A systematic review and meta-analysis. Emerg. Radiol. 2025, 32, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Akcali, A.; Ogan, B.; Buğra Sezen, C.; Metin, M. Giant left gastric artery aneurysm with intrathoracic extension. Saudi Med. J. 2025, 46, 199–201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, D.L.; Bermont, A.; Richter, V.; Shirin, H. Real world management of esophageal ulcers: Analysis of their presentation, etiology, and outcomes. Acta Gastroenterol. Belg. 2021, 84, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Rouviere, H.; Delmas, A. Human Anatomy, Descriptive, Topographical and Functional. Trunk, 11th ed.; Masson Publishing: Issy-les-Moulineaux, France, 2005. [Google Scholar]
- Dalley, A.F., II; Agur, A.M.R. Moore’s Clinically Oriented Anatomy, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2023. [Google Scholar]
- Loukas, M.; Louis, R.G., Jr.; Hullett, J.; Loiacano, M.; Skidd, P.; Wagner, T. An anatomical classification of the variations of the inferior phrenic vein. Surgical and radiologic anatomy. Surg. Radiol. Anat. 2005, 27, 566–574. [Google Scholar] [CrossRef]
- Frey, S.; De Mathelin, P.; Bachellier, P.; Addeo, P. Aberrant left gastric vein: What should surgeons know? Surg. Radiol. Anat. 2022, 44, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Karcaaltincaba, M. Aberrant left gastric vein is associated with hepatic artery variations. Abdom. Radiol. 2019, 44, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Karaosmanoglu, A.D.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Computed Tomography-Based Diagnosis of Gastric Vein Invasion in Patients with Gastric Cancer. Eurasian J. Med. 2018, 50, 91–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.Y.; Gao, B.L.; Song, B.; Fan, Q.Y.; Zhou, L.X.; Feng, P.Y.; Zhang, X.J.; Zhu, Q.F.; Xiang, C.; Peng, S.; et al. Evaluation of left gastric vein in Chinese healthy adults with multi-detector computed tomography. Postgrad. Med. 2016, 128, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Arhire, D.A.; Moraru, M.C.; Maxim, R.; Tarniceriu, C.C.; Partene Vicoleanu, S.A.; Haisan, A.; Nedelcu, I.; Nedelcu, A.H. Transient hepatic attenuation difference or fat sparing? Aberrant right gastric vein determining a pseudolesion at the border of the IInd/IIIrd liver segments. Review of developmental concepts. Folia Morphol. 2024, 83, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.W.; Chung, J.W.; Kim, H.C.; Choi, J.W.; Lee, M.; Hur, S.; Jae, H.J. Aberrant gastric venous drainage and associated atrophy of hepatic segment II: Computed tomography analysis of 2021 patients. Abdom. Radiol. 2020, 45, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Bezzi, M.; Broglia, L.; Lemos, A.A.; Rossi, P. Transjugular intrahepatic portosystemic shunt in portal vein thrombosis: Role of the right gastric vein with anomalous insertion. Cardiovasc. Intervent Radiol. 1995, 18, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Brandner, G.; Parrado, R.H.; Dias, A.P.; Pullat, R.C.; Lancaster, W.P. Gastric Outlet Obstruction From Preduodenal Portal Vein in an Adult. Am. Surg. 2023, 89, 6212–6214. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, M. Aberrant left gastric vein directly draining into the liver. Clin Anat. 2000, 13, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomaszewski, K.A.; Henry, B.M.; Kumar Ramakrishnan, P.; Roy, J.; Vikse, J.; Loukas, M.; Tubbs, R.S.; Walocha, J.A. Development of the Anatomical Quality Assurance (AQUA) checklist: Guidelines for reporting original anatomical studies. Clin. Anat. 2017, 30, 14–20. [Google Scholar] [CrossRef] [PubMed]
- von Hippel, P.T. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, R.; Gartlehner, G.; Grant, M.; Shamliyan, T.; Sedrakyan, A.; Wilt, T.J.; Griffith, L.; Oremus, M.; Raina, P.; Ismaila, A.; et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J. Clin. Epidemiol. 2011, 64, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Caty, L.; Denève, E.; Fontaine, C.; Guillem, P. Concurrent aberrant right gastric vein directly draining into the liver and variations of the hepatic artery. Surg. Radiol. Anat. 2004, 26, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Deger, S.; Bozer, A. Liver Pseudotumor Due to Aberrant Left Gastric Vein: A Case Report. J. Belg. Soc. Radiol. 2023, 107, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deneve, E.; Caty, L.; Fontaine, C.; Guillem, P. Simultaneous aberrant left and right gastric veins draining directly into the liver. Ann. Anat. 2003, 185, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Gabata, T.; Matsui, O.; Kadoya, M.; Ueda, K.; Kawamori, Y.; Yoshikawa, J.; Takashima, T. Aberrant gastric venous drainage in a focal spared area of segment IV in fatty liver: Demonstration with color Doppler sonography. Radiology 1997, 203, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, A.; Yoshimitsu, K.; Honda, H.; Kuroiwa, T.; Irie, H.; Tajima, T.; Jimi, M.; Chijiiwa, K.; Masuda, K. Pseudolesion in segment II of the liver observed on CT during arterial portography caused by the aberrant left gastric venous drainage. Abdom. Imaging 1999, 24, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, K.; Sun, S.; Berst, M.J.; Heery, S.D.; Fajardo, L.L. Portal vein occlusion with aberrant left gastric vein functioning as a hepatopetal collateral pathway. J. Vasc. Interv. Radiol. 2004, 15, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Kuwada, K.; Kuroda, S.; Kikuchi, S.; Hori, N.; Kubota, T.; Nishizaki, M.; Kagawa, S.; Fujiwara, T. Strategic approach to concurrent aberrant left gastric vein and aberrant left hepatic artery in laparoscopic distal gastrectomy for early gastric cancer: A case report. Asian J. Endosc. Surg. 2015, 8, 454–456. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J. Anatomic variations in the left gastric vein and their clinical significance during laparoscopic gastrectomy. Surg. Endosc. 2019, 33, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Matsui, O.; Kadoya, M.; Takahashi, S.; Yoshikawa, J.; Gabata, T.; Takashima, T.; Kitagawa, K. Focal sparing of segment IV in fatty livers shown by sonography and CT: Correlation with aberrant gastric venous drainage. AJR Am. J. Roentgenol. 1995, 164, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Matsui, O.; Takahashi, S.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Takashima, T.; Kitagawa, K. Pseudolesion in segment IV of the liver at CT during arterial portography: Correlation with aberrant gastric venous drainage. Radiology 1994, 193, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Matsui, O.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Takahashi, S.; Ueda, K.; Kawamori, Y.; Takashima, T.; Nakanuma, Y. Aberrant gastric venous drainage in cirrhotic livers: Imaging findings in focal areas of liver parenchyma. Radiology 1995, 197, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Anandpara, K.; Dey, A.K.; Kedar, P.; Hira, P.; Kale, S. Left Aberrant Gastric Vein Causing Isolated Left Hepatic Portal Venous Gas Secondary to an Incarcerated Diaphragmatic Hernia. Pol. J. Radiol. 2015, 80, 364–367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyaki, T.; Yamada, M.; Kumaki, K. Aberrant course of the left gastric vein in the human: Possibility of a persistent left portal vein. Cells Tissues Organs 1987, 130, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.C.; Fraum, T.J. Aberrant right gastric vein mimicking hepatic spread of prostate cancer on PSMA-PET/CT. Radiol. Case Rep. 2023, 18, 1140–1143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Natsume, T.; Shuto, K.; Kohno, T.; Ohira, G.; Tohma, T.; Sato, A.; Saito, H.; Ohta, T.; Kawahira, H.; Akai, T.; et al. Anatomic Variations of the Celiac Trunk and the Left Gastric Vein Assessing by Dual-Phase CT Angiography for Safety Laparoscopic Gastrectomy. J. Surg. Res. 2010, 158, 387–388. [Google Scholar] [CrossRef]
- Alfaro, A.J.; Herrera Ortiz, A.F.; Cardona Ortegón, J.D.; Varney, H.; Borrero León, R.; Torres, D.F.; Greiffenstein, J.; Dussan Tovar, C.A.; Aguirre, D.A. Aberrant Right and Left Gastric Veins as a Cause of Hepatic Pseudolesions: A Report of Three Cases. Cureus 2023, 15, e48455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rebibo, L.; Chivot, C.; Fuks, D.; Sabbagh, C.; Yzet, T.; Regimbeau, J.M. Three-dimensional computed tomography analysis of the left gastric vein in a pancreatectomy. HPB 2012, 14, 414–421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roi, D.J. Ultrasound anatomy of the left gastric vein. Clin. Radiol. 1993, 47, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Seong, N.J.; Chung, J.W.; Kim, H.C.; Park, J.H.; Jae, H.J.; An, S.B.; Cho, B.H. Right gastric venous drainage: Angiographic analysis in 100 patients. Korean J. Radiol. 2012, 13, 53–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terayama, N.; Matsui, O.; Tatsu, H.; Gabata, T.; Kinoshita, A.; Hasatani, K. Focal sparing of fatty liver in segment II associated with aberrant left gastric vein. Br. J. Radiol. 2004, 77, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Imaging of aberrant left gastric vein and associated pseudolesions of segments II and III of the liver and mimickers. Diagn. Interv. Radiol. 2015, 21, 105–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Chen, G.; Wu, P.; Zhu, J.; Peng, W.; Xing, C. CT imaging-based determination and classification of anatomic variations of left gastric vein. Surg. Radiol. Anat. 2017, 39, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Stefura, T.; Kacprzyk, A.; Droś, J.; Pędziwiatr, M.; Major, P.; Hołda, M.K. The venous trunk of henle (gastrocolic trunk): A systematic review and meta-analysis of its prevalence, dimensions, and tributary variations. Clin. Anat. 2018, 31, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Prado Neto, E.V.; Petroianu, A. Anatomical variations of portal venous system: Importance in surgical clinic. Arq. Bras. Cir. Dig. 2022, 35, e1666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García-Pagán, J.C. Derivación portosistémica, percutánea e intrahepática recubierta [Covered transjugular intrahepatic portosystemic shunts]. Gastroenterol. Hepatol. 2006, 29, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, T.; Nakamura, T.; Maeda, T. Aberrant gastric venous inflow to the left lobe of the liver parenchyma adjacent to the falciform ligament. Br. J. Radiol. 1999, 72, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Saad, P.F.; Razuk, A.; Telles, G.J.; Park, J.H.; Esteves, F.P.; Caffaro, R.A. Trashepatic left gastric vein embolization in the treatment of recurrent hemorrhaging in patients with schistosomiasis previously submitted to non-derivative surgery. Arq. Gastroenterol. 2012, 49, 238–244. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, K.Y.; Li, W.Q.; Li, Y.; Li, X.Y.; Ju, S. CT-based nomogram predicts esophageal gastric variceal bleeding in noncirrhotic portal hypertension caused by hepatic schistosomiasis. BMC Med. Inform. Decis. Mak. 2025, 25, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuki, M.; Kani, H.; Tatsugami, F.; Yoshikawa, S.; Narumi, Y. Preoperative assessment of vascular anatomy around the stomach by 3D imaging using MDCT before laparoscopy-assisted gastrectomy. Am. J. Roentgenol. 2004, 183, 145–151. [Google Scholar] [CrossRef]
- Harada, S.; Yamamoto, A.; Jogo, A.; Kageyama, K.; Nakano, M.; Murai, K.; Matsushita, K.; Nishida, N.; Kaminou, T.; Miki, Y. Transhepatic Antegrade Gastric Variceal Sclerotherapy: Comparing Outcomes with and without Initial Efferent Vein Embolization. J. Vasc. Interv. Radiol. 2025, 36, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.S.; Kavanagh, P.V.; Chen, M.Y. Noninvasive assessment of portomesenteric venous thrombosis: Current concepts and imaging strategies. J. Comput. Assist. Tomogr. 2002, 26, 392–401. Available online: https://journals.lww.com/jcat/fulltext/2002/05000/Systolically_Gated_3D_Phase_Contrast_MRA_of.00014.aspx (accessed on 19 May 2025). [CrossRef]
- Murphy, D.J.; Aghayev, A.; Steigner, M.L. Vascular CT and MRI: A practical guide to imaging protocols. Insights Imaging 2018, 9, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Ko, J.M.; Ahn, M.I.; Han, D.H.; Park, S.H. Dynamic CT and MRA findings of a case of portopulmonary venous anastomosis (PPVA) in a patient with portal hypertension: A case report and review of the literature. Acta Radiol. 2011, 52, 1044–1047. [Google Scholar] [CrossRef]
- Sahani, D.; Mehta, A.; Blake, M.; Prasad, S.; Harris, G. Preoperative hepatic vascular evaluation with CT and MR angiography: Implications for surgery. Radiographics 2004, 24, 1367–1380. [Google Scholar] [CrossRef]
- Young, V.; Rajeswaran, S. Management of portal hypertension in the pediatric population: A primer for the interventional radiologist. Semin. Interv. Radiol. 2018, 35, 174–184. [Google Scholar] [CrossRef]
- Laissy, J.P.; Trillaud, H.; Douek, P. MR angiography: Noninvasive vascular imaging of the abdomen. Abdom. Imaging 2002, 27, 553–569. [Google Scholar] [CrossRef]
- Malviya, K.K.; Verma, A. Importance of anatomical variation of the hepatic artery for complicated liver and pancreatic surgeries: A review emphasizing origin and branching. Diagnostics 2023, 13, 1233. [Google Scholar] [CrossRef] [PubMed]
- Patino, M.; Price, M.; Sahani, D. Computed tomography angiography of the hepatic, pancreatic, and splenic circulation. Radiol. Clin. N. Am. 2016, 54, 1173–1190. [Google Scholar] [CrossRef]
- Hoyos, S.; Vera, A.; Barragán, C.; Bejarano, D.; Lopez-Ruiz, A.M.; Grippi, F.; Mejia, A.; Molano, M.D.P. Surgical and interventional radiology management of vascular and biliary complications in liver transplantation: Narrative review. Dig. Dis. Interv. 2024, 8, 7–17. [Google Scholar] [CrossRef]
- Arkadopoulos, N.; Yiallourou, A.I.; Palialexis, C.; Stamatakis, E.; Kairi-Vassilatou, E.; Smyrniotis, V. Inferior vena cava obstruction and collateral circulation as unusual manifestations of hepatobiliary cystadenocarcinoma. Hepatobiliary & pancreatic diseases international. Hepatobiliary Pancreat Dis. Int. 2013, 12, 329–331. [Google Scholar] [CrossRef]
| Author (s) | Year | Geographic Region | Type Study/Sample (n) | Mean Age (Range)/Sex | GV Variants’ Classification |
|---|---|---|---|---|---|
| Ünal & Karcaaltincaba et al. | 2019 | Turkey | Abdominal CT scans/43,679 subjects | Not specified | 32 subjects with ALGV: Type 1: 22, Type 2: 3, and Type 3: 7 |
| Li et al. | 2016 | China | upper abdomen MDCTA/234 patients | 49.9 (18–81)/123 M and 111 F | Type 1: 108 (46.15%), Type 2: 72% (30.77%), Type 3: 34 (14.53%), and Type 4: 9 (3.85%) |
| Lee & Lee et al. | 2018 | South Korea | Gastrectomy for gastric AdenoCa/ 405 patients’ laparoscopic | 64 (30-92)/259 M and 146 F | Ia: 3.0% (n = 12), Ip: 48.1% (n = 195), II: 30.0% (n = 121), IIIa: 12.3% (n = 50), IIIp: 5.7% (n = 23) and IV: 1.0% (n = 4) |
| Kuwada et al. | 2015 | Japan | Preoperative Abdominal CT/1 Case | 60/F | 1/1 |
| Ishigami et al. | 2004 | USA | CTA/1 patient | 20/M | 1/1 |
| Frey et al. | 2022 | France | preoperative CT/3 patients | 49/M, 60/M, 59/M | 3/3 |
| Deneve et al. | 2003 | France | Autopsy/1 case | 64/F | 1/1 |
| Caty et al. | 2004 | France | Autopsy/1 case | 46/M | 1/1 |
| Choi et al. | 2020 | South Korea | CT/2021 patients | 62 (20–96)/1572 M and 449 F | Type 1: 31, Type 2: 21 |
| Arhire et al. | 2023 | Romania | Ultrasound and CT/2 patients | 59/M, 63/M | 1/1, 1/1 |
| Mittal et al. | 2015 | India | Ultrasound and CT/1 patient | 74/M | Type 1: 1 |
| Miyaki et al. | 1987 | Japan | dissection/245 patients | Not specified | Type 1: 1, Type 2: 1 |
| Ohkubo et al. | 2000 | Japan | Dissection/1 patient | 51/F | Type 1: 1 |
| Alfaro et al. | 2023 | Colombia | CT/3 patients | 62/F, 46/F, and 50/M | Type 1: 1, Type 2: 1, and Type 3: 1 |
| Seong et al. | 2012 | Korea | Gastric arteriography/100 patients | 56.2/73 M and 27 F | Type 1: 9, Type 2: 12 (2a: 3, 2b: 9), Type 3: 38 (3a: 22, 3b: 16), and Type 4: 7 |
| Unal et al. | 2015 | Turkey | Not specified | Not specified | Types 1, 2, and 3 |
| Unal et al. | 2018 | Turkey | CT/530 patients Venous invasion in 11 patients | 60/6 M and 5 F | Type 1: 3 Type 2: 2, Type 3: 2, Type 4: 2, and Type 5: 2 |
| Wu et al. | 2016 | China | CT/805 patients | 53/432 M and 393 F | Type 1: 401, Type 2: 166, Type 3: 161, Type 4: 59, Type 5: 12, Type 6: 6, and Type 7: 0 |
| Yamagami et al. | 1999 | Japan | CTA/1 patient | 50/M | Type 1: 1 |
| Muñoz & Fraum et al. | 2023 | United States | PSMA-PET/CT and MRI/1 patient | 53/M | Type 1: 1 |
| Bezzi et al. | 1995 | Italy | transjugular intrahepatic portosystemic shunt/2 patients | 50/M, 58/M | 2/2 |
| Deger & Bozer et al. | 2023 | Turkey | Abdominal US/1 patient | 49/M | 1/1 |
| Gabata et al. | 1997 | Japan | /17 patients with fatty liver | 56 (40 to 72 years)/12 M and 5 F | 5/17 |
| Hiwatashi et al. | 1999 | Japan | Angiography/1 patient | 54/M | 1/1 |
| Matsui et al. | 1994 | Japan | CT and CTAP (arterial portography)/Group A: 22 patients with pseudolesions in segment IV. Group B: 100 random patients without pseudolesions | 38–68 years/Group A: 15 M, 7 F 35–83 years/Group B: 67 M, 33 F | AGVD18 in group A AGVD 0/100 patients in group B |
| Matsui, Kadoya, Yoshikawa, et al. | 1995 | Japan | CT and CTAP/18 patients | 38–68 years/11M and 7 F | 17/18 and 1/18 |
| Matsui et al. | 1995 | Japan | CTAP and/or hepatic arteriography/17 patients | 43–73 years/8M, and 9 F | 7/17 |
| Natsume et al. | 2010 | Japan | 64-row MDCT (portal venography)/126 patients | 66/- | In 52 patients (44%), LCV flowed into the PV, 44 (37%) into the SV 22 (19%) into the junction of these two veins |
| Terayama et al. | 2004 | Japan | post-contrast helical CT/1 patient | 36/M | 1/1 |
| Roi et al. | 1993 | Wales | Abdomen US/86 patients | 3–83 years/24 M and 62 F | Drain Type 1: 26 (30%), Type 2: 28 (33%), Type 3: 32 (37%) Course (n = 83) Type 1A: 3, Type 1P: 21, Type 2A: 10, Type 2P: 17, Type 3A: 26 and Type 3P: 6 |
| Rebibo et al. | 2012 | France | CT/86 patients | Does not refer | Type A: 65%, Type B: 4.7%, and Type C: 30.3% |
| Author (s) | Year | Characteristics | Variant Type | Clinical Implications |
|---|---|---|---|---|
| Ünal & Karcaaltincaba et al. | 2019 | Type 1: Vein acting as a pure aberrant portal vein (APV) branching through the parenchyma. Type 2: The vein has a parenchymatous distribution and anastomosis to the left intrahepatic PV. Type 3: The vein has anastomosis to the left intrahepatic PV branch. | Drain | Hyperdensity at the posterior of segments II and III in type 1 (n = 20/22) and type 2 (n = 2/3) ALGV patients shows fat sparing from the third inflow effect. The ALGV maintains specific blood flow to liver segments during thrombosis. |
| Li et al. | 2016 | Type 1: The LGV originated from the PV. Type 2: The LGV originated from the SV. Type 3: The LGV originated from the angle between the PV and the SVs. Type 4: The LGV originated from the left branch of the PV. | Origin | Understanding the LGV anatomy is crucial before percutaneous transhepatic embolization of varicose veins or devascularization of the upper gastric region and abdominal esophagus. Moreover, in cases of esophageal variceal bleeding due to liver cirrhosis, LGV size indicates potential bleeding severity. |
| Lee & Lee et al. | 2018 | Type I: the LGV crossed the CHA and drained into the portal venous system (PVS). Type II: the LGV drained anteriorly to the CA Type III: The LGV crossed the SA and drained. Types I and III were further subdivided into anterior (a) and posterior (p) relative to CHA or SA. Type IV: the LGV drained directly into the liver parenchyma or the proximal PV near the PV bifurcation. | Course | Studying LGV variations before laparoscopic gastrectomy is crucial for preventing vein damage and preparing for potential hemorrhage. |
| Kuwada et al. | 2015 | The variant consisted of an ALGV and an ALHA directly entering the lateral segment of the liver via the hepatogastric ligament. The ALGV was divided at its entry point into the liver. | Drain | For curative lymph node dissection in gastric cancer, it is standard to cut the LGA and LGV at their origins. However, finding rare anomalies like concurrent ALGV and ALHA requires careful clinical consideration. |
| Ishigami et al. | 2004 | Large LGV contiguous to the superior lateral branch of the LPV through the gastrohepatic ligament, consistent with an ALGV. This aberrant vein is also connected to the posterior part of the umbilical portion of the LPV. | Drain | A retrospective analysis shows ALGV’s role as a decompression pathway, potentially reducing the severity of extensive variceal bleeding. This underscores the complexity of varicose vein pathophysiology and the need to evaluate anatomical abnormalities and their functional impact in treating portal hypertension and its complications. |
| Frey et al. | 2022 | Case 1: ALGV, which ends its course in the segment II parenchyma, corresponds to type 1 of the Unal classification, where it acts as a pure aberrant portal vein branching into liver sinusoids. Case 2: ALGV gives branches throughout the parenchyma and ends its course into the LPV, corresponding to type 2 of the Unal classification. Case 3: A large and single LGV is contiguous to the left branch of the portal vein, consistent with type 3 ALGV of the Unal classification. | Drain | ALGV serves as a hepatofugal collateral pathway in patients with advanced cirrhosis and portal hypertension. It also enables direct periportal spread of gastric tumors, affecting survival rates, especially in gastric cancer with venous invasion. Additionally, ALGV poses a risk of accidental hemorrhage during left gastric or hepatic surgeries. |
| Deneve et al. | 2003 | The LGV ascended from the lesser curvature and entered the left part of the porta hepatis. Similarly, the RGV ascended from the lesser curvature, passing in front of the common bile duct, and entered the porta hepatis directly. Both veins terminated in the intrahepatic segment of the LPV. | Drain | When the HPV becomes thrombosed, these variants may help maintain sufficient hepatopetal flow. As Bezzi suggests, they may be the only route for stenting a TIPS. |
| Caty et al. | 2004 | ARGV drains directly into the liver. While the LGV empties into the left aspect of the PV, the right one was observed to ascend from the lesser curvature of the stomach along the anterior right aspect of the common bile duct, directly reaching the porta hepatis. | Drain | The ARGV drains into the liver rather than the PV, potentially causing focal fatty infiltration and sparing in fatty liver disease. Imaging techniques, such as CT and ultrasound, can detect these anomalies, presenting as pseudolesions in the liver. |
| Choi et al. | 2020 |
Type 1: ARGV was observed in 31 patients (1.5%). Type 2: ALGV was observed in 21 patients (1.0%). | Drain | Segmental liver atrophy can create a benign pseudotumor, complicating diagnosis and misguiding care, especially for cancer patients. Furthermore, the area where the AGV drains may show focal sparing in fatty livers, focal fat deposition, or hyperplastic changes, mimicking hepatic tumors in imaging. Pseudolesions are often found in this area, influenced by different metabolic, toxic, and hormonal environments from varied venous blood supplies. |
| Arhire et al. | 2023 |
Case 1: The ARGV runs about 6 cm, curving backward to align with the left portal branch. Inside the venous ligament’s fissure, it extends towards the third liver segment, branching into small parenchymal vessels. Case 2: The ARGV runs approximately 6 cm in an anteroposterior direction, positioning itself anteromedial to the left branch of the portal vein. It then enters the third liver segment. In both cases, pseudolesions appeared as diffuse, homogeneous hyperdense areas with unclear borders at the II and III liver segment boundary, surrounded by hypodense, fatty hepatic parenchyma. | Drain | The ARGV may produce a pseudolesion due to hepatic blood inflow mismatch (third inflow and transient hepatic attenuation difference-related hemodynamic mechanisms) or an associated underlying metabolic cause, such as a focal fat-sparing area within diffuse hepatic fatty infiltration. These pseudolesions may mimic liver tumors, so it is vital to search for such an aberrant vessel to rule out other diagnoses. |
| Mittal et al. | 2015 | Type 1: ALGV joins directly to the left branch of the portal vein instead of draining into the main portal trunk. | Drain | The aberrant vein draining into the LPV instead of the main PV leads to isolated left hepatic portal venous gas, resulting in gastric pneumatosis and an incarcerated hiatal hernia. |
| Miyaki et al. | 1987 | Type 1: The vein enters the liver directly from the left side of the hilus. Type 2: The vein collects several branches from the lesser curvature. | Course | To understand the unusual course of the LGV in humans and to clarify whether the LGV originates from the omphalomesenteric or subintestinal vein. |
| Ohkubo et al. | 2000 | Type 1: The LGV originating from the lesser curvature of the stomach runs along the hepatic branch of the vagus nerve through the lesser omentum to reach the hepatic hilus and directly enters the liver. | Drain | Understanding anatomical knowledge of interportal venous communication is essential for properly treating bleeding esophageal varices or performing angiographic embolization. |
| Alfaro et al. | 2023 |
Type 1: Aberrant RGV drainage towards the liver causes a hypodense hepatic pseudolesion (HPS) located in hepatic segment IVb. Type 2: The LGV drains into the posterior margin of hepatic segment III, consistent with an ALGV causing a HPS localized to hepatic segments II and III. Type 3: The LGV drains into the posterior margin of hepatic segment III, consistent with an ALGV, and causes a small HPS localized to hepatic segment III. | Drain | AGVs may serve as an alternative venous drainage route in hypertensive gastropathy in cirrhotic patients or as a direct pathway for metastatic gastric cancer to the left liver. This variation can cause accidental bleeding during gastric or hepatic surgery, increasing surgery time and morbidity. Identifying AGVs on imaging is crucial to avoid misdiagnosis as liver lesions, which may lead to unnecessary procedures. |
| Seong et al. | 2012 |
Type 1: Smooth continuation as a single channel into the peripheral portal vein. Type 2: Collateral connection into the peripheral portal vein. 2a: Single collateral channel. 2b: Multiple collateral channels. Type 3: Superficial parenchymal blush formation in small areas without demonstrable portal branches. 3a: unifocal. 3b: multifocal. Type 4: Network formation around the sectional or segmental portal vein. | Drain | Aberrant gastric venous drainage is crucial due to pseudolesion formation in the portal phase of CT angiography, highlighting cavernous transformation in main portal thrombosis, and unexpected hemorrhage during hepatobiliary surgery from a missed aberrant gastric venous drainage. The ARGV may also serve as a direct metastatic pathway for gastric cancer in the lesser curvature and a potential route for hepatofugal arterioportal shunt in main portal vein tumor thrombosis. Additionally, it can serve as an alternative for stent placement in a transjugular intrahepatic portosystemic shunt with main portal thrombosis. |
| Unal et al. | 2015 |
Type 1: The vein functions as a pure aberrant portal vein, branching out and flowing through the sinusoids. Type 2: The vein exhibits a parenchymatous distribution with anastomosis to the portal vein. Type 3: The vein follows a more cranial course, connecting to intrahepatic portal vein branches. | Drain | ALGV in gastric cancer patients can cause tumor spread to the liver. ALGV-related pseudolesions affecting the posterior segments II and III can mimic metastases; therefore, MRI can differentiate between pseudolesions and true lesions. |
| Unal et al. | 2018 | Type 1: short gastric vein invaded. Patients survived 6, 7, and 256 days. Type 2: gastric vein invaded. Patients survived 60 and 439 days. Type 3: ALGV invaded. Patients survived 105 and 187 days. Type 4: SMV via the right gastroepiploic vein invaded. Patients survived 120 and 1275 days. Type 5: Portal vein via the gastric vein. Patients survived 537 days (second patient N/ac). | Drain | Evaluate the value of CT-based diagnosis for venous invasion in gastric cancer patients, noting that survival rates are significantly low for those with ALGV and short gastric vein invasion. Thus, ALGV or short gastric vein invasion on CT may indicate a poor prognosis. |
| Wu et al. | 2016 |
Type 1: LGV runs dorsal to the common hepatic artery. Type 2: LGV runs ventral to SA. Type 3: LGV runs between the common hepatic arteries and the SA. Type 4: LGV runs dorsal to SA. Type 5: LGV runs cranially into the LPV or hepatic parenchyma. Type 6: LGV runs ventral to the common hepatic artery. Type 7: Arterial variations impairing the reference frame function of the common hepatic artery and SA. | Variations of course | Development of a new classification system for variations of LGV. This system may help in the scientific description of LGV variations and facilitate the diagnosis of gastric cancer. |
| Yamagami et al. | 1999 | Type 1: Right gastric vein drains directly to the left lobe of the liver parenchyma around the falciform ligament. | Drain | The gastric vein right draining into the liver parenchyma around the falciform ligament may influence non-tumor abnormalities seen on CT angiography. |
| Muñoz & Fraum et al. | 2023 | Type 1: Right gastric vein directly perfuses a part of hepatic segment IV with intense uptake of the radiotracer in PSMA-PET/CT | Drain | Additional vascular supply to portions of the hepatic parenchyma may result in differential enhancement patterns on dynamic post-contrast imaging studies. ARGV represents an important consideration when assessing for metastatic disease because variants in hepatic vasculature can sometimes be mistaken for more serious pathologies. |
| Bezzi et al. | 1995 | To maintain flow, anomalous anastomoses between the RGV and the right or left portal vein branches were preserved. | Drain | Both cases reported are of a transjugular intrahepatic portosystemic shunt where the right gastric vein drains into branches of the portal vein and supplies its flow when a thrombosis occurs in the portal vein. |
| Deger & Bozer et al. | 2023 | ALGV | Drain | The ALGV causes pseudolesions in segments 2 and 3 of the liver parenchyma. |
| Gabata et al. | 1997 | An AGV ascended within the hepatoduodenal ligament, anterior to the main portal vein, reaching the porta hepatis. It then directly drained into the focal spared area at the posterior edge of segment IV. | Drain associated with fatty liver. | In cases of preserved focal area in the posterior border of segment IV in fatty liver, presents direct drainage to the liver in the AGV. |
| Hiwatashi et al. | 1999 | An AGV drains into segment II of the liver. | Drain associates a pseudolesion | In patients with metastasis data in the posterior lobes of the liver, as observed on CT, ultrasound, and venous angiography, an ALGV was identified that drained into segment II of the liver, generating pseudolesions that mimicked metastasis. |
| Matsui et al. | 1994 | The ARGV drains into segment IV of the liver. | Drain associates a pseudolesion. | 17 patients out of 22 had a vein coming from the pylorus, the right gastric vein, with aberrant drainage directly into segment IV of the liver. 6 of these patients had direct intrahepatic drainage into a portal branch. |
| Matsui, Kadoya, Yoshikawa, et al. | 1995 | The ARGV drains into segments IV and I of the liver. | Drain | 17/18 patients had aberrant drainage of the right gastric vein in the posterior aspect of segment 4 of the liver, while only one patient had aberrant drainage in segment 1. |
| Matsui et al. | 1995 | The ARGV drains into the area of focal preservation. | Drain | Aberrant drainage of the direct right gastric vein in focal areas of the liver in patients with fatty liver. |
| Natsume et al. | 2010 | 52/126 LCV flowed into the portal vein 44/126 LCV flowed into the splenic vein, 22/126 flowed into the junction of these two veins | Drain | Utilizing various imaging techniques is essential for the successful execution of critical surgical procedures, as well as understanding the potential variations among patients. |
| Terayama et al. | 2004 | ALGV runs along the hepatogastric ligament toward the left side of the hepatic hilus, enters the II segment in the liver, and joins the intrahepatic portal venous branch. The corresponding area was focally spared of fatty liver. | Drain | LGV causes pseudolesions in segments II and IV of the liver on CT during arterial portography, and it may reveal a perfusion defect in the corresponding area. |
| Roi et al. | 1993 | Type 1: LGV drains in the portal vein (PV) Type 2: LGV drains in the splenoportal junction. Type 3: LGV drain in the splenic vein (SV) Type 1A: anterior LGV to the PV. Type 1P: posterior LGV to PV. Type 2A: anterior LGV to splenoportal junction. Type 2P: posterior LGV to splenoportal junction. Type 3A: anterior LGV to SV Type 3P: posterior LGV to SV. | Drain and course | Sonography of the termination of the LGV, despite the highly variable drainage site, enables an easy and accurate definition of its anatomical relationship with the adjacent vessels. |
| Rebibo et al. | 2012 | Type A: termination on the portal vein (PV) Type B: termination on the splenomesenteric trunk (SMT) Type C: termination on the splenic vein (SV) | Drain | Understanding the distribution and drainage area of the gastric vein is crucial, particularly during surgical resections of the pancreas, to avoid impacting the stomach’s drainage. This can be achieved through imaging techniques. |
| Author | Year | Total, n | Prevalence (%) |
|---|---|---|---|
| Miyaki et al. | 1987 | 245 | 2 |
| Matsui, Kadoya, Yoshikawa, et al. | 1995 | 122 | 18 |
| Matsui et al. | 1995 | 18 | 1 |
| Seong et al. | 2012 | 100 | 7 |
| Rebibo et al. | 2012 | 86 | 26 |
| Li et al. | 2016 | 234 | 43 |
| Wu et al. | 2016 | 805 | 77 |
| Unal et al. | 2018 | 530 | 11 |
| Lee & Lee et al. | 2018 | 405 | 12 |
| Ünal & Karcaaltincaba et al. | 2019 | 43,679 | 32 |
| Choi et al. | 2020 | 2021 | 52 |
| Parameters | Number of Studies and Subjects | Prevalence (%) | 95% CI | I2 | p-Value |
|---|---|---|---|---|---|
| Overall | 11 (48,245) | 8.32% | 3.12–13.17 | 98.92% | - |
| Cadaveric | 1 (245) | 0.082% | - | - | p = 0.0001 |
| Imaging | 10 (48,000) | 8.18% | 3.49–12.91 | 89.11% | |
| Asia | 8 (3950) | 6.37% | 4.19–8.98 | 88.12% | p = 0.032 |
| Africa | 0 | - | - | - | |
| Europe | 3 (44,295) | 2.11% | 0.77–3.99 | 91.35% | |
| America | 0 | - | - | - | |
| Oceania | 0 | - | - | - | |
| Male | 7 (2552) | 5.29% | 3.12–9.33 | 77.11% | p = 0.024 |
| Female | 7 (1173) | 2.43% | 1.10–4.13 | 84.12% | |
| Gastric vein left | 10 (48,000) | 8.18% | 7.01–10.12 | 77.10% | p = 0.012 |
| Gastric veinright | 3 (459) | 3.29% | 2.21–4.13 | 88.12% |
| Author(s) | Year | Type of Consideration: Pathological, Surgical, Advantageous, Imaging | Description |
|---|---|---|---|
| Miyaki et al. | 1987 | Imaging | To determine the aberrant course of the LGV in humans by imaging and to elucidate whether the LGV derives from the omphalomesenteric vein or the subintestinal vein |
| Roi et al. | 1993 | Imaging | Ultrasonography of the LGV termination, despite significant variability in the drainage site, enables an easy and precise definition of its anatomical relationship with the adjacent vessels. |
| Matsui et al. | 1994 | Surgical | Segment IV receives a large blood supply; therefore, in the event of surgery in that area, it is necessary to check for any GV variant. |
| Bezzi et al. | 1995 | Surgical/Advantageous | In cases of hypertension of the portal vein system, if it is not possible to cannulate and the patient has an anomalous insertion of the RGV in the branches of the HPV system, the RGV can be cannulated to relieve portal hypertension |
| Matsui et al. | 1995 | Not indicated | Not indicated |
| Matsui, Kadoya, Yoshikawa, et al. | 1995 | Pathological | Relationship between the appearance of posterior masses in segment IV of a fatty liver and the aberrant drainage of the RGV in the same segment. |
| Gabata et al. | 1997 | Pathological | Focal preserved area at the posterior border of segment IV in fatty liver, showing direct drainage to the liver through the AGV |
| Yamagami et al. | 1999 | Imaging | The RGV draining into the hepatic parenchyma around the falciform ligament may influence the appearance of non-tumor abnormalities on CTA |
| Hiwatashi et al. | 1999 | Pathological | The relevance of using imaging and angiography to detect variations in GV drainage in case of pseudolesions, and thus avoid invasive procedures |
| Ohkubo et al. | 2000 | Surgical | Anatomical knowledge of interportal venous communication is essential to treat bleeding esophageal varices or angiographic embolization adequately. |
| Deneve et al. | 2003 | Advantageous | When the main HPV is thrombosed, ALGV and ARGV can contribute to maintaining sufficient hepatic flow and can be used as the sole route for stenting a TIPS |
| Ishigami et al. | 2004 | Advantageous | ALGV has a crucial role as a decompression pathway, possibly reducing the severity of extensive variceal bleeding |
| Caty et al. | 2004 | Pathological | The ARGV, by draining directly into the liver rather than the HPV, may contribute to focal fatty infiltration and focal sparing in fatty liver |
| Terayama et al. | 2004 | Pathological | LGV causes pseudolesions in segments II and IV of the liver, which are visible on a CT scan during arterial portography, and may reveal a perfusion defect in the corresponding area. |
| Natsume et al. | 2010 | Imaging | 3DCTA is a valuable and essential modality for visualizing the precise anatomy surrounding the stomach preoperatively and for conducting safe operations. |
| Seong et al. | 2012 | Pathological | Aberrant gastric venous drainage is crucial for radiologists and clinicians due to the formation of pseudolesions from cavernous transformation in main hepatic vein thrombosis, and unexpected hemorrhage during hepatobiliary surgery from unidentified AGVs. The ARGV may also serve as a direct metastatic route for gastric cancer on the lesser curvature and an arterioportal bypass in tumor thrombosis of the main HPV. |
| Rebibo et al. | 2012 | Surgical | Preoperative analysis of the LGV is valuable because the vein can be identified in every case. Understanding the anatomical location of the termination enables subsequent resection to be initiated in a low-risk area. |
| Kuwada et al. | 2015 | Surgical | In the setting of curative lymph node dissection for gastric cancer, identifying aberrant LGV and ALHA requires careful clinical consideration. In such cases, the approach involved dividing the ALGV at its root while selectively preserving the ALHA |
| Mittal et al. | 2015 | Pathological | The aberrant vein draining into the LPV instead of the main HPV results in isolated left hepatic venous portal gas, resulting in gastric pneumatosis and an incarcerated hiatal hernia |
| Unal et al. | 2015 | Pathological | The ALGV in patients with gastric cancer may lead to the direct spread of the tumor to the liver. Hepatic pseudolesions associated with ALGV affecting the posterior aspect of liver segments II and III may mimic metastasis |
| Li et al. | 2016 | Pathological | Variceal bleeding is a complication in individuals with portal hypertension, with the LGV being the primary source of blood supply to esophagogastric varices. Furthermore, esophageal variceal bleeding occurs more frequently in patients with an enlarged LGV diameter exceeding 5–6 mm, a parameter indicative of portal hypertension |
| Wu et al. | 2016 | Not indicated | Not indicated |
| Lee & Lee et al. | 2018 | Surgical | Type II (Lee classification) LGV has a relatively lower risk of injury during laparoscopic gastrectomy; there is no need to perform a complete lymph node dissection around the CA |
| Unal et al. | 2018 | Pathological | The ALGV in a patient with gastric cancer may indicate a worse prognosis |
| Ünal & Karcaaltincaba et al. | 2019 | Advantageous | The presence of the ALGV type III variant maintained the flow of the LPV in patients with main HPV thrombosis |
| Choi et al. | 2020 | Pathological | The segment drained by the AGV may exhibit focal sparing in fatty livers, focal fat deposition, or hyperplastic changes, which can mimic liver tumors on imaging studies. Segment II atrophy was found more frequently in patients with AGVs |
| Frey et al. | 2022 | Pathological | Segment II atrophy is observed in 33.3% of patients with ALGV. ALGV provides a direct route for periportal dissemination of the gastric tumor |
| Arhire et al. | 2023 | Pathological | ARGV can produce pseudoinjury due to a mismatch in hepatic blood flow (hemodynamic mechanisms related to the third flow and the transient hepatic attenuation difference), but also due to an associated and underlying metabolic cause |
| Alfaro et al. | 2023 | Advantageous/Pathological | AGVs could act as an alternative route for venous drainage in hypertensive gastropathy in cirrhotic patients or as a direct metastatic route for gastric cancer on the left side of the liver |
| Deger & Bozer et al. | 2023 | Imaging | The importance of recognizing vascular anomalies, such as ALGV, in the liver vasculature and linking them to pseudolesions in the liver parenchyma is crucial for avoiding invasive procedures. |
| Muñoz & Fraum et al. | 2023 | Imaging | ARGV represents a crucial consideration when evaluating metastatic disease, as variants in the hepatic vasculature can sometimes be misdiagnosed as more severe pathologies. |
| Author(s) | Year | Sample n | Study Type Imaging, Cadaveric, or Surgery |
|---|---|---|---|
| Miyaki et al. | 1987 | 245 | Cadaveric |
| Roi et al. | 1993 | 86 | Imaging |
| Matsui et al. | 1994 | 122 | imaging |
| Bezzi et al. | 1995 | 2 | imaging |
| Matsui et al. | 1995 | 18 | imaging |
| Matsui, Kadoya, Yoshikawa, et al. | 1995 | 17 | imaging |
| Gabata et al. | 1997 | 17 | imaging |
| Yamagami et al. | 1999 | 1 | Imaging |
| Hiwatashi et al. | 1999 | 1 | imaging |
| Ohkubo et al. | 2000 | 1 | Cadaveric |
| Deneve et al. | 2003 | 1 | Cadaveric |
| Ishigami et al. | 2004 | 1 | imaging |
| Caty et al. | 2004 | 1 | Cadaveric |
| Ishigami et al. | 2004 | 1 | imaging |
| Terayama et al. | 2004 | 1 | Imaging |
| Natsume et al. | 2010 | 126 | imaging |
| Seong et al. | 2012 | 100 | Imaging |
| Rebibo et al. | 2012 | 86 | imaging |
| Kuwada et al. | 2015 | 1 | imaging |
| Mittal et al. | 2015 | 1 | Cadaveric |
| Unal et al. | 2015 | not specified | not specified |
| Li et al. | 2016 | 234 | Imaging |
| Wu et al. | 2016 | 805 | Imaging |
| Lee & Lee | 2018 | 405 | in vivo |
| Unal et al. | 2018 | 530 | Imaging |
| Ünal & Karcaaltincaba et al. | 2019 | 43,679 | Imaging |
| Choi et al. | 2020 | 2021 | Cadaveric |
| Frey et al. | 2022 | 3 | imaging |
| Arhire et al. | 2023 | 2 | Cadaveric |
| Alfaro et al. | 2023 | 3 | Imaging |
| Deger & Bozer et al. | 2023 | 1 | imaging |
| Muñoz & Fraum et al. | 2023 | 1 | imaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruna-Mejias, A.; Salgado-Torres, C.; Cáceres-Gálvez, C.; Rodriguez-Osorio, B.; Orellana-Donoso, M.; Nova-Baeza, P.; Suazo-Santibañez, A.; Oyanedel-Amaro, G.; Sanchis-Gimeno, J.; Piagkou, M.; et al. The Gastric Vein Variants: An Evidence-Based Systematic Review of Prevalence and Clinical Considerations. J. Clin. Med. 2025, 14, 3630. https://doi.org/10.3390/jcm14113630
Bruna-Mejias A, Salgado-Torres C, Cáceres-Gálvez C, Rodriguez-Osorio B, Orellana-Donoso M, Nova-Baeza P, Suazo-Santibañez A, Oyanedel-Amaro G, Sanchis-Gimeno J, Piagkou M, et al. The Gastric Vein Variants: An Evidence-Based Systematic Review of Prevalence and Clinical Considerations. Journal of Clinical Medicine. 2025; 14(11):3630. https://doi.org/10.3390/jcm14113630
Chicago/Turabian StyleBruna-Mejias, Alejandro, Cristian Salgado-Torres, Constanza Cáceres-Gálvez, Benjamin Rodriguez-Osorio, Mathias Orellana-Donoso, Pablo Nova-Baeza, Alejandra Suazo-Santibañez, Gustavo Oyanedel-Amaro, Juan Sanchis-Gimeno, Maria Piagkou, and et al. 2025. "The Gastric Vein Variants: An Evidence-Based Systematic Review of Prevalence and Clinical Considerations" Journal of Clinical Medicine 14, no. 11: 3630. https://doi.org/10.3390/jcm14113630
APA StyleBruna-Mejias, A., Salgado-Torres, C., Cáceres-Gálvez, C., Rodriguez-Osorio, B., Orellana-Donoso, M., Nova-Baeza, P., Suazo-Santibañez, A., Oyanedel-Amaro, G., Sanchis-Gimeno, J., Piagkou, M., Triantafyllou, G., Konschake, M., & Valenzuela-Fuenzalida, J. J. (2025). The Gastric Vein Variants: An Evidence-Based Systematic Review of Prevalence and Clinical Considerations. Journal of Clinical Medicine, 14(11), 3630. https://doi.org/10.3390/jcm14113630







