Intraosseous and Intra-Articular Platelet-Rich Plasma for Severe Knee Osteoarthritis: A Real-World-Outcomes Initiative
Abstract
1. Introduction
2. Methods
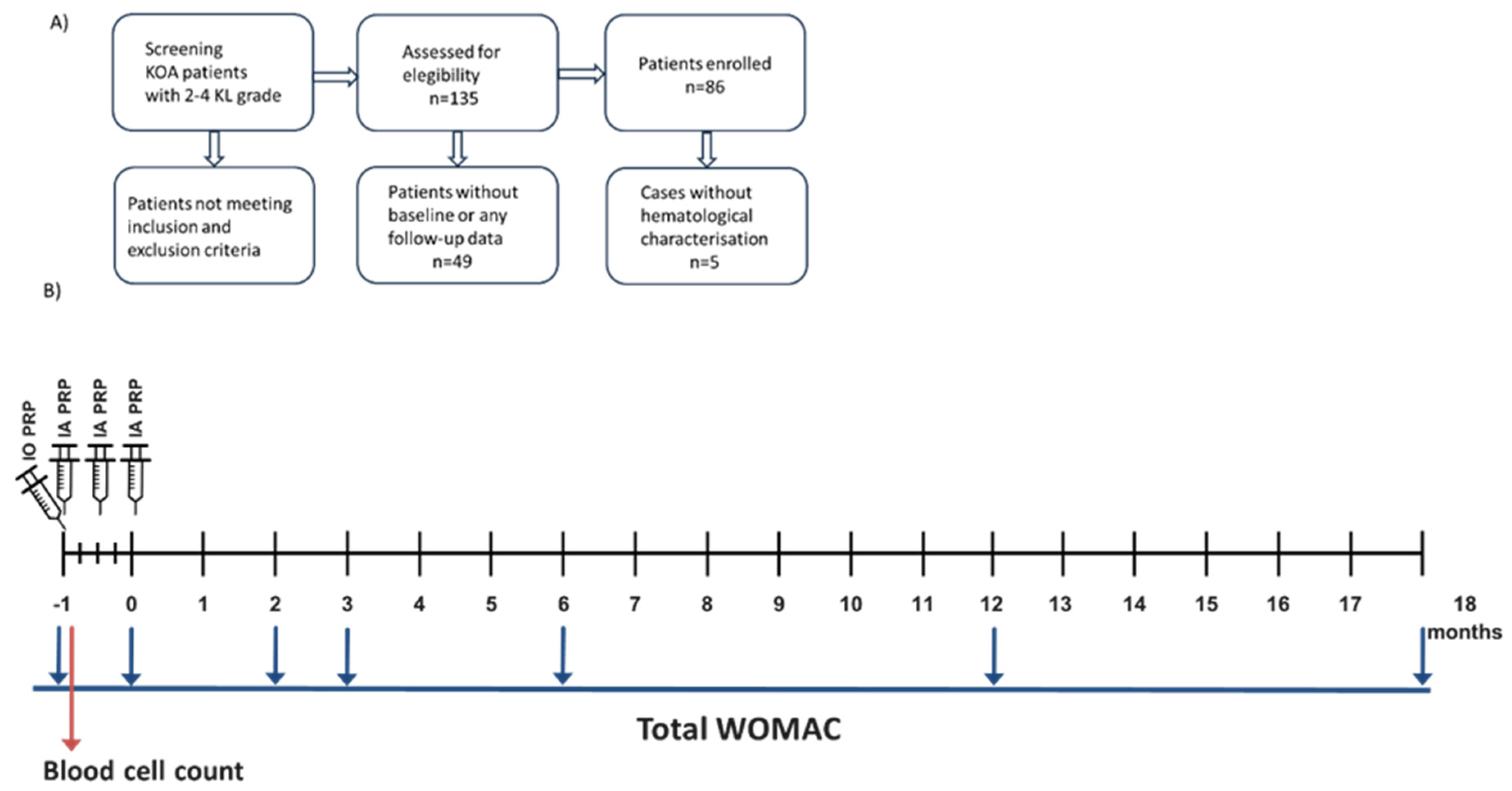
2.1. Study Design
2.2. Inclusion and Exclusion Criteria for Patients
2.3. Clinical Evaluation
2.4. PRP Obtention and Implantation
2.5. PRP Characterization and Hematological Counts
2.6. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
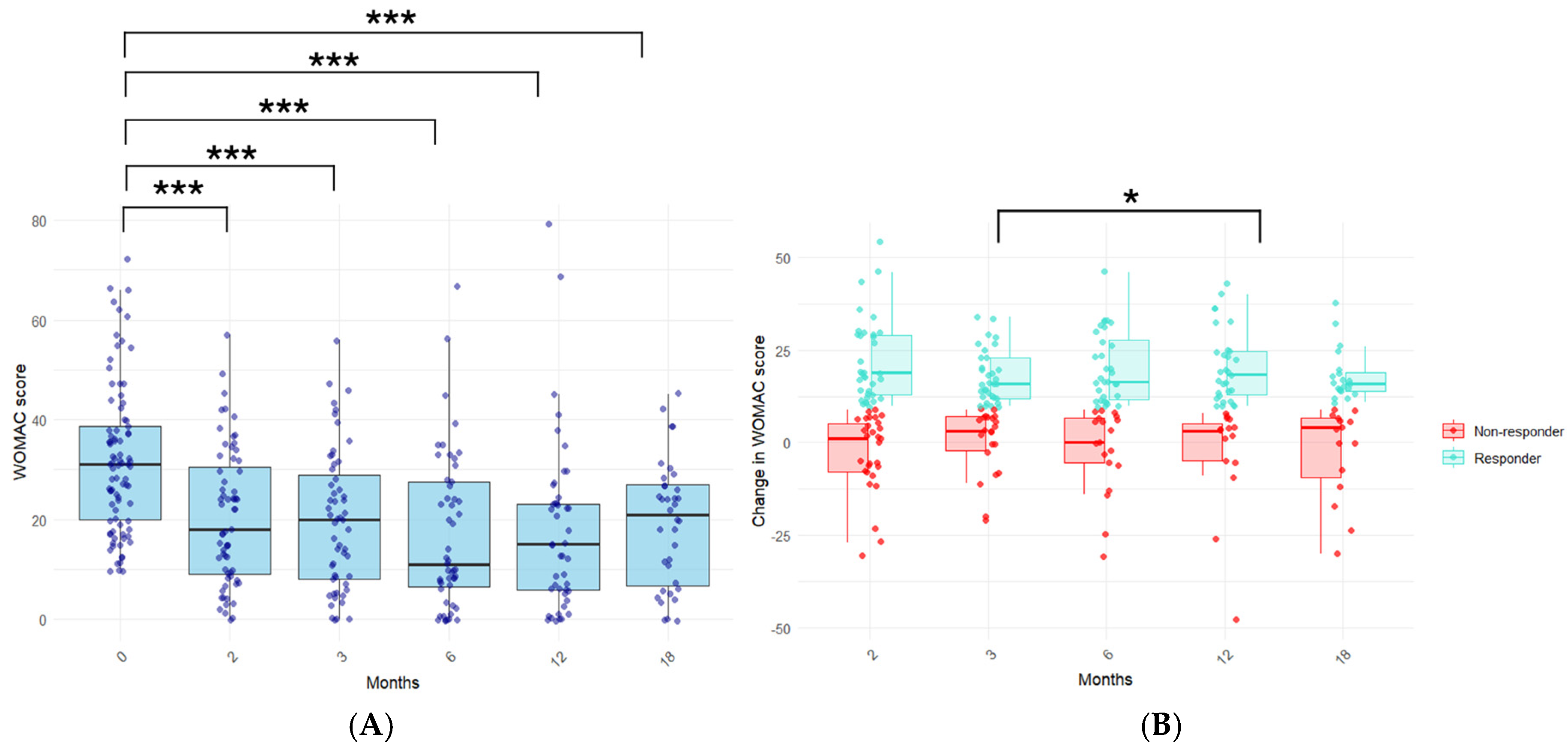
3.2. Evaluation of the Long-Term Clinical Response to PRP Treatment in KOA Patients
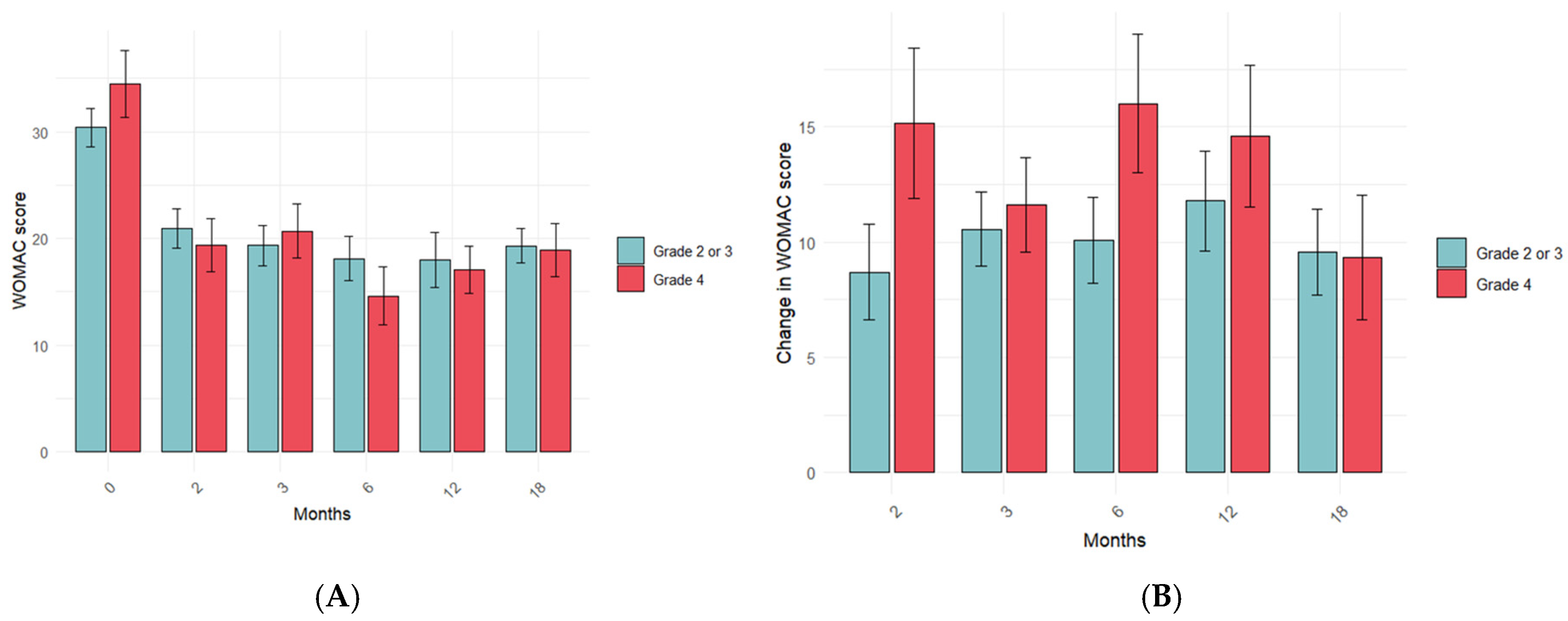
3.3. Influence of PRP Characteristics on the Treatment Response in the Long Term
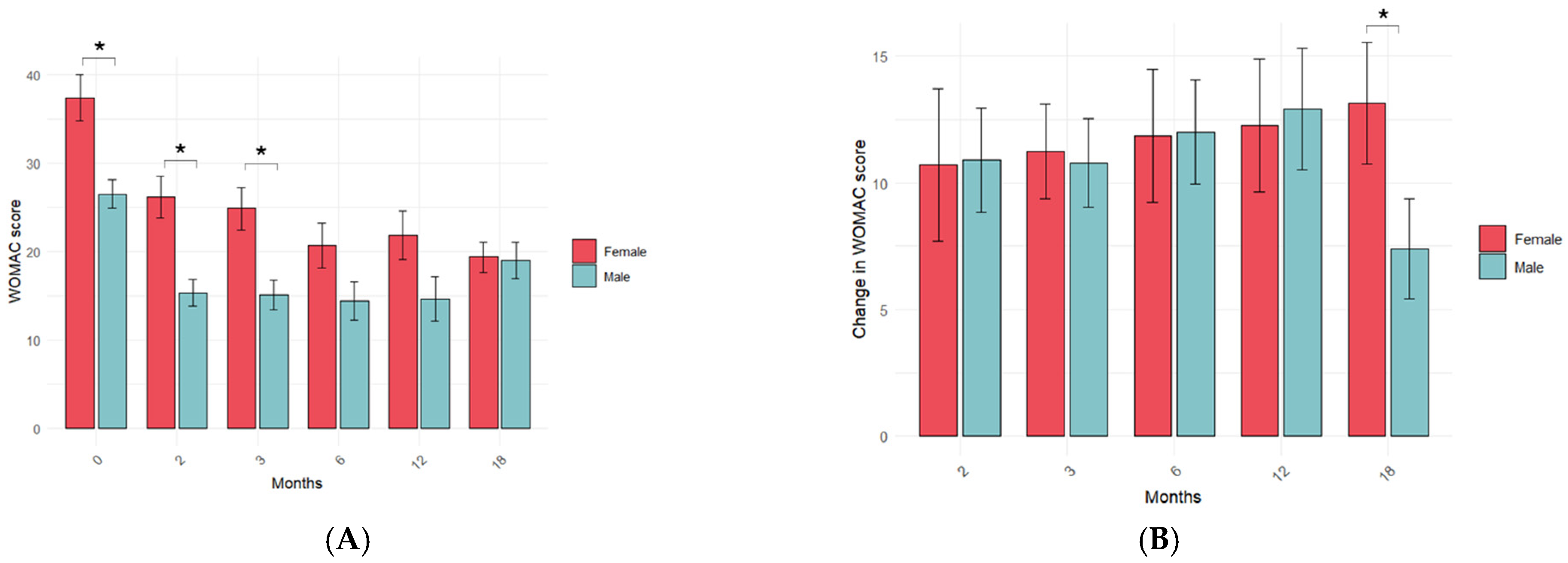
3.4. Influence of KOA Patient Variables on the PRP Treatment Response in the Long Term
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cucchiarini, M.; de Girolamo, L.; Filardo, G.; Oliveira, J.M.; Orth, P.; Pape, D.; Reboul, P. Basic science of osteoarthritis. J. Exp. Orthop. 2016, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.; Wragg, N.M.; Wilson, S.L. The use of PRP injections in the management of knee osteoarthritis. Cell Tissue Res. 2019, 376, 143–152. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef]
- Fu, H.; Wang, W.; Chen, Y. Treatment effects of platelet-rich plasma combined with arthroscopic microfracture for knee cartilage injury. Am. J. Transl. Res. 2024, 16, 6457. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Liao, C.; Lai, Q.; Peng, J.; Guo, N. Microfracture surgery combined with platelet-rich plasma injection in treating osteochondral lesions of talus: A system review and update meta analysis. Foot Ankle Surg. 2024, 30, 21–26. [Google Scholar] [CrossRef]
- Barman, A.; Bandyopadhyay, D.; Mohakud, S.; Sahoo, J.; Maiti, R.; Mukherjee, S.; Prakash, S.; Roy, S.S.; Viswanath, A. Comparison of clinical outcome, cartilage turnover, and inflammatory activity following either intra-articular or a combination of intra-articular with intra-osseous platelet-rich plasma injections in osteoarthritis knee: A randomized, clinical trial. Injury 2023, 54, 728–737. [Google Scholar] [CrossRef]
- Xiong, Y.; Gong, C.; Peng, X.; Liu, X.; Su, X.; Tao, X.; Li, Y.; Wen, Y.; Li, W. Efficacy and safety of platelet-rich plasma injections for the treatment of osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Front. Med. 2023, 10, 1204144. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Clement, N.D.; Bardgett, M.; Weir, D.; Holland, J.; Gerrand, C.; Deehan, D.J. What is the minimum clinically important difference for the womac index after TKA? Clin. Orthop. Relat. Res. 2018, 476, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Anitua, E.; Delgado, D.; Sanchez, P.; Prado, R.; Goiriena, J.J.; Prosper, F.; Orive, G.; Padilla, S. A new strategy to tackle severe knee osteoarthritis: Combination of intra-articular and intraosseous injections of Platelet Rich Plasma. Expert Opin. Biol. Ther. 2016, 16, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; Bansal, A.; Agarwal, D.; Preoteasa, I. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci. Rep. 2021, 11, 3971. [Google Scholar] [CrossRef]
- Patel, S.; Gahlaut, S.; Thami, T.; Chouhan, D.K.; Jain, A.; Dhillon, M.S. Comparison of Conventional Dose Versus Superdose Platelet-Rich Plasma for Knee Osteoarthritis: A Prospective, Triple-Blind, Randomized Clinical Trial. Orthop. J. Sports Med. 2024, 12, 23259671241227863. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, J.; Li, J.; Fu, W. The Combined Intraosseous Administration of Orthobiologics Outperformed Isolated Intra-articular Injections in Alleviating Pain and Cartilage Degeneration in a Rat Model of MIA-Induced Knee Osteoarthritis. Am. J. Sports Med. 2024, 52, 140–154. [Google Scholar] [CrossRef]
- Sánchez, M.; Delgado, D.; Pompei, O.; Pérez, J.C.; Sánchez, P.; Garate, A.; Bilbao, A.M.; Fiz, N.; Padilla, S. Treating Severe Knee Osteoarthritis with Combination of Intra-Osseous and Intra-Articular Infiltrations of Platelet-Rich Plasma: An Observational Study. Cartilage 2018, 10, 245. [Google Scholar] [CrossRef]
- Berrigan, W.; Tao, F.; Kopcow, J.; Park, A.L.; Allen, I.; Tahir, P.; Reddy, A.; Bailowitz, Z. The Effect of Platelet Dose on Outcomes After Platelet Rich Plasma Injections for Musculoskeletal Conditions: A Systematic Review and Meta-Analysis. Curr. Rev. Musculoskelet. Med. 2024, 17, 570–588. [Google Scholar] [CrossRef]
- Singh, A.; Chakravarty, S.; Sehgal, D.; Rust, B.; Sharieff, K.A.; Singh, A.; Chakravarty, S.; Sehgal, D.; Rust, B.D.; Sharieff, K.A. Optimal Dosage of Platelet-Rich Plasma Injections in Patients With Osteoarthritis of the Knee: A Scoping Review. Cureus 2024, 16, e75497. [Google Scholar] [CrossRef]
- Mautner, K.; Colberg, R.E.; Malanga, G.; Borg-Stein, J.P.; Harmon, K.G.; Dharamsi, A.S.; Chu, S.; Homer, P. Outcomes After Ultrasound-Guided Platelet-Rich Plasma Injections for Chronic Tendinopathy: A Multicenter, Retrospective Review. Phys. Med. Rehabil. 2013, 5, 169–175. [Google Scholar] [CrossRef]
- Bensa, A.; Previtali, D.; Sangiorgio, A.; Boffa, A.; Salerno, M.; Filardo, G. PRP Injections for the Treatment of Knee Osteoarthritis: The Improvement Is Clinically Significant and Influenced by Platelet Concentration: A Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2025, 53, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Boffa, A.; De Marziani, L.; Andriolo, L.; Di Martino, A.; Romandini, I.; Zaffagnini, S.; Filardo, G. Influence of Platelet Concentration on the Clinical Outcome of Platelet-Rich Plasma Injections in Knee Osteoarthritis. Am. J. Sports Med. 2024, 52, 3223–3231. [Google Scholar] [CrossRef] [PubMed]
- Bec, C.; Rousset, A.; Brandin, T.; François, P.; Rabarimeriarijaona, S.; Dumoulin, C.; Heleu, G.; Grimaud, F.; Veran, J.; Magalon, G.; et al. A Retrospective Analysis of Characteristic Features of Responders and Impaired Patients to a Single Injection of Pure Platelet-Rich Plasma in Knee Osteoarthritis. J. Clin. Med. 2021, 10, 1748. [Google Scholar] [CrossRef]
- Romandini, I.; Boffa, A.; Di Martino, A.; Andriolo, L.; Cenacchi, A.; Sangiorgi, E.; Orazi, S.; Pizzuti, V.; Zaffagnini, S.; Filardo, G. Leukocytes Do Not Influence the Safety and Efficacy of Platelet-Rich Plasma Injections for the Treatment of Knee Osteoarthritis: A Double-Blind Randomized Controlled Trial. Am. J. Sports Med. 2024, 52, 3212–3222. [Google Scholar] [CrossRef]
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-rich PRP for knee osteoarthritis: Current concepts. J. Clin. Orthop. Trauma 2019, 10, S179–S182. [Google Scholar] [CrossRef]
- Gupta, A.; Viswanath, A.; Kumar, G.H. Leukocyte-Poor Platelet-Rich Plasma for the Management of Knee Osteoarthritis: A Retrospective Study with 12 Months of Follow-Up. Cureus 2024, 16, e69662. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Buda, R.; Timoncini, A.; Di Martino, A.; Cenacchi, A.; Fornasari, P.M.; Giannini, S.; Marcacci, M. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 528–535. [Google Scholar] [CrossRef]
- Sánchez, M.; Jorquera, C.; de Dicastillo, L.L.; Martínez, N.; Espregueira-Mendes, J.; Vergés, J.; Azofra, J.; Delgado, D. Women show a positive response to platelet-rich plasma despite presenting more painful knee osteoarthritis than men. Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cuadros, M.E.; Pérez-Moro, O.S.; Alonso-Sardón, M.; Iglesias-De-Sena, H.; Mirón-Canelo, J.A. Age and Sex Affect Osteoarthritis and the Outcome on Knee Replacement. MOJ Orthop. Rheumatol. 2017, 9, 14–26. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- O’Donnell, C.; Migliore, E.; Grandi, F.C.; Koltsov, J.; Lingampalli, N.; Cisar, C.; Indelli, P.F.; Sebastiano, V.; Robinson, W.H.; Bhutani, N.; et al. Platelet-Rich Plasma (PRP) from Older Males with Knee Osteoarthritis Depresses Chondrocyte Metabolism and Upregulates Inflammation. J. Orthop. Res. 2019, 37, 1760. [Google Scholar] [CrossRef] [PubMed]
- Kamada, K.; Matsushita, T.; Yamashita, T.; Matsumoto, T.; Iwaguro, H.; Kuroda, R.; Sobajima, S. Factors affecting the therapeutic effects of multiple intra-articular injections of platelet-rich-plasma for knee osteoarthritis. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2024, 38, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Pereira Ruiz, M.T.; Vaccaro, F.; Guitaldi, R.; Di Martino, A.; Cenacchi, A.; Fornasari, P.M.; Marcacci, M. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: Single- versus double-spinning approach. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2082–2091. [Google Scholar] [CrossRef] [PubMed]

| Sex | |
|---|---|
| N-Miss | 1 |
| Women | 42 (49%) |
| Men | 43 (51%) |
| Age | |
| Mean (SD) | 56.6 (12.9) |
| Range | 15–85 |
| KL severity grade | |
| Grade 2 | 10 (11.6%) |
| Grade 3 | 46 (53.5%) |
| Grade 4 | 30 (34.8%) |
| Blood | PRP | |
|---|---|---|
| Platelets | ||
| N-miss | 5 | 5 |
| Mean (SD) | 226.4 (61.5) | 497 (140.4) |
| Range | 94–479 | 117–850 |
| Leukocytes | ||
| N-miss | 4 | 4 |
| Mean (SD) | 5.56 (1.46) | 1.92 (5.57) |
| Range | 2.03–10.0 | 0.01–6.17 |
| Erythrocytes | ||
| N-miss | 4 | 4 |
| Mean (SD) | 3865 (780) | 0.015 (0.009) |
| Range | 3680–5730 | 0.00–0.06 |
| Months | Responders | Non-Responders | p-Value | |
|---|---|---|---|---|
| WOMAC score | 2 | 14.9 | 27 | <0.001 |
| 3 | 16.2 | 25.3 | <0.05 | |
| 6 | 10.6 | 27.8 | <0.001 | |
| 12 | 12.3 | 28.5 | <0.005 | |
| 18 | 14.8 | 25.3 | <0.05 | |
| Change in WOMAC score | 2 | 22.1 | −2.8 | <0.001 |
| 3 | 18 | 0.4 | <0.001 | |
| 6 | 20.3 | 2.5 | <0.001 | |
| 12 | 20.5 | −3.1 | <0.001 | |
| 18 | 18 | −2.5 | <0.001 | |
| PRP leukocytes (×106/mL) | 2 | 2.12 | 2.56 | 0.66 |
| 3 | 3.37 | 0.96 | 0.21 | |
| 6 | 2.87 | 1.78 | 0.24 | |
| 12 | 3.62 | 0.85 | 0.96 | |
| 18 | 2.42 | 1.68 | 0.79 | |
| PRP erythrocytes (×106/mL) | 2 | 0.02 | 0.01 | 0.60 |
| 3 | 0.01 | 0.02 | 0.48 | |
| 6 | 0.01 | 0.02 | 0.067 | |
| 12 | 0.02 | 0.01 | 0.30 | |
| 18 | 0.02 | 0.01 | 0.87 | |
| PRP platelets (×106/mL) | 2 | 502.29 | 497.86 | 0.90 |
| 3 | 481.87 | 482.29 | 0.99 | |
| 6 | 487.74 | 458.76 | 0.57 | |
| 12 | 486.28 | 508.21 | 0.97 | |
| 18 | 488.09 | 533.57 | 0.59 | |
| Age | 2 | 57.4 | 55.3 | 0.24 |
| 3 | 55.5 | 60.2 | 0.34 | |
| 6 | 54.9 | 59.2 | 0.47 | |
| 12 | 53.3 | 58.6 | 0.63 | |
| 18 | 56.5 | 54.7 | 0.31 |
| Time (Months) | Low Platelet Dose (3.8 ± 0.9 × 109 Platelets/Injection) | High Platelet Dose (6.0 ± 1.3 × 109 Platelets/Injection) | p-Value | |||
|---|---|---|---|---|---|---|
| WOMAC score | 0 | 31.2 | 33.5 | 0.23 | ||
| 2 | 21.4 | 21.3 | 0.85 | |||
| 3 | 19.4 | 21.7 | 0.55 | |||
| 6 | 16.0 | 18.7 | 0.78 | |||
| 12 | 16.2 | 19.4 | 0.57 | |||
| 18 | 17.2 | 21.7 | 0.29 | |||
| Change in WOMAC score | 2 | 9.6 | 12.0 | 0.56 | ||
| 3 | 10.3 | 11.8 | 0.89 | |||
| 6 | 12.3 | 12.1 | 0.98 | |||
| 12 | 12.8 | 12.9 | 0.90 | |||
| 18 | 10.4 | 8.7 | 0.87 | |||
| Responders | Yes | No | Yes | No | p-value | |
| Relative frequencies (%) | 2 | 25.8 | 24.2 | 29.0 | 21.0 | 0.61 |
| 3 | 30.8 | 23.1 | 28.8 | 17.3 | 0.69 | |
| 6 | 31.3 | 20.8 | 33.3 | 14.6 | 0.49 | |
| 12 | 30.2 | 18.6 | 37.2 | 14.0 | 0.45 | |
| 18 | 31.4 | 17.1 | 28.6 | 22.9 | 0.58 | |
| (A) | ||||||
|---|---|---|---|---|---|---|
| KL Severity Degree | 2–3 | 4 | p-Value | |||
| Responders | Yes | No | Yes | No | ||
| Relative frequencies (%) | 2 | 34.4 | 32.8 | 20.3 | 12.5 | 0.42 |
| 3 | 40 | 23.6 | 20 | 16.4 | 0.57 | |
| 6 | 41.2 | 27.5 | 21.6 | 9.8 | 0.55 | |
| 12 | 44.4 | 24.4 | 22.2 | 8.9 | 0.65 | |
| 18 | 38.9 | 27.8 | 19.4 | 13.9 | 0.99 | |
| (B) | ||||||
| Sex | Women | Men | p-value | |||
| Responders | Yes | No | Yes | No | ||
| Relative frequencies (%) | 2 | 25 | 21.9 | 29.7 | 23.4 | 0.84 |
| 3 | 27.8 | 20.4 | 33.3 | 18.5 | 0.62 | |
| 6 | 27.4 | 13.7 | 35.3 | 23.5 | 0.63 | |
| 12 | 22.2 | 20 | 44.4 | 13.3 | 0.08 | |
| 18 | 30.6 | 5.6 | 27.8 | 36.1 | <0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalán, J.M.; Escarrer-Garau, G.; Estrany-Celià, M.d.M.; Parra, C.; Arbona-González, L.; Mercader-Barceló, J.; Dos-Anjos, S. Intraosseous and Intra-Articular Platelet-Rich Plasma for Severe Knee Osteoarthritis: A Real-World-Outcomes Initiative. J. Clin. Med. 2025, 14, 3627. https://doi.org/10.3390/jcm14113627
Catalán JM, Escarrer-Garau G, Estrany-Celià MdM, Parra C, Arbona-González L, Mercader-Barceló J, Dos-Anjos S. Intraosseous and Intra-Articular Platelet-Rich Plasma for Severe Knee Osteoarthritis: A Real-World-Outcomes Initiative. Journal of Clinical Medicine. 2025; 14(11):3627. https://doi.org/10.3390/jcm14113627
Chicago/Turabian StyleCatalán, José Miguel, Gabriel Escarrer-Garau, Maria del Mar Estrany-Celià, Catalina Parra, Laura Arbona-González, Josep Mercader-Barceló, and Severiano Dos-Anjos. 2025. "Intraosseous and Intra-Articular Platelet-Rich Plasma for Severe Knee Osteoarthritis: A Real-World-Outcomes Initiative" Journal of Clinical Medicine 14, no. 11: 3627. https://doi.org/10.3390/jcm14113627
APA StyleCatalán, J. M., Escarrer-Garau, G., Estrany-Celià, M. d. M., Parra, C., Arbona-González, L., Mercader-Barceló, J., & Dos-Anjos, S. (2025). Intraosseous and Intra-Articular Platelet-Rich Plasma for Severe Knee Osteoarthritis: A Real-World-Outcomes Initiative. Journal of Clinical Medicine, 14(11), 3627. https://doi.org/10.3390/jcm14113627






