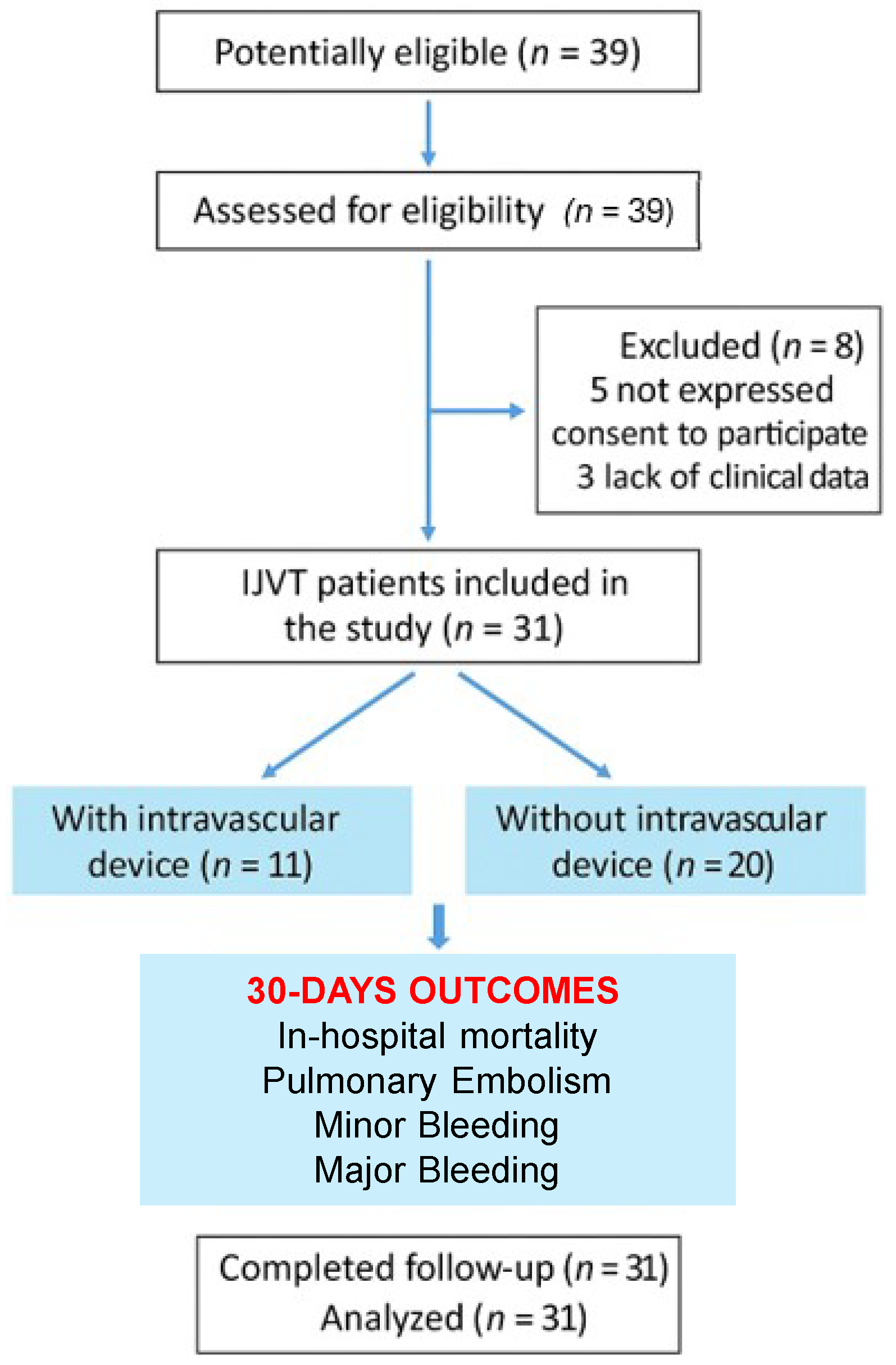
1. Introduction
Venous thromboembolism (VTE) represents a significant clinical challenge, particularly in patients with cancer, where the risk of thrombosis is markedly increased [
1,
2]. The presence of malignancy has been shown to elevate the risk of VTE by four to seven times compared to the general population, making it one of the most common and serious complications encountered in oncological care [
1,
2,
3]. While lower extremity deep vein thrombosis (DVT) is well-studied, upper extremity venous thrombosis (UEVT) and, in particular, internal jugular vein thrombosis (IJVT) remain relatively underexplored. The upper extremity venous system, including the internal jugular vein, poses unique diagnostic and therapeutic challenges, especially in cancer patients where central venous access devices (CVADs) are often utilized for chemotherapy and supportive care [
4]. The use of peripherally inserted central catheters (PICCs) and chest ports, while essential for treatment delivery, significantly increases the risk of catheter-related thrombosis [
4]. Among cancer patients, IJVT is an uncommon but serious event [
5], with complications such as pulmonary embolism (PE), superior vena cava (SVC) syndrome, and post-thrombotic syndrome reported in up to 50% of cases [
6,
7]. Central venous catheters (CVCs) are a critical risk factor, often associated with endothelial injury and local inflammation, which predispose to thrombus formation [
8]. Studies indicate that the complication rate is particularly high when the internal jugular vein is involved, with data suggesting that isolated jugular thrombosis carries a higher risk of adverse outcomes compared to other upper extremity veins [
6,
8]. Despite the recognized association between cancer, CVADs, and IJVT, the clinical course and biomarker profiles of patients with catheter-related thrombosis remain inadequately characterized. In addition, the impact of malignancy stage and other patient-specific factors on the risk of thrombosis and subsequent complications is not fully understood. Given the critical implications for patient management and prognosis, it is essential to further investigate the clinical characteristics and outcomes of IJVT in the context of malignancy and CVAD use. This study aimed to analyze the clinical characteristics of patients with newly diagnosed IJVT, in particular to evaluate mortality, development of PE and incidence of bleeding at 30 days from diagnosis. Secondly, we performed sub-analyses according to the presence of vascular devices, mortality, development of pulmonary embolism and active cancer. We also evaluated the therapeutic choices regarding the prescription and type of anticoagulant therapy.
5. Discussion
In this prospective cohort, we investigated the clinical characteristics, laboratory parameters, and clinical outcomes of patients diagnosed with IJVT. Consistent with prior literature, our findings indicate that cancer and CVADs are key contributors to thrombosis risk. Notably, one-third of our cohort had active malignancy, and CVADs were present in 35.5% of cases. Despite the recognized role of device-related endothelial injury in thrombus formation, sub-analyses did not reveal higher mortality or PE rates in the CVAD group. However, patients with a CVAD demonstrated a distinctive biomarker profile (lower D-dimer, higher platelet counts, and increased CRP), suggesting a possible localized thrombotic and inflammatory process. Moreover, the elevated CRP levels observed in patients with CVADs may reflect not only localized inflammation due to device-related endothelial injury but also a higher degree of systemic illness. Liver and kidney function markers were included to ensure that baseline systemic differences between groups did not confound the primary outcomes. Renal impairment, in particular, is known to be associated with altered inflammatory and thrombotic responses. The inclusion of these parameters supports a more comprehensive assessment of the patients’ clinical status and strengthens the validity of the observed associations between CVAD and prothrombotic laboratory findings.
Patients requiring central venous access are often more clinically fragile, with more complex medical conditions, which may independently contribute to an increased inflammatory profile. Although our study design did not include illness severity scores, this hypothesis is consistent with the clinical context and deserves further investigation.
Although it may be a known trigger [
10,
11], we have not recorded cases of IJVT related or secondary to heparin-induced thrombocytopenia.
Notably, there was not fully standardized thromboprophylaxis protocol universally applied across the participating institutions. However, institutional guidelines recommended risk-stratified prophylaxis based on patient comorbidities, type of admission (medical vs. surgical), and presence of central venous access devices. LMWH was the most commonly used agent, in line with national and international guidelines [
12,
13]. The observed variability in anticoagulant use among patients reflects individualized clinical decisions and highlights the need for more uniform thromboprophylaxis pathways in high-risk inpatients, especially those with intravascular devices.
Therapeutic regimens varied, with LMWH used most frequently, reflecting a preference for agents with a proven safety record and established efficacy in malignancy-associated thrombosis. The alternative agents FDP and apixaban were employed in five patients (16.1%) and three patients (9.7%), respectively, while edoxaban was used in only one case. No prescriptions for dabigatran or rivaroxaban were observed, highlighting a selective preference for certain direct oral anticoagulants (DOACs), likely dictated by specific clinical factors (e.g., renal function, interaction with other therapies, or tumor type). Among the four patients who were prescribed a DOAC, two were already taking it (one patient on apixaban for atrial fibrillation and one patient on apixaban for pulmonary embolism). VKA therapy was noted in three patients (9.7%), indicating limited use of this option, presumably due to the substantial monitoring requirements (INR checks) and the growing availability of more convenient alternatives. Similarly, the use of ASA in three patients (9.7%) suggests a targeted approach for individuals with specific indications (for example, cardiovascular prevention), rather than as monotherapy for venous thrombotic events. It should also be noted that four patients (12.9%) did not receive any pharmacological anticoagulant treatment. This group may reflect complex clinical scenarios—such as patients with a high bleeding risk, limited prognosis, or explicit refusal of therapy—or situations in which the risk/benefit ratio of anticoagulation was deemed unfavorable. No patient was managed with UFH, indicating that current clinical practice generally favors a fixed-dose subcutaneous regimen (e.g., LMWH) over continuous UFH infusion, which is typically reserved for particularly unstable circumstances or cases requiring rapid dose adjustments. Overall, the findings demonstrate a preference for LMWH as the primary treatment strategy, with modest use of DOACs and other agents (FDP, ASA, VKA) based on each patient’s indications and clinical status. This variability in therapeutic choices likely reflects the complexity of clinical presentations and underscores the need for shared protocols or additional prospective data to determine the optimal strategy in specific patient subgroups. No statistically significant differences emerged between patients who died and those who survived, nor between those who developed PE and those who did not, indicating that IJVT severity may be multifactorial. Similarly, while active cancer is well-established as a risk amplifier for venous thromboembolism, sub-analyses did not detect clear laboratory differentiations, perhaps reflecting the small sample size or the heterogeneity of tumor types.
Overall, the data highlight the complex interplay of risk factors—malignancy, device use, and individual patient comorbidities—and the importance of tailored therapeutic strategies. They also reinforce the need for clinicians to maintain a high index of suspicion for IJVT, particularly in hospitalized patients with catheters and/or cancer, given its potential to progress to serious complications, including pulmonary embolism.