Multicenter Evaluation of the First Validated German-Language Fatigue Questionnaire for Patients with Chronic Inflammatory Bowel Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Demographics and Study Design
2.2. Statistics
3. Results
3.1. Population
3.2. Responses in the IBD-F
3.3. Comparison of IBD-F Responses with the Patient Population
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nocerino, A.; Nguyen, A.; Agrawal, M.; Mone, A.; Lakhani, K.; Swaminath, A. Fatigue in Inflammatory Bowel Diseases: Etiologies and Management. Adv. Ther. 2020, 37, 97–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goërtz, Y.M.J.; Braamse, A.M.J.; Spruit, M.A.; Janssen, D.J.A.; Ebadi, Z.; Van Herck, M.; Burtin, C.; Peters, J.B.; Sprangers, M.A.G.; Lamers, F.; et al. Fatigue in patients with chronic disease: Results from the population-based Lifelines Cohort Study. Sci. Rep. 2021, 11, 20977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lönnfors, S.; Vermeire, S.; Greco, M.; Hommes, D.; Bell, C.; Avedano, L. IBD and health-related quality of life—Discovering the true impact. J. Crohns Colitis 2014, 8, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Borren, N.Z.; van der Woude, C.J.; Ananthakrishnan, A.N. Fatigue in IBD: Epidemiology, pathophysiology and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Vogelaar, L.; de Haar, C.; Aerts, B.R.; Peppelenbosch, M.P.; Timman, R.; Hanssen, B.E.; van der Woude, C.J. Fatigue in patients with inflammatory bowel disease is associated with distinct differences in immune parameters. Clin. Exp. Gastroenterol. 2017, 10, 83–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, S.; Blanchard, A.; Walker, J.R.; Graff, L.A.; Miller, N.; Bernstein, C.N. Common symptoms and stressors among individuals with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2011, 9, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Norton, C.; Czuber-Dochan, W.; Bassett, P.; Berliner, S.; Bredin, F.; Darvell, M.; Forbes, A.; Gay, M.; Ream, E.; Terry, H. Assessing fatigue in inflammatory bowel disease: Comparison of three fatigue scales. Aliment. Pharmacol. Ther. 2015, 42, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Huppertz-Hauss, G.; Høivik, M.L.; Jelsness-Jørgensen, L.P.; Opheim, R.; Henriksen, M.; Høie, O.; Hovde, Ø.; Kempski-Monstad, I.; Solberg, I.C.; Jahnsen, J.; et al. Fatigue in a population-based cohort of patients with inflammatory bowel disease 20 years after diagnosis: The IBSEN study. Scand. J. Gastroenterol. 2017, 52, 351–358, Erratum in Scand. J. Gastroenterol. 2017, 52, i. https://doi.org/10.1080/00365521.2017.1294316. [Google Scholar] [CrossRef] [PubMed]
- Graff, L.A.; Clara, I.; Walker, J.R.; Lix, L.; Carr, R.; Miller, N.; Rogala, L.; Bernstein, C.N. Changes in fatigue over 2 years are associated with activity of inflammatory bowel disease and psychological factors. Clin. Gastroenterol. Hepatol. 2013, 11, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Gasche, C.; Lomer, M.C.; Cavill, I.; Weiss, G. Iron, anaemia, and inflammatory bowel diseases. Gut 2004, 53, 1190–1197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Huijts, M.; Duits, A.; Staals, J.; van Oostenbrugge, R.J. Association of vitamin B12 deficiency with fatigue and depression after lacunar stroke. PLoS ONE 2012, 7, e30519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Frigstad, S.O.; Høivik, M.L.; Jahnsen, J.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, Ø.; Bernklev, T.; Moum, B.; et al. Fatigue is not associated with vitamin D deficiency in inflammatory bowel disease patients. World J. Gastroenterol. 2018, 24, 3293–3301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narula, N.; Cooray, M.; Anglin, R.; Muqtadir, Z.; Narula, A.; Marshall, J.K. Impact of High-Dose Vitamin D3 Supplementation in Patients with Crohn’s Disease in Remission: A Pilot Randomized Double-Blind Controlled Study. Dig. Dis. Sci. 2017, 62, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Zoller, H.; Wolf, M.; Blumenstein, I.; Primas, C.; Lindgren, S.; Thomsen, L.L.; Reinisch, W.; Iqbal, T. Hypophosphataemia following ferric derisomaltose and ferric carboxymaltose in patients with iron deficiency anaemia due to inflammatory bowel disease (PHOSPHARE-IBD): A randomised clinical trial. Gut 2023, 72, 644–653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Atreya, R.; Bettenworth, D.; Bokemeyer, B.; Dignass, A.; Ehehalt, R.; Germer, C.; Grunert, P.C.; Helwig, U.; Horisberger, K.; et al. Aktualisierte S3-Leitlinie “Diagnostik und Therapie des Morbus Crohn” der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) (Version 4.1)—Living guideline. Z. Gastroenterol. 2024, 62, 1229–1318. [Google Scholar]
- Bager, P.; Hvas, C.L.; Rud, C.L.; Dahlerup, J.F. Randomised clinical trial: High-dose oral thiamine versus placebo for chronic fatigue in patients with quiescent inflammatory bowel disease. Aliment. Pharmacol. Ther. 2021, 53, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oketola, B.; Akinrolie, O.; Webber, S.; Askin, N.; Rabbani, R.; Abou-Setta, A.M.; Singh, H. Physical Activity for Quiescent and Mildly Active Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Can. Assoc. Gastroenterol. 2023, 6, 162–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Czuber-Dochan, W.; Norton, C.; Bredin, F.; Darvell, M.; Nathan, I.; Terry, H. Healthcare professionals’ perceptions of fatigue experienced by people with IBD. J. Crohn’s Colitis 2014, 8, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Jelsness-Jørgensen, L.P.; Bernklev, T.; Henriksen, M.; Torp, R.; Moum, B.A. Chronic fatigue is more prevalent in patients with inflammatory bowel disease than in healthy controls. Inflamm. Bowel Dis. 2011, 17, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Smets, E.M.; Garssen, B.; Bonke, B.; De Haes, J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Czuber-Dochan, W.; Norton, C.; Bassett, P.; Berliner, S.; Bredin, F.; Darvell, M.; Forbes, A.; Gay, M.; Nathan, I.; Ream, E.; et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J. Crohn’s Colitis 2014, 8, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Scholz, K.A.M.; Thomann, A.K.; Teich, N.; Baumann, H.; Walldorf, J.; Büning, C.; Rosania, R.; Canbay, A.; Arnim, U.V. Validation of the German Inflammatory Bowel Disease Fatigue (IBD-F) Questionnaire. Z. Gastroenterol. 2023, 61, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm. Bowel Dis. 2004, 10, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Varbobitis, I.; Kokkotis, G.; Gizis, M.; Perlepe, N.; Laoudi, E.; Bletsa, M.; Bekiari, D.; Koutsounas, I.; Kounadis, G.; Xourafas, V.; et al. The IBD-F Patient Self-Assessment Scale Accurately Depicts the Level of Fatigue and Predicts a Negative Effect on the Quality of Life of Patients with IBD in Clinical Remission. Inflamm. Bowel Dis. 2021, 27, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; Kłopocka, M.; Michalak, A.; Cichoz-Lach, H.; Talar-Wojnarowska, R.; Domz Ał-Magrowska, D.; Konecki, Ł.; Filipiuk, A.; Krogulecki, M.; Kopertowska-Majchrzak, M.; et al. Effectiveness and safety outcomes after long-term (54 weeks) vedolizumab therapy for Crohn’s disease: A prospective, real-world observational study including patient-reported outcomes (POLONEZ II). Ther. Adv. Gastroenterol. 2024, 17, 17562848241293938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amiot, A.; Chaibi, S.; Bouhnik, Y.; Serrero, M.; Filippi, J.; Roblin, X.; Bourrier, A.; Bouguen, G.; Franchimont, D.; Savoye, G.; et al. GETAID-patient experience study group Prevalence Determinants of Fatigue in Patients with IBD: ACross-Sectional Survey from the GETAID. J. Crohn’s Colitis 2023, 17, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Befrits, R.; Wikman, O.; Lindgren, S.; Moum, B.; Hjortswang, H.; Hjollund, N.H.; Dahlerup, J.F. Fatigue in out-patients with inflammatory bowel disease is common and multifactorial. Aliment. Pharmacol. Ther. 2012, 35, 133–141. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, P.; Torres-Costoso, A.I.; Fernández-Rodríguez, R.; Guzmán-Pavón, M.J.; de Arenas-Arroyo, S.N.; Basco-López, J.Á.; Reina-Gutiérrez, S. Effect of Vitamin D Supplementation on Fatigue in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bager, P.; Vestergaard, C.; Juul, T.; Dahlerup, J.F. Population-based normative data for the inflammatory bowel disease fatigue scale—IBD-F. Scand. J. Gastroenterol. 2018, 53, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.L.; Zoëga, H.; Shah, S.A.; Leleiko, N.; Lidofsky, S.; Bright, R.; Flowers, N.; Law, M.; Moniz, H.; Merrick, M.; et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment. Pharmacol. Ther. 2014, 39, 811–822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bager, P.; Hvas, C.L.; Rud, C.L.; Dahlerup, J.F. Long-term maintenance treatment with 300 mg thiamine for fatigue in patients with inflammatory bowel disease: Results from an open-label extension of the TARIF study. Scand. J. Gastroenterol. 2022, 57, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, T.; Carlsen, A.; Terje Kvaloy, J.; Bolstad, B.; Warren, D.J.; Aabakken, L.; Lundin, K.; Karlsen, L.; Steinsbo, O.; Omdal, R. Fatigue in Inflammatory Bowel Disease: No Effect of Serum Concentrations of Infliximab, Adalimumab or Anti-Drug Antibodies During Maintenance Therapy. Scand. J. Immunol. 2025, 101, e70029. [Google Scholar] [CrossRef]
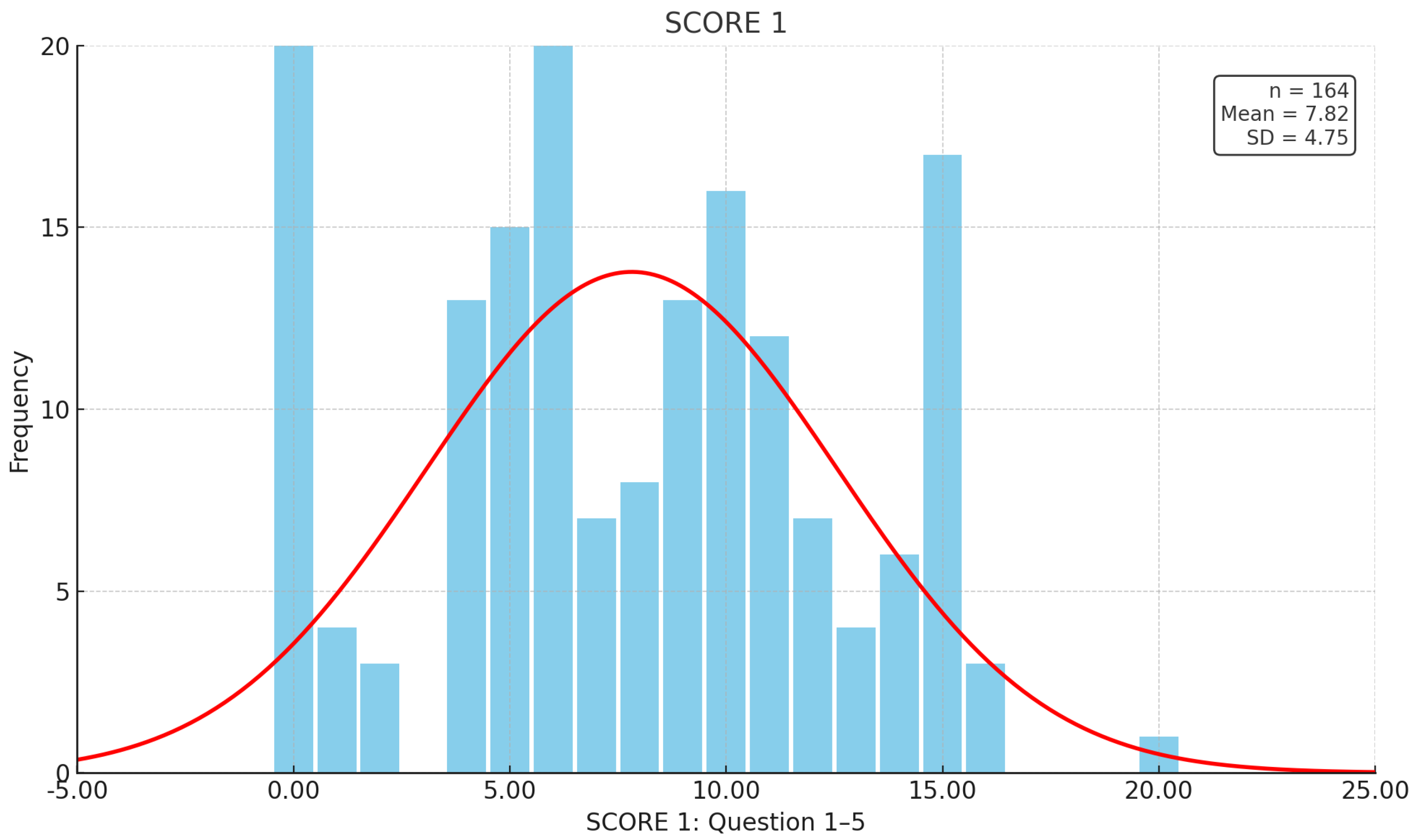
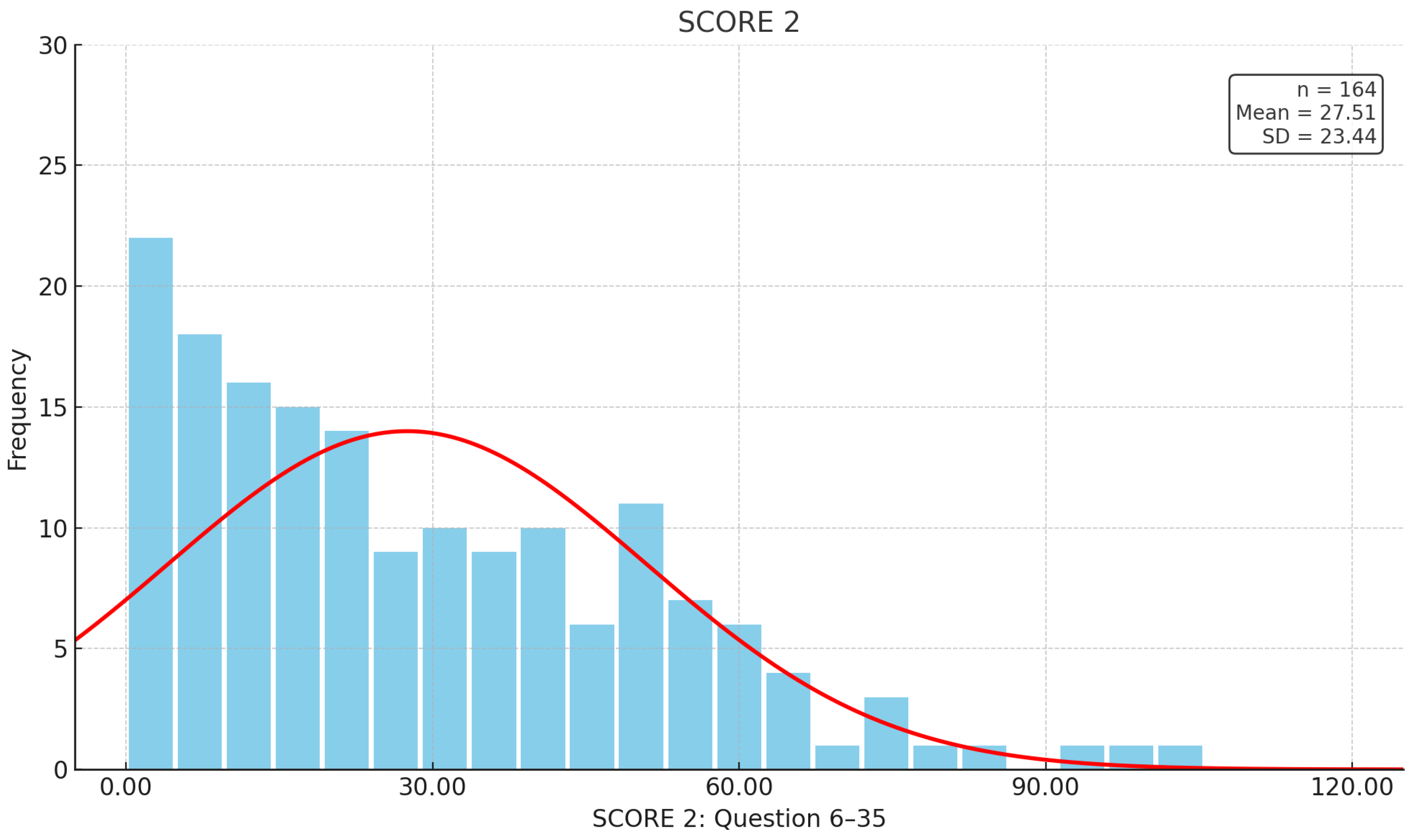
| Patient-related data (total n = 164) | n | Gender | ||
| 86 | Male | 52.4% | ||
| 78 | Female | 47.6% | ||
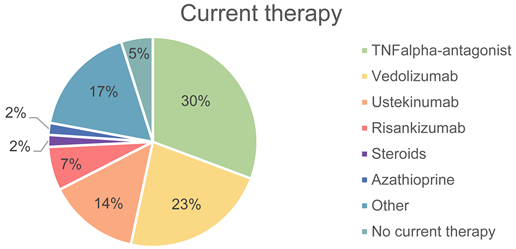
| Current therapy (n = 163) | n | Therapy | ||
| 50 | TNF-alpha antagonist | 30.7% | ||
| 37 | Vedolizumab | 22.7% | ||
| 23 | Ustekinumab | 14.1% | ||
| 11 | Risankizumab | 6.7% | ||
| 3 | Steroids | 1.8% | ||
| 3 | Azathioprine | 1.8% | ||
| 28 | Other | 17.2% | ||
| 8 | No current therapy | 4.9% | ||
| Current disease activity (n = 104) based on histology | n | Disease | ||
| 24 | None | 23.1% | ||
| 30 | Light | 28.8% | ||
| 28 | Moderate | 26.9% | ||
| 22 | High | 21.2% | ||
| Laboratory values | n | Laboratory values | Average values | Reference range |
| 119 | Vitamin D | 29.6 ng/mL (SD 11.5) | 20–70 ng/mL | |
| 145 | Vitamin B12 | 494.5 pg/mL (SD 287.8) | 191–663 pg/mL | |
| 82 | Folic acid | 8.6 ng/mL (SD 5.0) | 3.9–26.8 ng/mL | |
| 139 | Ferritin | 133.7 ng/mL (SD 185.0) | 30–400 ng/mL | |
| 121 | Transferrin | 257.9 mg/dL (SD 34.8) | 200–360 mg/dL | |
| 121 | Transferrin saturation | 24.6% (SD 10.0) | 16–45% | |
| 161 | Hb | 13.9 g/dL (SD 1.7) | 14–18 g/dL | |
| 135 | Albumin | 4.4 g/dL (SD 0.3) | 3.5–5.2 g/dL | |
| 159 | CRP | 0.5 mg/dL (SD 1.2) | <0.5 mg/dL | |
| 75 | fCalprotectin | 263.3 µg/g (SD 304.2) | <50 µg/g | |
 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, M.; Schulz, F.; Tadic, V.; Muzalyova, A.; Classen, J.; Denzer, U.; Blumenstein, I.; Schnoy, E. Multicenter Evaluation of the First Validated German-Language Fatigue Questionnaire for Patients with Chronic Inflammatory Bowel Diseases. J. Clin. Med. 2025, 14, 3618. https://doi.org/10.3390/jcm14113618
Müller M, Schulz F, Tadic V, Muzalyova A, Classen J, Denzer U, Blumenstein I, Schnoy E. Multicenter Evaluation of the First Validated German-Language Fatigue Questionnaire for Patients with Chronic Inflammatory Bowel Diseases. Journal of Clinical Medicine. 2025; 14(11):3618. https://doi.org/10.3390/jcm14113618
Chicago/Turabian StyleMüller, Magnus, Franziska Schulz, Vidan Tadic, Anna Muzalyova, Johanna Classen, Ulrike Denzer, Irina Blumenstein, and Elisabeth Schnoy. 2025. "Multicenter Evaluation of the First Validated German-Language Fatigue Questionnaire for Patients with Chronic Inflammatory Bowel Diseases" Journal of Clinical Medicine 14, no. 11: 3618. https://doi.org/10.3390/jcm14113618
APA StyleMüller, M., Schulz, F., Tadic, V., Muzalyova, A., Classen, J., Denzer, U., Blumenstein, I., & Schnoy, E. (2025). Multicenter Evaluation of the First Validated German-Language Fatigue Questionnaire for Patients with Chronic Inflammatory Bowel Diseases. Journal of Clinical Medicine, 14(11), 3618. https://doi.org/10.3390/jcm14113618






