Clinical Value of Bioactive Adrenomedullin and Proenkephalin A in Patients with Left Ventricular Assist Devices: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Techniques
Minimally Invasive Approach
2.3. Measurement of penKid and bio-ADM
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Participants
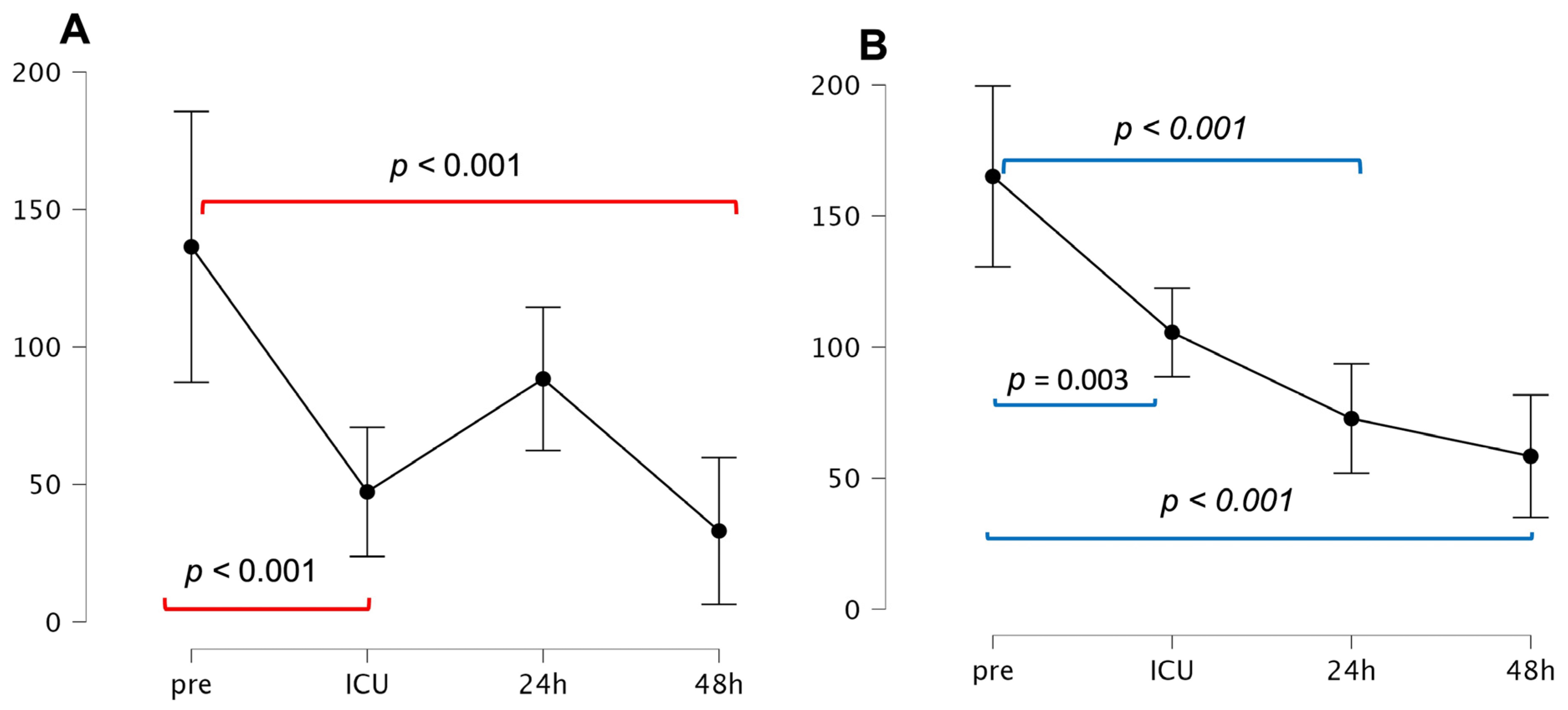
3.2. Time Course of penKid and bio-ADM
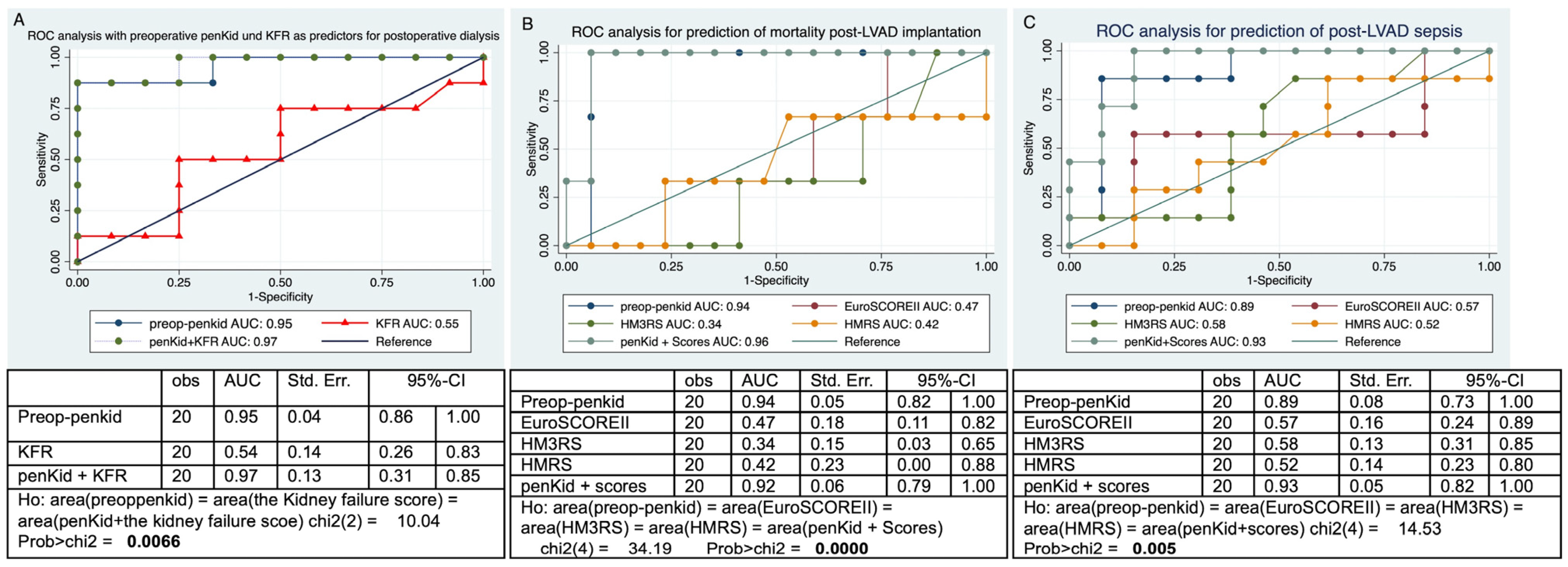
3.3. Preoperative Plasma bio-ADM Predicts Postoperative RHF and Hospital Readmission Due to HF
3.4. Preoperative Plasma penKid Predicts Postoperative AKI with the Need for Dialysis, 30-Day Mortality, and Postoperative Sepsis After LVAD Implantation
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 95%-CI | 95% confidence interval |
| AHT | Arterial hypertension |
| AST | Aspartate aminotransferase |
| AKI | Acute kidney injury |
| AUC | area under the curve |
| BMI | Body mass index Kg/m2 |
| BSA | Body surface area m2 |
| BUN | Blood urea nitrogen |
| Bio-ADM | Bioactive adrenomedullin |
| CPB | Cardiopulmonary bypass |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| CRITT score | CVP greater than 15 mm Hg (C); severe RV dysfunction (R); preoperative mechanical ventilation/intubation (I); severe tricuspid regurgitation (T); and tachycardia (T) |
| CVD | Cerebrovascular disease |
| CVP | Central venous pressure |
| DCM | Dilative cardiomyopathy |
| EDTA | ethylenediaminetetraacetic acid blood sample |
| eGFR | estimated glomerular filtration rate |
| EuroSCORE II | European system for cardiac operative risk evaluation II |
| EURORHFS | The European Registry for Patients with Mechanical Circulatory Support right heart failure score |
| IDDM | Insulin-dependent diabetes mellitus |
| HLP | Hyperlipoproteinemia |
| HMII | HeartMate II |
| HM3 | HeartMate 3 |
| HMRS | The HeartMate II-risk-score |
| HM3RS | The HeartMate 3-risk-score |
| HF | Heart failure |
| ICU | Intensive care unit |
| iNO | Inhaled nitroxide |
| INTERMACS | the Interagency Registry for Mechanically Assisted Circulatory Support |
| INR | International normalized ratio |
| ICM | Ischemic cardiomyopathy |
| KFR | kidney failure risk score |
| LVADs | Left ventricular assist devices |
| LOS | Length of stay |
| MRHFS | Michigan-right-heart-failure risk score |
| mPAP | mean pulmonary artery pressure |
| OR | Odds ratio |
| PAD | Peripheral arterial disease |
| Penkid | Proenkephalin A |
| PHT | Pulmonary hypertension |
| RAP/PCWP | right atrium pressure/postcapillary wedge pressure ratio |
| ROC | Receiver operating characteristic analysis |
| RHF | Right heart failure |
| RVAD | Right ventricular assist device |
| RVFAC | RV fractional area change |
| sPAP | Systolic pulmonary pressure |
| TASV | Tricuspid annular systolic velocity |
| TAPSE | Tricuspid annular plane systolic excursion |
| TIA | Transitory ischemic attack |
References
- Kanwar, M.K.; Lohmueller, L.C.; Kormos, R.L.; Loghmanpour, N.A.; Benza, R.L.; Mentz, R.J.; Bailey, S.H.; Murali, S.; Antaki, J.F. Low Accuracy of the HeartMate Risk Score for Predicting Mortality Using the INTERMACS Registry Data. ASAIO J. 2017, 63, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Cowger, J.; Sundareswaran, K.; Rogers, J.G.; Park, S.J.; Pagani, F.D.; Bhat, G.; Jaski, B.; Farrar, D.J.; Slaughter, M.S. Predicting survival in patients receiving continuous flow left ventricular assist devices: The HeartMate II risk score. J. Am. Coll. Cardiol. 2013, 61, 313–321. [Google Scholar] [CrossRef]
- Thomas, S.S.; Nahumi, N.; Han, J.; Lippel, M.; Colombo, P.; Yuzefpolskaya, M.; Takayama, H.; Naka, Y.; Uriel, N.; Jorde, U.P. Pre-operative mortality risk assessment in patients with continuous-flow left ventricular assist devices: Application of the HeartMate II risk score. J. Heart Lung Transplant. 2014, 33, 675–681. [Google Scholar] [CrossRef]
- Matsue, Y.; Ter Maaten, J.M.; Struck, J.; Metra, M.; O’Connor, C.M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Cleland, J.G.; et al. Clinical Correlates and Prognostic Value of Proenkephalin in Acute and Chronic Heart Failure. J. Card. Fail. 2017, 23, 231–239. [Google Scholar] [CrossRef]
- Emmens, J.E.; ter Maaten, J.M.; Damman, K.; van Veldhuisen, D.J.; de Boer, R.A.; Struck, J.; Bergmann, A.; Sama, I.E.; Streng, K.W.; Anker, S.D.; et al. Proenkephalin, an Opioid System Surrogate, as a Novel Comprehensive Renal Marker in Heart Failure. Circ. Heart Fail. 2019, 12, e005544. [Google Scholar] [CrossRef]
- Ng, L.L.; Squire, I.B.; Jones, D.J.L.; Cao, T.H.; Chan, D.C.S.; Sandhu, J.K.; Quinn, P.A.; Davies, J.E.; Struck, J.; Hartmann, O.; et al. Proenkephalin, Renal Dysfunction, and Prognosis in Patients With Acute Heart Failure: A GREAT Network Study. J. Am. Coll. Cardiol. 2017, 69, 56–69. [Google Scholar] [CrossRef]
- Voordes, G.; Davison, B.; Biegus, J.; Edwards, C.; Damman, K.; ter Maaten, J.; Mebazaa, A.; Takagi, K.; Adamo, M.; Ambrosy, A.P.; et al. Biologically active adrenomedullin as a marker for residual congestion and early rehospitalization in patients hospitalized for acute heart failure: Data from STRONG-HF. Eur. J. Heart Fail. 2024, 26, 1480–1492. [Google Scholar] [CrossRef]
- Núñez, J.; de la Espriella, R.; Rossignol, P.; Voors, A.A.; Mullens, W.; Metra, M.; Chioncel, O.; Januzzi, J.L.; Mueller, C.; Richards, A.M.; et al. Congestion in heart failure: A circulating biomarker-based perspective. A review from the Biomarkers Working Group of the Heart Failure Association, European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 1751–1766. [Google Scholar] [CrossRef]
- Pandhi, P.; Ter Maaten, J.M.; Emmens, J.E.; Struck, J.; Bergmann, A.; Cleland, J.G.; Givertz, M.M.; Metra, M.; O’Connor, C.M.; Teerlink, J.R.; et al. Clinical value of pre-discharge bio-adrenomedullin as a marker of residual congestion and high risk of heart failure hospital readmission. Eur. J. Heart Fail. 2020, 22, 683–691. [Google Scholar] [CrossRef]
- Ahmad, U.; Khattab, M.A.; Schaelte, G.; Goetzenich, A.; Foldenauer, A.C.; Moza, A.; Tewarie, L.; Stoppe, C.; Autschbach, R.; Schnoering, H.; et al. Combining Minimally Invasive Surgery With Ultra-Fast-Track Anesthesia in HeartMate 3 Patients: A Pilot Study. Circ. Heart Fail. 2022, 15, e008358. [Google Scholar] [CrossRef]
- Schmitto, J.D.; Krabatsch, T.; Damme, L.; Netuka, I. Less invasive HeartMate 3 left ventricular assist device implantation. J. Thorac. Dis. 2018, 10, S1692–S1695. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Bergmann, D.; Schulte, J.; Zayat, R.; Marx, G.; Simon, T.P.; Mossanen, J.; Brücken, A.; Stoppe, C. Proenkephalin A and bioactive adrenomedullin are useful for risk prognostication in cardiac surgery. Front. Cardiovasc. Med. 2022, 9, 1017867. [Google Scholar] [CrossRef]
- Weber, J.; Sachse, J.; Bergmann, S.; Sparwaßer, A.; Struck, J.; Bergmann, A. Sandwich Immunoassay for Bioactive Plasma Adrenomedullin. J. Appl. Lab. Med. 2017, 2, 222–233. [Google Scholar] [CrossRef]
- Marino, R.; Struck, J.; Maisel, A.S.; Magrini, L.; Bergmann, A.; Di Somma, S. Plasma adrenomedullin is associated with short-term mortality and vasopressor requirement in patients admitted with sepsis. Crit. Care 2014, 18, R34. [Google Scholar] [CrossRef]
- Donato, L.J.; Meeusen, J.W.; Lieske, J.C.; Bergmann, D.; Sparwaßer, A.; Jaffe, A.S. Analytical performance of an immunoassay to measure proenkephalin. Clin. Biochem. 2018, 58, 72–77. [Google Scholar] [CrossRef]
- Intermacs, S. INTERMACS Adverse Event Definitions. Available online: https://intermacs.kirso.net/intermacs-documents/ (accessed on 1 November 2024).
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am. J. Kidney Dis. 2022, 79, 268–288.e1. [Google Scholar] [CrossRef]
- Tangri, N.; Grams, M.E.; Levey, A.S.; Coresh, J.; Appel, L.J.; Astor, B.C.; Chodick, G.; Collins, A.J.; Djurdjev, O.; Elley, C.R.; et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-analysis. JAMA 2016, 315, 164–174. [Google Scholar] [CrossRef]
- Mehra, M.R.; Nayak, A.; Morris, A.A.; Lanfear, D.E.; Nemeh, H.; Desai, S.; Bansal, A.; Guerrero-Miranda, C.; Hall, S.; Cleveland, J.C.; et al. Prediction of Survival After Implantation of a Fully Magnetically Levitated Left Ventricular Assist Device. JACC Heart Fail. 2022, 10, 948–959. [Google Scholar] [CrossRef]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac Surg. 2012, 41, 734–744; discussion 744–735. [Google Scholar] [CrossRef]
- Matthews, J.C.; Koelling, T.M.; Pagani, F.D.; Aaronson, K.D. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J. Am. Coll. Cardiol. 2008, 51, 2163–2172. [Google Scholar] [CrossRef]
- Atluri, P.; Goldstone, A.B.; Fairman, A.S.; MacArthur, J.W.; Shudo, Y.; Cohen, J.E.; Acker, A.L.; Hiesinger, W.; Howard, J.L.; Acker, M.A.; et al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann. Thorac. Surg. 2013, 96, 857–863; discussion 863–854. [Google Scholar] [CrossRef] [PubMed]
- Soliman, O.I.I.; Akin, S.; Muslem, R.; Boersma, E.; Manintveld, O.C.; Krabatsch, T.; Gummert, J.F.; de By, T.; Bogers, A.; Zijlstra, F.; et al. Derivation and Validation of a Novel Right-Sided Heart Failure Model After Implantation of Continuous Flow Left Ventricular Assist Devices: The EUROMACS (European Registry for Patients with Mechanical Circulatory Support) Right-Sided Heart Failure Risk Score. Circulation 2018, 137, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Storrow, A.B.; Hartmann, O.; Barrett, T.W.; Fermann, G.J.; Maisel, A.S.; Struck, J.; Bergmann, A.; Collins, S.P. Plasma bioactive adrenomedullin as a prognostic biomarker in acute heart failure. Am. J. Emerg. Med. 2016, 34, 257–262. [Google Scholar] [CrossRef]
- Crisanti, L.; Mueller, C. Cardio-renal risk stratification and acute kidney injury in acute coronary syndromes. Eur. Heart J. 2025, 46, 55–57. [Google Scholar] [CrossRef]
- Geven, C.; Kox, M.; Pickkers, P. Adrenomedullin and Adrenomedullin-Targeted Therapy As Treatment Strategies Relevant for Sepsis. Front. Immunol. 2018, 9, 292. [Google Scholar] [CrossRef]
- Molvin, J.; Jujic, A.; Navarin, S.; Melander, O.; Zoccoli, G.; Hartmann, O.; Bergmann, A.; Struck, J.; Bachus, E.; Di Somma, S.; et al. Bioactive adrenomedullin, proenkephalin A and clinical outcomes in an acute heart failure setting. Open Heart 2019, 6, e001048. [Google Scholar] [CrossRef]
- Bravo, C.A.; Navarro, A.G.; Dhaliwal, K.K.; Khorsandi, M.; Keenan, J.E.; Mudigonda, P.; O’Brien, K.D.; Mahr, C. Right heart failure after left ventricular assist device: From mechanisms to treatments. Front. Cardiovasc. Med. 2022, 9, 1023549. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.P.; Kelkar, A.; Weinberger, J.F.; Morris, A.A.; Georgiopoulou, V.V.; Markham, D.W.; Butler, J.; Vega, J.D.; Smith, A.L. Validation of clinical scores for right ventricular failure prediction after implantation of continuous-flow left ventricular assist devices. J. Heart Lung Transplant. 2015, 34, 1595–1603. [Google Scholar] [CrossRef]
- Shah, K.S.; Taub, P.; Patel, M.; Rehfeldt, M.; Struck, J.; Clopton, P.; Mehta, R.L.; Maisel, A.S. Proenkephalin predicts acute kidney injury in cardiac surgery patients. Clin. Nephrol. 2015, 83, 29–35. [Google Scholar] [CrossRef]
- van den Brink, O.W.; Delbridge, L.M.; Rosenfeldt, F.L.; Penny, D.; Esmore, D.S.; Quick, D.; Kaye, D.M.; Pepe, S. Endogenous cardiac opioids: Enkephalins in adaptation and protection of the heart. Heart Lung Circ. 2003, 12, 178–187. [Google Scholar] [CrossRef]
- Peacock, W.F.; Hollander, J.E.; Diercks, D.B.; Lopatin, M.; Fonarow, G.; Emerman, C.L. Morphine and outcomes in acute decompensated heart failure: An ADHERE analysis. Emerg. Med. J. 2008, 25, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Head, S.J.; Osnabrugge, R.L.; Howell, N.J.; Freemantle, N.; Bridgewater, B.; Pagano, D.; Kappetein, A.P. A systematic review of risk prediction in adult cardiac surgery: Considerations for future model development. Eur. J. Cardiothorac Surg. 2013, 43, e121–e129. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Jacobs, J.P.; Alam, S.S.; Thiessen-Philbrook, H.; Everett, A.; Likosky, D.S.; Lobdell, K.; Wyler von Ballmoos, M.C.; Parker, D.M.; Garg, A.X.; et al. Utility of Biomarkers to Improve Prediction of Readmission or Mortality After Cardiac Surgery. Ann. Thorac. Surg. 2018, 106, 1294–1301. [Google Scholar] [CrossRef]


| Age years | 65.25 ± 10.68 |
| Female n (%) | 5 (25.0) |
| ICM n (%) | 13 (65.0) |
| DCM n (%) | 7 (35.0) |
| IDDM n (%) | 4 (20.0) |
| PAD n (%) | 3 (15.0) |
| CVD n (%) | 1 (5.0) |
| AHT n (%) | 13 (65.0) |
| Nicotine n (%) | 11 (55.0) |
| Preoperative Apoplex n (%) | 2 (10.0) |
| HLP n (%) | 6 (30.0) |
| COPD n (%) | 4 (20.0) |
| PHT n (%) | 15 (75.0) |
| Prior cardiac surgery n (%) | 2 (10.0) |
| INTERMACS 2 n (%) | 2 (10.0) |
| INTERMACS 3 n (%) | 7 (35.0) |
| INTERMACS 4 n (%) | 11 (55.0) |
| BMI kg/m2 | 29.05 ± 6.06 |
| BSA m2 | 2.09 ± 0.30 |
| EF % | 20.40 ± 4.52 |
| EuroSCORE II % | 11.86 ± 6.79 |
| HM3RS | 3.37 ± 1.43 |
| HMRS | 1.33 ± 0.80 |
| MRHFS | 1.23 ± 1.63 |
| CRITT score | 0.95 ± 0.69 |
| EURORHFS | 2.98 ± 2.20 |
| The kidney failure equations | 3.22 ± 6.95 |
| CKD | 5 (25%) |
| LVEDD cm | 6.90 ± 1.53 |
| RVFAC % | 35.86 ± 10.36 |
| TASV cm/s | 12.00 ± 14.22 |
| TAPSE cm | 9.57 ± 6.23 |
| Sphericity index | 0.74 ± 0.15 |
| Preoperative laboratory: | |
| Creatinine mg/dL | 1.35 ± 0.65 |
| eGFR mL/min/1.73 m2 | 68.77 ± 18.36 |
| BUN mg/dL | 50 (39, 57.50) |
| Albumin g/dL | 3.46 ± 0.75 |
| INR | 1.12 ± 0.26 |
| Thrombocytes/nL | 220 (179.25, 268.75) |
| AST U/L | 29.50 (22, 34.50) |
| Hematocrit % | 40.12 ± 5.78 |
| Bilirubin mg/dL | 0.72 ± 0.49 |
| Preoperative right heart catheterization: | |
| CVP mmHg | 12.45 ± 6.39 |
| RV pressure mmHg | 46.21 ± 13.79 |
| mPAP mmHg | 35.40 ± 16.28 |
| sPAP mmHg | 48.19 ± 16.20 |
| PCWP mmHg | 23.25 ± 9.74 |
| RA/PCWP | 0.63 ± 0.38 |
| Perioperative data: | |
| CPB time minute | 129.30 ± 62.68 |
| Cross clamp time | 3.60 ± 12.84 |
| ICU days | 8.38 (4, 23.69) |
| Hospital lOS days | 22 (16, 33) |
| Inotropic Days | 7.88 (2.75, 18.25) |
| iNO Hours | 15.50 (0, 69.25) |
| ICU readmission n (%) | 3 (15.0) |
| Postop Pneumonia n (%) | 14 (70.0) |
| Postop Sepsis n (%) | 4 (20.0) |
| Delirium n (%) | 4 (20.0) |
| AKI n (%) | 10 (50.0) |
| Postop dialysis n (%) | 8 (40.0) |
| re-thorax n (%) | 7 (35.0) |
| Right-Heart-Failure n (%) | 6 (30.0) |
| RVAD Implantation n (%) | 2 (10.0) |
| Device thrombus n (%) | 1 (5.0) |
| Ischemic stroke n (%) | 1 (5.0) |
| Hemorrhagic stroke n (%) | 1(5.0) |
| TIA n (%) | 1(5.0) |
| 30 days mortality | 3 (15) |
| Overall mortality within 2 years | 3 (15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogan, L.; Abugameh, A.; Kolashov, A.; Moza, A.; Goetzenich, A.; Stoppe, C.; Shoaib, M.; Bergmann, D.; Spillner, J.; Khattab, M.A.; et al. Clinical Value of Bioactive Adrenomedullin and Proenkephalin A in Patients with Left Ventricular Assist Devices: An Observational Study. J. Clin. Med. 2025, 14, 3613. https://doi.org/10.3390/jcm14103613
Dogan L, Abugameh A, Kolashov A, Moza A, Goetzenich A, Stoppe C, Shoaib M, Bergmann D, Spillner J, Khattab MA, et al. Clinical Value of Bioactive Adrenomedullin and Proenkephalin A in Patients with Left Ventricular Assist Devices: An Observational Study. Journal of Clinical Medicine. 2025; 14(10):3613. https://doi.org/10.3390/jcm14103613
Chicago/Turabian StyleDogan, Leyla, Ahmad Abugameh, Alish Kolashov, Ajay Moza, Andreas Goetzenich, Christian Stoppe, Mohammed Shoaib, Deborah Bergmann, Jan Spillner, Mohammad Amen Khattab, and et al. 2025. "Clinical Value of Bioactive Adrenomedullin and Proenkephalin A in Patients with Left Ventricular Assist Devices: An Observational Study" Journal of Clinical Medicine 14, no. 10: 3613. https://doi.org/10.3390/jcm14103613
APA StyleDogan, L., Abugameh, A., Kolashov, A., Moza, A., Goetzenich, A., Stoppe, C., Shoaib, M., Bergmann, D., Spillner, J., Khattab, M. A., & Zayat, R. (2025). Clinical Value of Bioactive Adrenomedullin and Proenkephalin A in Patients with Left Ventricular Assist Devices: An Observational Study. Journal of Clinical Medicine, 14(10), 3613. https://doi.org/10.3390/jcm14103613






