The Feasibility of a Guideline-Directed Medical Therapy Rapid Up-Titration Programme Among Real-World Heart Failure Patients: A Multicentre Observational Study
Abstract
1. Introduction
- -
- The GDMT applied and target doses (TDs) achieved during the RTP;
- -
- The safety of RTP considering the fulfilment of the “safety indicators” used in the STRONG-HF trial [16] and the occurrence of serious adverse events;
- -
- The effect of the occurrence of the safety indicators, frailty syndrome, and presence of multimorbidity and HF categories on the success of RTP;
- -
- Changes in the quality of life (QoL) of patients;
- -
- Changes in the value of the left ventricular ejection fraction (LVEF) due to the effect of the RTP.
2. Materials and Methods
2.1. Study Population and Design
2.2. Statistical Analysis
3. Results
4. Discussion
4.1. Main Findings
4.2. GDMT and Its Rapid Up-Titration in HF Patients
4.3. Fulfilment of Safety Indicators During the RTP
4.4. The Effect of RTP on QoL
4.5. Changes in LVEF Due to RTP
4.6. The Effect of Frailty Syndrome on GDMT Implementation During the RTP
4.7. The Effect of Comorbidities on GDMT Implementation During the RTP
4.8. The Effect of the HF Category on GDMT Implementation During the RTP
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindberg, F.; Savarese, G. Opportunities and challenges in preventing heart failure: When is risk modifiable? Eur. Heart J. 2025, 46, 1537–1539. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Abdin, A.; Anker, S.D.; Butler, J.; Coats, A.J.S.; Kindermann, I.; Lainscak, M.; Lund, L.H.; Metra, M.; Mullens, W.; Rosano, G.; et al. ‘Time is prognosis’ in heart failure: Time-to-treatment initiation as a modifiable risk factor. ESC Heart Fail. 2021, 8, 4444–4453. [Google Scholar] [CrossRef]
- Tang, A.B.; Ziaeian, B.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Global Impact of Optimal Implementation of Guideline-Directed Medical Therapy in Heart Failure. JAMA Cardiol. 2024, 9, 1154–1158. [Google Scholar] [CrossRef]
- Krum, H.; Roecker, E.B.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Coats, A.J.; Katus, H.A.; Fowler, M.B.; Packer, M. Effects of initiating carvedilol in patients with severe chronic heart failure: Results from the COPERNICUS Study. JAMA 2003, 289, 712–718. [Google Scholar] [CrossRef]
- Lam, P.H.; Packer, M.; Fonarow, G.C.; Faselis, C.; Allman, R.M.; Morgan, C.J.; Singh, S.N.; Pitt, B.; Ahmed, A. Early Effects of Starting Doses of Enalapril in Patients with Chronic Heart Failure in the SOLVD Treatment Trial. Am. J. Med. 2020, 133, e25–e31. [Google Scholar] [CrossRef]
- Packer, M.; McMurray, J.J.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015, 131, 54–61. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Berg, D.D.; Jhund, P.S.; Docherty, K.F.; Murphy, S.A.; Verma, S.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; et al. Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients with Heart Failure with Reduced Ejection Fraction. JAMA Cardiol. 2021, 6, 499–507. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á. The new slogen in the baseline pharmacological therapy of heart failure with reduced ejection fraction: ASAP! Cardiol. Hung. 2023, 53, 502–506. [Google Scholar] [CrossRef]
- Shahid, I.; Fonarow, G.C.; Greene, S.J. Time to Quadruple Guideline-Directed Medical Therapy as a Key Performance Measure for Heart Failure. J. Card. Fail. 2023, 29, 730–733. [Google Scholar] [CrossRef]
- Cotter, G.; Davison, B.; Cohen-Solal, A.; Freund, Y.; Mebazaa, A. Targeting the ‘vulnerable’ period–first 3-6 months after an acute heart failure admission–the light gets brighter. Eur. J. Heart Fail. 2023, 25, 30–34. [Google Scholar] [CrossRef]
- Gergely, G.T.; Bánfi-Bacsárdi, F.; Komáromi, A.; Pilecky, D.; Boldizsár, E.M.; Flegler, D.; Kazay, Á.; Füzesi, T.; Forrai, Z.; Vértes, V.; et al. Rapid up-titration of guide-directed medical therapy after a heart failure hospitalisation. Orv. Hetil. 2024, 165, 1197–1205. [Google Scholar] [CrossRef]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- Butler, J.; Talha, K.M.; Fonarow, G.C. STRONG-HF and Implementing Heart Failure Therapies: Godspeed … with Care. Circulation 2023, 147, 1189–1191. [Google Scholar] [CrossRef]
- Bánfi-Bacsárdi, F.; Forrai, Z.; Kazay, Á.; Füzesi, T.; Vámos, M.; Gergely, T.G.; Pilecky, D.; Komáromi, A.; Szőnyi, M.D.; Papp, E.; et al. Eligibility for Rapid Up-Titration of Guideline-Directed Medical Therapy of Real-World Patients Hospitalised for Heart Failure. Cardiology 2024, 1–11. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
- Spertus, J.A.; Jones, P.G. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 469–476. [Google Scholar] [CrossRef]
- Spertus, J.A.; Jones, P.G.; Sandhu, A.T.; Arnold, S.V. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Jankowska, E.; Hill, L.; Piepoli, M.; Doehner, W.; Anker, S.D.; Lainscak, M.; Jaarsma, T.; Ponikowski, P.; Rosano, G.M.C.; et al. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur. J. Heart Fail. 2019, 21, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Rickham, P.P. Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br. Med. J. 1964, 2, 177. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- ACC/AHA Joint Committee. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J. Card. Fail. 2022, 28, e1–e167. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure with Reduced Ejection Fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef]
- Greene, S.J.; Ezekowitz, J.A.; Anstrom, K.J.; Demyanenko, V.; Givertz, M.M.; Pina, I.L.; O’Connor, C.M.; Koglin, J.; Roessig, L.; Hernandez, A.F.; et al. Medical Therapy During Hospitalization for Heart Failure with Reduced Ejection Fraction: The VICTORIA Registry. J. Card. Fail. 2022, 28, 1063–1077. [Google Scholar] [CrossRef]
- Bánfi-Bacsárdi, F.; Muk, B.; Pilecky, D.; Duray, G.Z.; Kiss, R.G.; Nyolczas, N. The Optimization of Guideline-Directed Medical Therapy during Hospitalization among Patients with Heart Failure with Reduced Ejection Fraction in Daily Clinical Practice. Cardiology 2023, 148, 27–37. [Google Scholar] [CrossRef]
- Joseph, P.; Roy, A.; Lonn, E.; Störk, S.; Floras, J.; Mielniczuk, L.; Rouleau, J.L.; Zhu, J.; Dzudie, A.; Balasubramanian, K.; et al. Global Variations in Heart Failure Etiology, Management, and Outcomes. JAMA 2023, 329, 1650–1661. [Google Scholar] [CrossRef]
- Pierce, J.B.; Vaduganathan, M.; Fonarow, G.C.; Ikeaba, U.; Chiswell, K.; Butler, J.; DeVore, A.D.; Heidenreich, P.A.; Huang, J.C.; Kittleson, M.M.; et al. Contemporary Use of Sodium-Glucose Cotransporter-2 Inhibitor Therapy Among Patients Hospitalized for Heart Failure with Reduced Ejection Fraction in the US: The Get with The Guidelines-Heart Failure Registry. JAMA Cardiol. 2023, 8, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Ayodele, I.; Pierce, J.B.; Khan, M.S.; Lewsey, S.C.; Yancy, C.W.; Alhanti, B.; Van Spall, H.G.C.; Allen, L.A.; Fonarow, G.C. Eligibility and Projected Benefits of Rapid Initiation of Quadruple Therapy for Newly Diagnosed Heart Failure. JACC Heart Fail. 2024, 12, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Muk, B.; Pilecky, D.; Bánfi-Bacsárdi, F.; Füzesi, T.; Gergely, G.T.; Komáromi, A.; Papp, E.; Szőnyi, M.D.; Forrai, Z.; Kazay, Á.; et al. The changes in the pharmacotherapy of heart failure with reduced ejection fraction and its effect on prognosis: Experience in the Hungarian clinical practice. Orv. Hetil. 2024, 165, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, S.; Basile, C.; Marzano, F.; Di Santo, M.; Galasso, G.; Ameri, P.; Nodari, S.; Santoro, G.; Severino, P.; Guerra, F.; et al. Guideline-directed medical therapy patterns in heart failure with reduced ejection fraction: The OPTIMA-HF registry. Eur. Heart J. 2024, 45, ehae666-1091. [Google Scholar] [CrossRef]
- Packer, M.; McMurray, J.J.V. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur. J. Heart Fail. 2021, 23, 882–894. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Fonarow, G.C. Simultaneous or Rapid Sequence Initiation of Quadruple Medical Therapy for Heart Failure-Optimizing Therapy with the Need for Speed. JAMA Cardiol. 2021, 6, 743–744. [Google Scholar] [CrossRef]
- Shen, L.; Jhund, P.S.; Docherty, K.F.; Vaduganathan, M.; Petrie, M.C.; Desai, A.S.; Køber, L.; Schou, M.; Packer, M.; Solomon, S.D.; et al. Accelerated and personalized therapy for heart failure with reduced ejection fraction. Eur. Heart J. 2022, 43, 2573–2587. [Google Scholar] [CrossRef]
- Kimmoun, A.; Cotter, G.; Davison, B.; Takagi, K.; Addad, F.; Celutkiene, J.; Chioncel, O.; Solal, A.C.; Diaz, R.; Damasceno, A.; et al. Safety, Tolerability and efficacy of Rapid Optimization, helped by NT-proBNP and GDF-15, of Heart Failure therapies (STRONG-HF): Rationale and design for a multicentre, randomized, parallel-group study. Eur. J. Heart Fail. 2019, 21, 1459–1467. [Google Scholar] [CrossRef]
- Pagnesi, M.; Metra, M.; Cohen-Solal, A.; Edwards, C.; Adamo, M.; Tomasoni, D.; Lam, C.S.P.; Chioncel, O.; Diaz, R.; Filippatos, G.; et al. Uptitrating Treatment After Heart Failure Hospitalization Across the Spectrum of Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2023, 81, 2131–2144. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Mebazaa, A.; Davison, B.; Edwards, C.; Adamo, M.; Arrigo, M.; Barros, M.; Biegus, J.; Čelutkienė, J.; Čerlinskaitė-Bajorė, K.; et al. Early changes in renal function during rapid up-titration of guideline-directed medical therapy following an admission for acute heart failure. Eur. J. Heart Fail. 2023, 25, 2230–2242. [Google Scholar] [CrossRef]
- Pagnesi, M.; Vilamajó, O.A.G.; Meiriño, A.; Dumont, C.A.; Mebazaa, A.; Davison, B.; Adamo, M.; Arrigo, M.; Barros, M.; Biegus, J.; et al. Blood pressure and intensive treatment up-titration after acute heart failure hospitalization: Insights from the STRONG-HF trial. Eur. J. Heart Fail. 2024, 26, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Pagnesi, M.; Mebazaa, A.; Davison, B.; Edwards, C.; Tomasoni, D.; Arrigo, M.; Barros, M.; Biegus, J.; Celutkiene, J.; et al. NT-proBNP and high intensity care for acute heart failure: The STRONG-HF trial. Eur. Heart J. 2023, 44, 2947–2962. [Google Scholar] [CrossRef]
- Čerlinskaitė-Bajorė, K.; Lam, C.S.P.; Sliwa, K.; Adamo, M.; Ter Maaten, J.M.; Léopold, V.; Mebazaa, A.; Davison, B.; Edwards, C.; Arrigo, M.; et al. Sex-specific analysis of the rapid up-titration of guideline-directed medical therapies after a hospitalization for acute heart failure: Insights from the STRONG-HF trial. Eur. J. Heart Fail. 2023, 25, 1156–1165. [Google Scholar] [CrossRef]
- Farmakis, D.; Davison, B.; Fountoulaki, K.; Liori, S.; Chioncel, O.; Metra, M.; Celutkiene, J.; Cohen-Solal, A.; Damasceno, A.; Diaz, R.; et al. Rapid Uptitration of Guideline-Directed Medical Therapies in Acute Heart Failure With and Without Atrial Fibrillation. JACC Heart Fail. 2024, 12, 1845–1858. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Chang, A.J.; Davison, B.; Voors, A.; Cohen-Solal, A.; Damasceno, A.; Kimmoun, A.; Lam, C.S.P.; Edwards, C.; Tomasoni, D.; et al. Titration of Medications After Acute Heart Failure Is Safe, Tolerated, and Effective Regardless of Risk. JACC Heart Fail. 2024, 12, 1566–1582. [Google Scholar] [CrossRef]
- Spahillari, A.; Cohen, L.P.; Lin, C.; Liu, Y.; Tringale, A.; Sheppard, K.E.; Ko, C.; Khairnar, R.; Williamson, K.M.; Wasfy, J.H.; et al. Efficacy, Safety and Mechanistic Impact of a Heart Failure Guideline-Directed Medical Therapy Clinic. JACC Heart Fail. 2024, 13, 554–568. [Google Scholar] [CrossRef]
- Shahid, I.; Khan, M.S.; Fonarow, G.C.; Butler, J.; Greene, S.J. Bridging gaps and optimizing implementation of guideline-directed medical therapy for heart failure. Prog. Cardiovasc. Dis. 2024, 82, 61–69. [Google Scholar] [CrossRef]
- Muk, B.; Bánfi-Bacsárdi, F.; Vámos, M.; Pilecky, D.; Majoros, Z.; Török, G.M.; Vágány, D.; Polgár, B.; Solymossi, B.; Borsányi, T.D.; et al. The Impact of Specialised Heart Failure Outpatient Care on the Long-Term Application of Guideline-Directed Medical Therapy and on Prognosis in Heart Failure with Reduced Ejection Fraction. Diagnostics 2024, 14, 131. [Google Scholar] [CrossRef]
- Bánfi-Bacsárdi, F.; Vámos, M.; Majoros, Z.; Török, G.; Pilecky, D.; Duray, G.Z.; Kiss, R.G.; Nyolczas, N.; Muk, B. The effect of kidney function on the optimization of medical therapy and on mortality in heart failure with reduced ejection fraction. Orv. Hetil. 2023, 164, 1387–1396. [Google Scholar] [CrossRef]
- Tomasoni, D.; Davison, B.; Adamo, M.; Pagnesi, M.; Mebazaa, A.; Edwards, C.; Arrigo, M.; Barros, M.; Biegus, J.; Čelutkienė, J.; et al. Safety Indicators in Patients Receiving High-intensity Care After Hospital Admission for Acute Heart Failure: The STRONG-HF Trial. J. Card. Fail. 2024, 30, 525–537. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Moura, B.; Metra, M.; Böhm, M.; Bauersachs, J.; Ben Gal, T.; Adamopoulos, S.; Abdelhamid, M.; Bistola, V.; Čelutkienė, J.; et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Usman, M.S.; Anker, M.S.; Butler, J.; Böhm, M.; Abraham, W.T.; Adamo, M.; Chopra, V.K.; Cicoira, M.; Cosentino, F.; et al. Patient phenotype profiling in heart failure with preserved ejection fraction to guide therapeutic decision making. A scientific statement of the Heart Failure Association, the European Heart Rhythm Association of the European Society of Cardiology, and the European Society of Hypertension. Eur. J. Heart Fail. 2023, 25, 936–955. [Google Scholar] [CrossRef] [PubMed]
- Banfi-Bacsardi, F.; Muk, B.; Majoros, Z.S.; Torok, G.; Pilecky, D.; Duray, G.Z.; Kiss, R.G.; Nyolczas, N. The effect of long-term application of guideline-directed medical therapy on systolic blood pressure in hospitalized patients with heart failure with reduced ejection fraction. Eur. Heart J. 2023, 44, ehad655-991. [Google Scholar] [CrossRef]
- Böhm, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Mahfoud, F.; Brueckmann, M.; Jamal, W.; Ofstad, A.P.; et al. Empagliflozin Improves Cardiovascular and Renal Outcomes in Heart Failure Irrespective of Systolic Blood Pressure. J. Am. Coll. Cardiol. 2021, 78, 1337–1348. [Google Scholar] [CrossRef]
- Serenelli, M.; Jackson, A.; Dewan, P.; Jhund, P.S.; Petrie, M.C.; Rossignol, P.; Campo, G.; Pitt, B.; Zannad, F.; Ferreira, J.P.; et al. Mineralocorticoid Receptor Antagonists, Blood Pressure, and Outcomes in Heart Failure with Reduced Ejection Fraction. JACC Heart Fail. 2020, 8, 188–198. [Google Scholar] [CrossRef]
- Melendo-Viu, M.; Dobarro, D.; Marchán López, Á.; Domínguez, L.M.; Raposeiras-Roubín, S.; Abu-Assi, E.; Cardero-González, C.; Pérez-Expósito, L.; Cespón Fernández, M.; Parada Barcia, J.A.; et al. Hypotension at heart failure discharge: Should it be a limiting factor for drug titration? Int. J. Cardiol. 2023, 386, 59–64. [Google Scholar] [CrossRef]
- Girerd, N.; Coiro, S.; Benson, L.; Savarese, G.; Dahlström, U.; Rossignol, P.; Lund, L.H. Hypotension in heart failure is less harmful if associated with high or increasing doses of heart failure medication: Insights from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2024, 26, 359–369. [Google Scholar] [CrossRef]
- Mullens, W.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Fudim, M.; Iacoviello, M.; Franke, J.; Flammer, A.J.; et al. Renal effects of guideline-directed medical therapies in heart failure: A consensus document from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 603–619. [Google Scholar] [CrossRef]
- Shen, L.; Kristensen, S.L.; Bengtsson, O.; Böhm, M.; de Boer, R.A.; Docherty, K.F.; Inzucchi, S.E.; Katova, T.; Køber, L.; Kosiborod, M.N.; et al. Dapagliflozin in HFrEF Patients Treated with Mineralocorticoid Receptor Antagonists: An Analysis of DAPA-HF. JACC Heart Fail. 2021, 9, 254–264. [Google Scholar] [CrossRef]
- Desai, A.S.; Vardeny, O.; Claggett, B.; McMurray, J.J.; Packer, M.; Swedberg, K.; Rouleau, J.L.; Zile, M.R.; Lefkowitz, M.; Shi, V.; et al. Reduced Risk of Hyperkalemia During Treatment of Heart Failure with Mineralocorticoid Receptor Antagonists by Use of Sacubitril/Valsartan Compared with Enalapril: A Secondary Analysis of the PARADIGM-HF Trial. JAMA Cardiol. 2017, 2, 79–85. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Zannad, F.; Butler, J.; Filipattos, G.; Ritter, I.; Schüler, E.; Kraus, B.J.; Pocock, S.J.; Anker, S.D.; Packer, M. Empagliflozin and serum potassium in heart failure: An analysis from EMPEROR-Pooled. Eur. Heart J. 2022, 43, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Fuery, M.A.; Leifer, E.S.; Samsky, M.D.; Sen, S.; O’Connor, C.M.; Fiuzat, M.; Ezekowitz, J.; Piña, I.; Whellan, D.; Mark, D.; et al. Prognostic Impact of Repeated NT-proBNP Measurements in Patients with Heart Failure with Reduced Ejection Fraction. JACC Heart Fail. 2024, 12, 479–487. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Ahmad, T.; Mulder, H.; Coles, A.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Mark, D.B.; et al. Natriuretic Peptide Response and Outcomes in Chronic Heart Failure with Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2019, 74, 1205–1217. [Google Scholar] [CrossRef]
- Čelutkienė, J.; Čerlinskaitė-Bajorė, K.; Cotter, G.; Edwards, C.; Adamo, M.; Arrigo, M.; Barros, M.; Biegus, J.; Chioncel, O.; Cohen-Solal, A.; et al. Impact of Rapid Up-Titration of Guideline-Directed Medical Therapies on Quality of Life: Insights From the STRONG-HF Trial. Circ. Heart Fail. 2024, 17, e011221. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Camacho, A.; Piña, I.L.; Rocha, R.; Williamson, K.M.; Maisel, A.S.; Felker, G.M.; Prescott, M.F.; Butler, J.; Solomon, S.D. Reverse Cardiac Remodeling and Outcome After Initiation of Sacubitril/Valsartan. Circ. Heart Fail. 2020, 13, e006946. [Google Scholar] [CrossRef]
- Kodsi, M.; Makarious, D.; Gan, G.C.H.; Choudhary, P.; Thomas, L. Cardiac reverse remodelling by imaging parameters with recent changes to guideline medical therapy in heart failure. ESC Heart Fail. 2023, 10, 3258–3275. [Google Scholar] [CrossRef]
- Boulet, J.; Mehra, M.R. Left Ventricular Reverse Remodeling in Heart Failure: Remission to Recovery. Struct. Heart 2021, 5, 466–481. [Google Scholar] [CrossRef]
- Chan, A.K.; Sanderson, J.E.; Wang, T.; Lam, W.; Yip, G.; Wang, M.; Lam, Y.Y.; Zhang, Y.; Yeung, L.; Wu, E.B.; et al. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 591–596. [Google Scholar] [CrossRef]
- Groenning, B.A.; Nilsson, J.C.; Sondergaard, L.; Fritz-Hansen, T.; Larsson, H.B.; Hildebrandt, P.R. Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J. Am. Coll. Cardiol. 2000, 36, 2072–2080. [Google Scholar] [CrossRef]
- Fan, G.; Guo, D.L. The effect of sodium-glucose cotransporter-2 inhibitors on cardiac structure remodeling and function: A meta-analysis of randomized controlled trials. Eur. J. Intern. Med. 2023, 114, 49–57. [Google Scholar] [CrossRef]
- Greenberg, B.; Quinones, M.A.; Koilpillai, C.; Limacher, M.; Shindler, D.; Benedict, C.; Shelton, B. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation 1995, 91, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Solymossi, B.; Muk, B.; Sepp, R.; Habon, T.; Borbély, A.; Heltai, K.; Majoros, Z.; Járai, Z.; Vágány, D.; Szatmári, Á.; et al. Incidence and predictors of heart failure with improved ejection fraction category in a HFrEF patient population. ESC Heart Fail. 2024, 11, 783–794. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ling, Y.; Guo, W.; Li, Q.; Yu, S.; Huang, H.; Zhang, R.; Gong, Z.; Liu, J.; Mo, L.; et al. Prevalence and Prognosis of HFimpEF Developed From Patients with Heart Failure with Reduced Ejection Fraction: Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 757596. [Google Scholar] [CrossRef]
- Banfi-Bacsardi, F.; Fuzesi, T.; Kazay, A.; Torok, G.M.; Pilecky, D.; Vamos, M.; Gergely, T.G.; Forrai, Z.S.; Majoros, Z.S.; Borsanyi, T.D.; et al. The development and long-term maintenance of heart failure with improved ejection fraction after a heart failure hospitalization: How are mortality rates affected? Eur. Heart J. 2024, 45, ehae666-941. [Google Scholar] [CrossRef]
- Veltmann, C.; Duncker, D.; Doering, M.; Gummadi, S.; Robertson, M.; Wittlinger, T.; Colley, B.J.; Perings, C.; Jonsson, O.; Bauersachs, J.; et al. Therapy duration and improvement of ventricular function in de novo heart failure: The Heart Failure Optimization study. Eur. Heart J. 2024, 45, 2771–2781. [Google Scholar] [CrossRef]
- Khan, M.S.; Segar, M.W.; Usman, M.S.; Singh, S.; Greene, S.J.; Fonarow, G.C.; Anker, S.D.; Felker, G.M.; Januzzi, J.L., Jr.; Butler, J.; et al. Frailty, Guideline-Directed Medical Therapy, and Outcomes in HFrEF: From the GUIDE-IT Trial. JACC Heart Fail. 2022, 10, 266–275. [Google Scholar] [CrossRef]
- Bánfi-Bacsárdi, F.; Kazay, Á.; Gergely, T.G.; Forrai, Z.; Füzesi, T.P.; Hanuska, L.F.; Schäffer, P.P.; Pilecky, D.; Vámos, M.; Vértes, V.; et al. Therapeutic Consequences and Prognostic Impact of Multimorbidity in Heart Failure: Time to Act. J. Clin. Med. 2024, 14, 139. [Google Scholar] [CrossRef]
- Gerhardt, T.; Gerhardt, L.M.S.; Ouwerkerk, W.; Roth, G.A.; Dickstein, K.; Collins, S.P.; Cleland, J.G.F.; Dahlstrom, U.; Tay, W.T.; Ertl, G.; et al. Multimorbidity in patients with acute heart failure across world regions and country income levels (REPORT-HF): A prospective, multicentre, global cohort study. Lancet Glob. Health 2023, 11, e1874–e1884. [Google Scholar] [CrossRef]
- Tomasoni, D.; Vitale, C.; Guidetti, F.; Benson, L.; Braunschweig, F.; Dahlström, U.; Melin, M.; Rosano, G.M.C.; Lund, L.H.; Metra, M.; et al. The role of multimorbidity in patients with heart failure across the left ventricular ejection fraction spectrum: Data from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2024, 26, 854–868. [Google Scholar] [CrossRef]

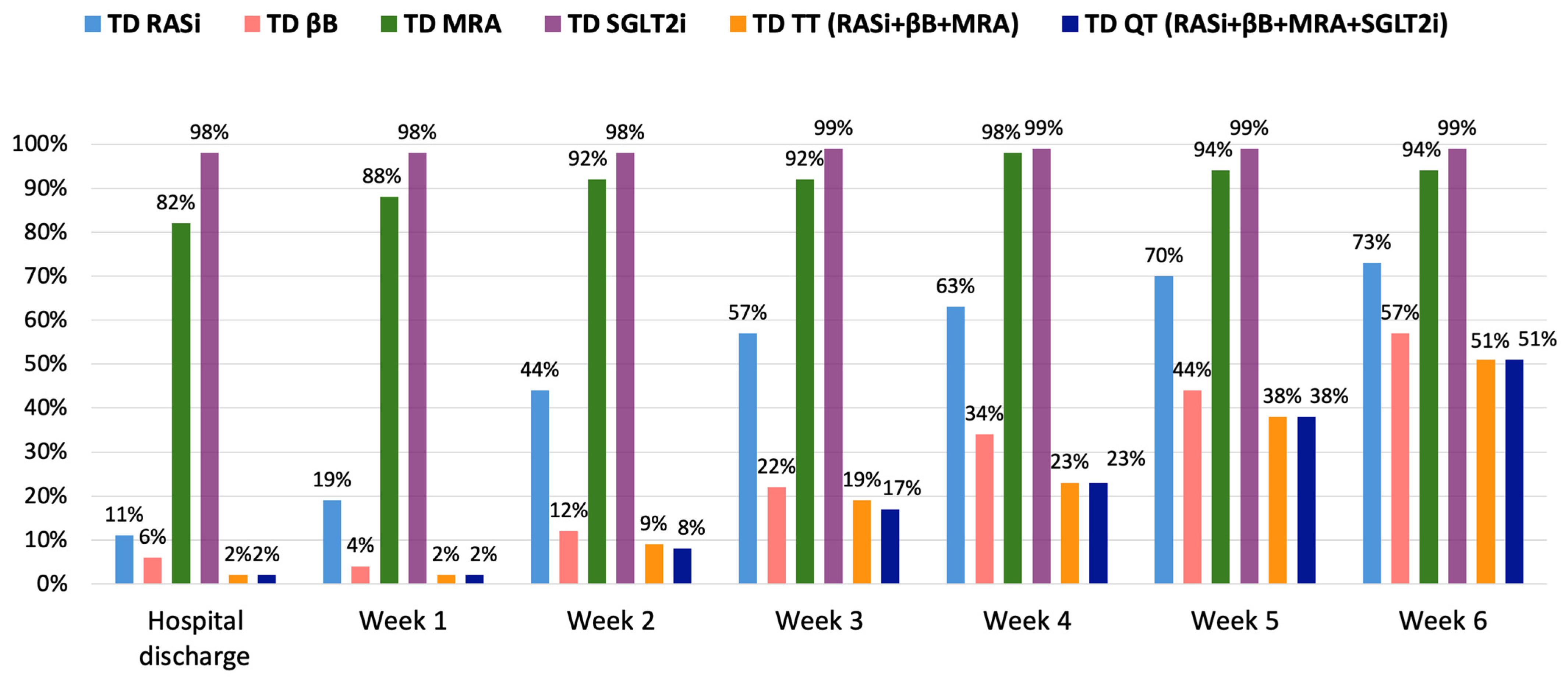
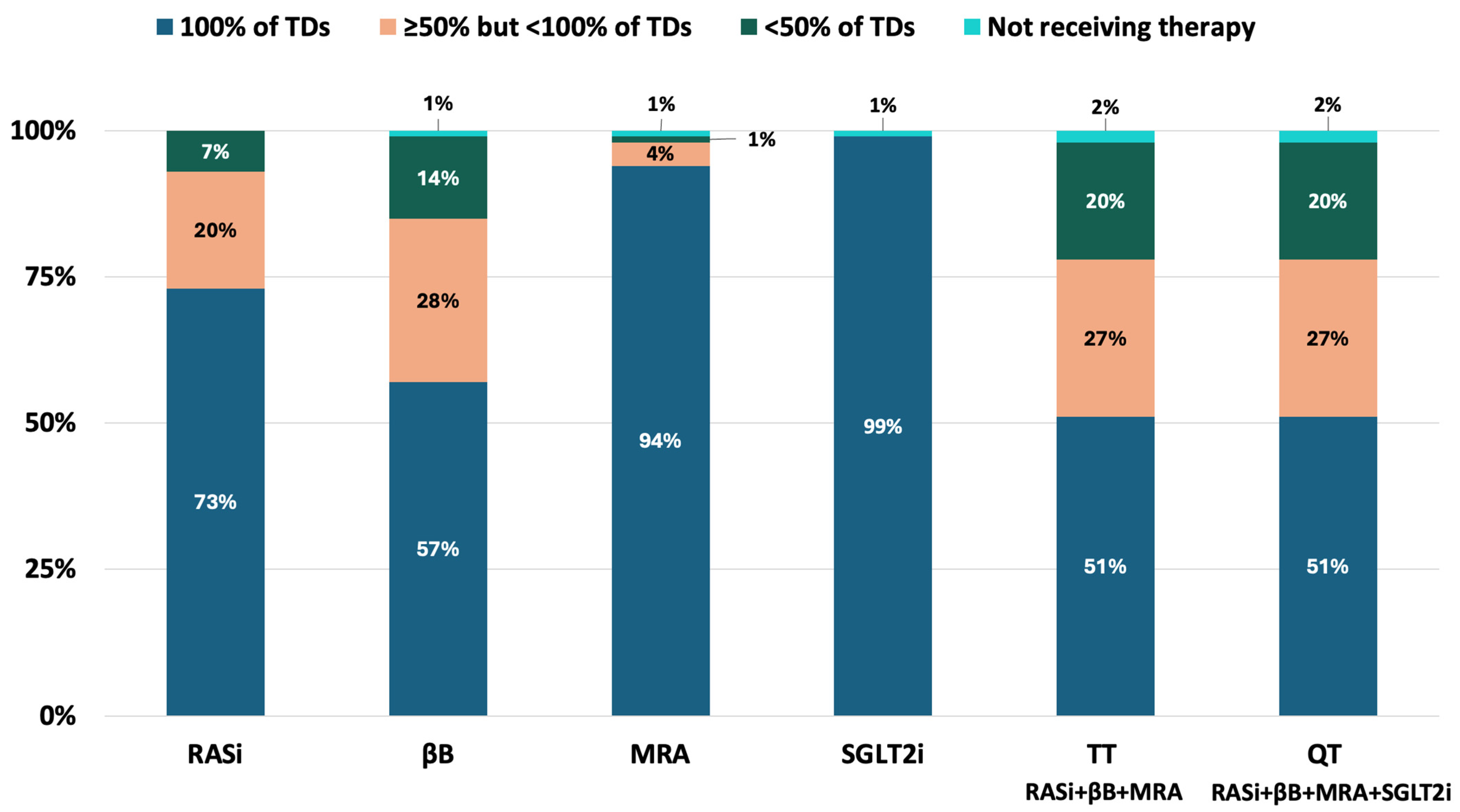
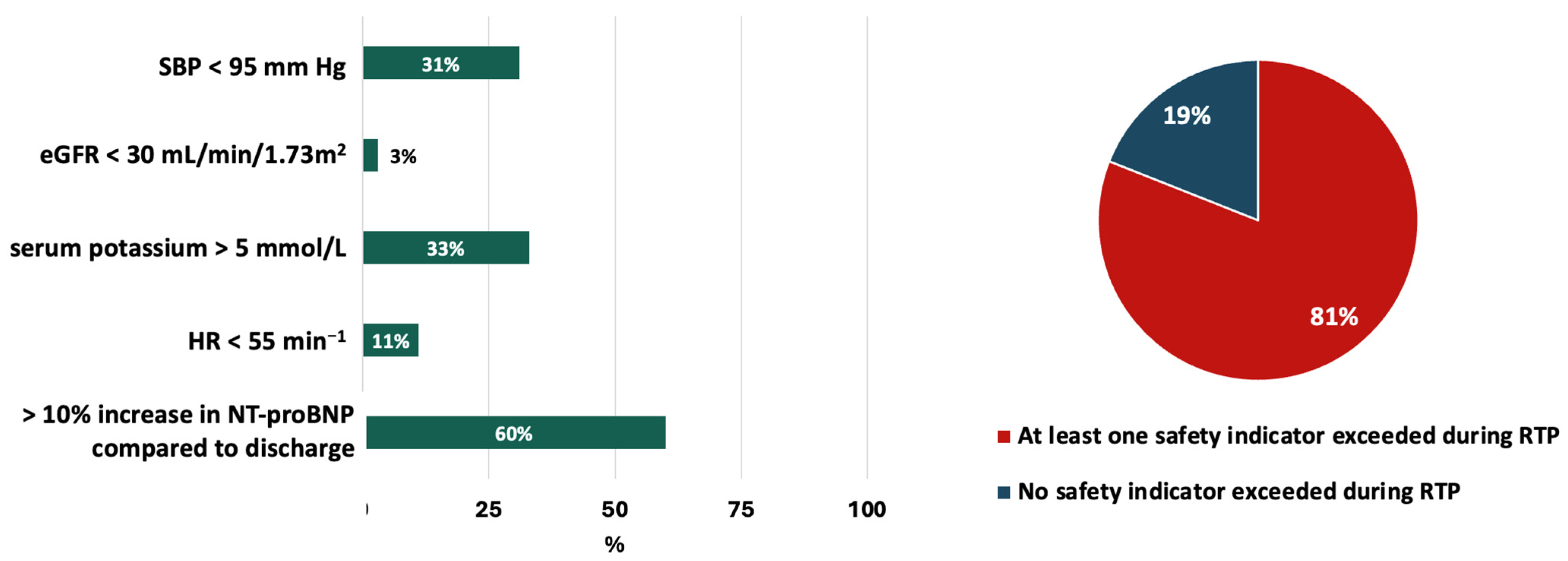
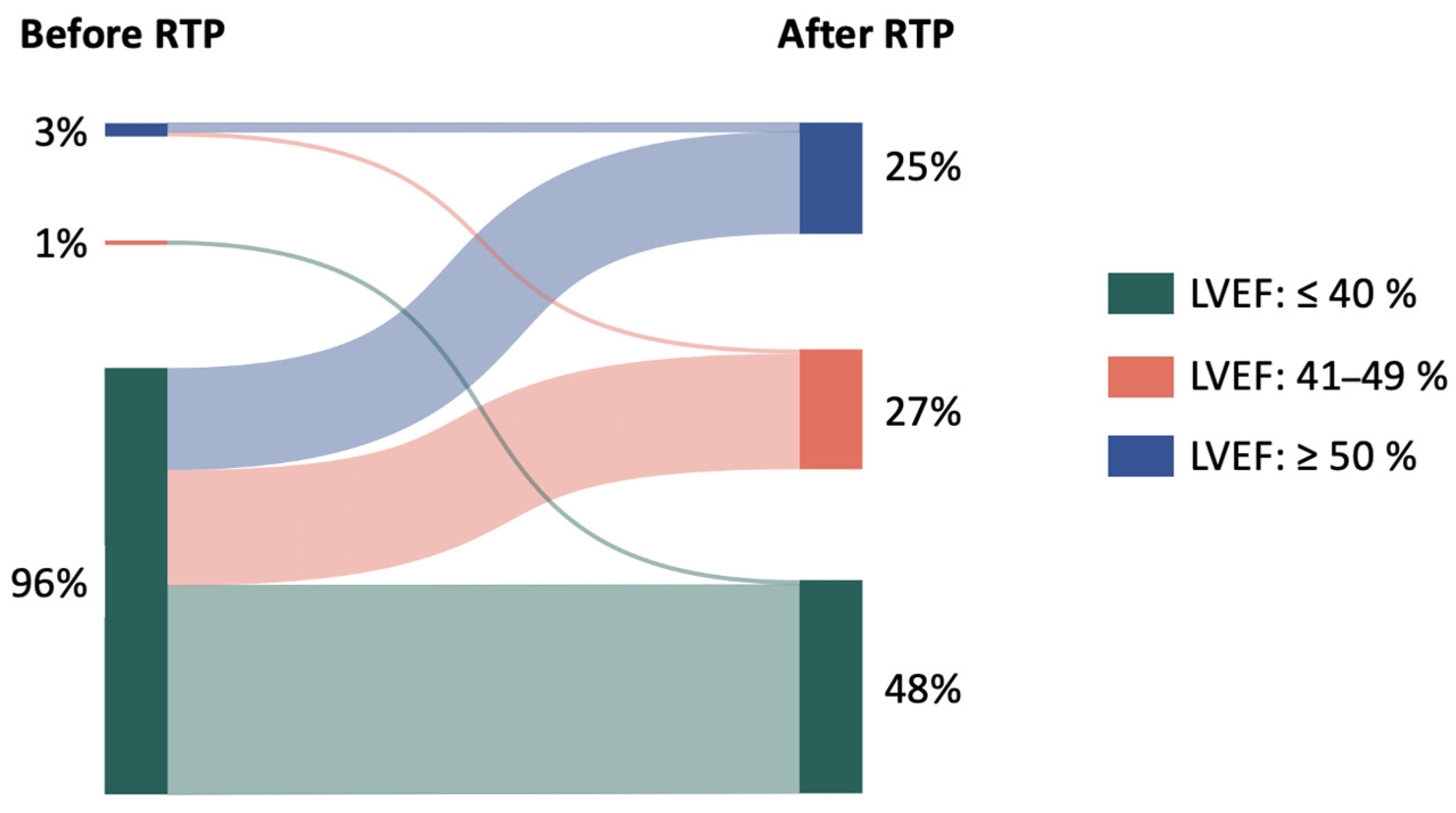
| Parameters | Total Cohort (n = 90) |
|---|---|
| Male sex (%) | 82 |
| Age, median [IQR], years | 56 [49–63] |
| De novo heart failure (%) | 63 |
| LVEF, median [IQR], % | 24 [20–32] |
| HFrEF (%) | 96 |
| HFmrEF (%) | 1 |
| HFpEF (%) | 3 |
| Duration of hospitalisation, median [IQR], days | 9 [7–13] |
| Comorbidities | |
| Coronary artery disease (%) | 28 |
| Hypertension (%) | 68 |
| Atrial fibrillation/flutter (%) | 41 |
| Stroke (%) | 1 |
| PAD (%) | 2 |
| Severe VHD (%) | 7 |
| Previously diagnosed chronic kidney disease (%) | 8 |
| eGFR < 60 mL/min/1.73 m2 (%) | 28 |
| Dyslipidaemia (%) | 71 |
| Iron deficiency (%) | 68 |
| Obesity (%) | 66 |
| Diabetes (%) | 34 |
| Hyperuricaemia (%) | 23 |
| Anaemia (%) | 11 |
| Hypo-/hyperthyroidism (%) | 11 |
| Asthma/COPD (%) | 10 |
| Sleep-disordered breathing (%) | 3 |
| ≥3 NCCMs (%) | 58 |
| At admission | |
| Heart rate, median [IQR], min−1 | 97 [87–114] |
| Systolic blood pressure, median [IQR], mmHg | 126 [114–140] |
| Serum creatinine, median [IQR], μmol/L | 98 [85–115] |
| eGFR, median [IQR], mL/min/1.73 m2 | 71 [59–85] |
| Serum potassium, median [IQR], mmol/L | 4.1 [3.8–4.5] |
| Serum sodium, median [IQR], mmol/L | 139 [136–140] |
| Haemoglobin, median [IQR], g/L | 150 [138–161] |
| NT-proBNP, median [IQR], pg/mL | 4095 [2352–8160] |
| RASi (%) | 54 |
| ACEi/ARB (%) | 52 |
| ARNI (%) | 2 |
| βB (%) | 48 |
| MRA (%) | 38 |
| TT (%) | 28 |
| SGLT2i (%) | 23 |
| QT (%) | 17 |
| TD RASi (%) * | 21 |
| TD ACEi/ARB (%) * | 21 |
| TD ARNI (%) * | 0 |
| TD βB (%) * | 7 |
| TD MRA (%) * | 12 |
| TD TT (%) * | 2 |
| TD SGLT2i (%) * | 23 |
| TD QT (%) * | 1 |
| CRT-D/CRT-P (%) | 1 |
| ICD (without CRT-D) (%) | 4 |
| At discharge | |
| Heart rate, median [IQR], min−1 | 78 [70–85] |
| Systolic blood pressure, median [IQR], mmHg | 112 [105–121] |
| Serum creatinine, median [IQR], μmol/L | 103 [87–119] |
| eGFR, median [IQR], mL/min/1.73 m2 | 67 [55–83] |
| Serum potassium, median [IQR], mmol/L | 4.4 [4.1–4.7] |
| Serum sodium, median [IQR], mmol/L | 139 [136–141] |
| NT-proBNP, median [IQR], pg/mL | 1390 [735–2835] |
| RASi (%) | 100 |
| ACEi/ARB (%) | 69 |
| ARNI (%) | 31 |
| βB (%) | 97 |
| MRA (%) | 99 |
| TT (%) | 96 |
| SGLT2i (%) | 98 |
| QT (%) | 94 |
| TD RASi (%) * | 11 |
| TD ACEi/ARB (%) * | 11 |
| TD ARNI (%) * | 0 |
| TD βB (%) * | 6 |
| TD MRA (%) * | 82 |
| TD TT (%) * | 2 |
| TD SGLT2i (%) * | 98 |
| TD QT (%) * | 2 |
| Loop diuretics (%) | 99 |
| Thiazide diuretics (%) | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bánfi-Bacsárdi, F.; Ráduly, A.P.; Borbély, A.; Nyolczas, N.; Szilágyi, A.; Gergely, T.G.; Forrai, Z.; Papp, J.; Rátosi, O.; Rácz, T.; et al. The Feasibility of a Guideline-Directed Medical Therapy Rapid Up-Titration Programme Among Real-World Heart Failure Patients: A Multicentre Observational Study. J. Clin. Med. 2025, 14, 3611. https://doi.org/10.3390/jcm14103611
Bánfi-Bacsárdi F, Ráduly AP, Borbély A, Nyolczas N, Szilágyi A, Gergely TG, Forrai Z, Papp J, Rátosi O, Rácz T, et al. The Feasibility of a Guideline-Directed Medical Therapy Rapid Up-Titration Programme Among Real-World Heart Failure Patients: A Multicentre Observational Study. Journal of Clinical Medicine. 2025; 14(10):3611. https://doi.org/10.3390/jcm14103611
Chicago/Turabian StyleBánfi-Bacsárdi, Fanni, Arnold Péter Ráduly, Attila Borbély, Noémi Nyolczas, Attila Szilágyi, Tamás G. Gergely, Zsolt Forrai, Judit Papp, Orsolya Rátosi, Tünde Rácz, and et al. 2025. "The Feasibility of a Guideline-Directed Medical Therapy Rapid Up-Titration Programme Among Real-World Heart Failure Patients: A Multicentre Observational Study" Journal of Clinical Medicine 14, no. 10: 3611. https://doi.org/10.3390/jcm14103611
APA StyleBánfi-Bacsárdi, F., Ráduly, A. P., Borbély, A., Nyolczas, N., Szilágyi, A., Gergely, T. G., Forrai, Z., Papp, J., Rátosi, O., Rácz, T., Hati, K., Kocsis, I., Csanádi, Z., Duray, G. Z., Andréka, P., Piróth, Z., & Muk, B. (2025). The Feasibility of a Guideline-Directed Medical Therapy Rapid Up-Titration Programme Among Real-World Heart Failure Patients: A Multicentre Observational Study. Journal of Clinical Medicine, 14(10), 3611. https://doi.org/10.3390/jcm14103611







