Efficacy and Safety of Transcranial Magnetic Stimulation for Treating Late-Life Depression: A Scoping Review
Abstract
:1. Introduction
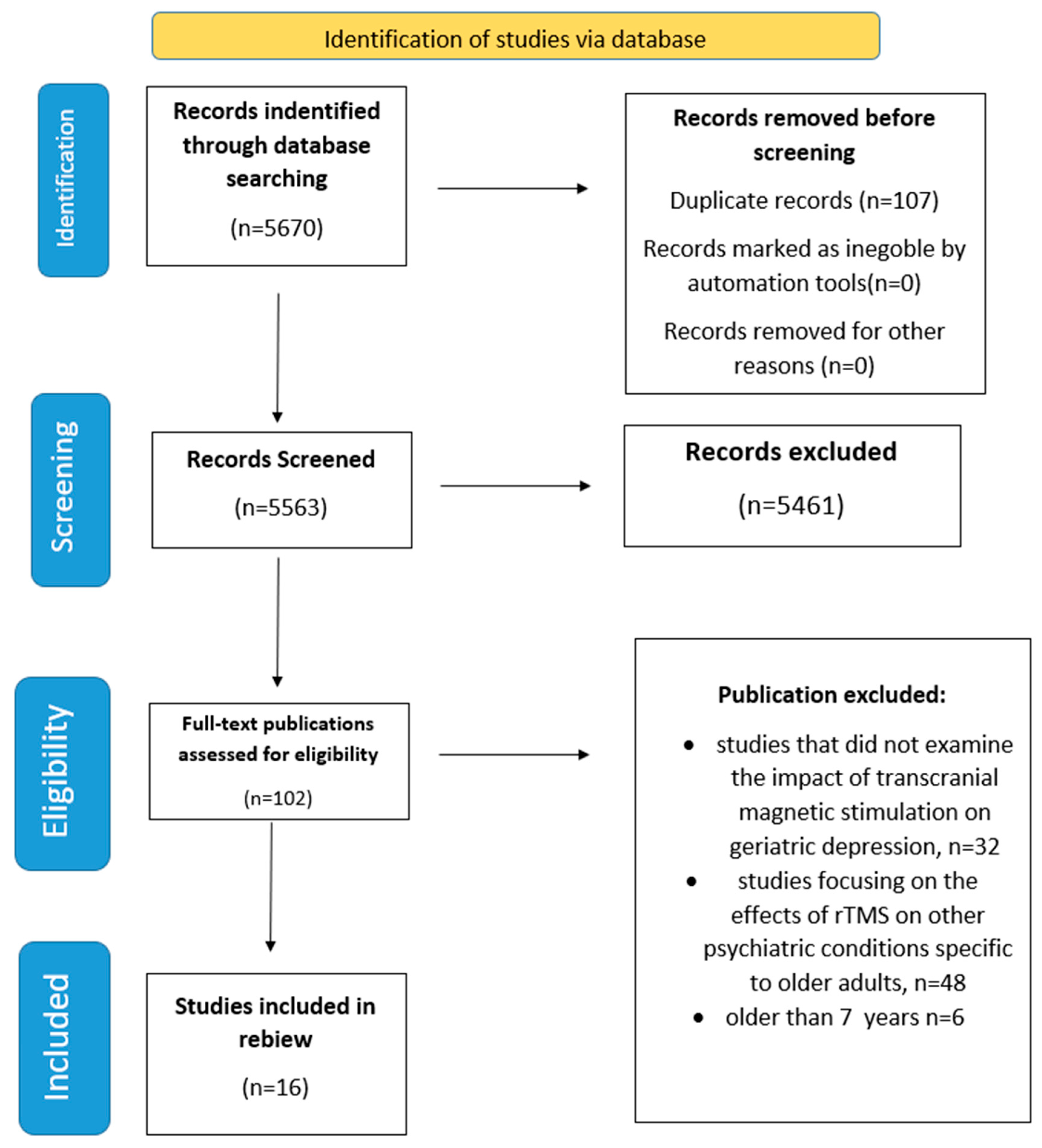
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection Process
3. Results
3.1. Adverse Reactions
3.2. Association with Pharmacological Treatment
3.3. Efficacy and TMS Protocols
3.3.1. Efficacy and TMS/rTMS Protocols
3.3.2. Efficacy and Deep TMS Protocols
3.3.3. TBS Protocols
3.4. Efficacy and Neuroplasticity Measurement
3.5. Efficacy and Neuroimaging
4. Discussion
Clinical Implications and Patient-Centered Recommendations
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galletly, C.; Fitzgerald, P.; Clarke, P.; Gill, S.; Burton, C.; Turnbull, C. A Practical Guide to Setting up a Repetitive Transcranial Magnetic Stimulation (RTMS) Service. Australas. Psychiatry 2010, 18, 314–317. [Google Scholar] [CrossRef]
- Koutsomitros, T.; Evagorou, O.; Schuhmann, T.; Zamar, A.; Sack, A.T. Advances in Transcranial Magnetic Stimulation (TMS) and Its Applications in Resistant Depression. Psychiatriki 2021, 32, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Luan, D.; Zhao, M.-G.; Shi, Y.-C.; Li, L.; Cao, Y.-J.; Feng, H.-X.; Zhang, Z.-J. Mechanisms of Repetitive Transcranial Magnetic Stimulation for Anti-Depression: Evidence from Preclinical Studies. World J. Psychiatry 2020, 10, 223–233. [Google Scholar] [CrossRef]
- Hutton, T.M. The Clinical Application of Transcranial Magnetic Stimulation. Psychiatr. Ann. 2014, 44, 305–309. [Google Scholar] [CrossRef]
- Dai, L.; Wang, P.; Zhang, P.; Guo, Q.; Du, H.; Li, F.; He, X.; Luan, R. The Therapeutic Effect of Repetitive Transcranial Magnetic Stimulation in Elderly Depression Patients. Medicine 2020, 99, E21493. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, R.; Kaufman, A.H. Diagnosis and Treatment of Late-Life Depression. Psychiatr. Times 2014, 31, 1. [Google Scholar]
- Farrugia, M.E.; Di Marco, M.; Kersel, D.; Carmichael, C. A Physical and Psychological Approach to Managing Fatigue in Myasthenia Gravis: A Pilot Study. J. Neuromuscul. Dis. 2018, 5, 373–385. [Google Scholar] [CrossRef]
- Depression in Late Life: Not A Natural Part of Aging—American Association for Geriatric Psychiatry. Available online: https://aagponline.org/patient-article/depression-in-late-life-not-a-natural-part-of-aging/?fbclid=IwY2xjawJDwn9leHRuA2FlbQIxMAABHU_Nd47sEKwdAB48TdSoLvepBcHK9TtK803qW3tFHQX8Msi_xQkNh-V (accessed on 7 April 2025).
- Vintilă, B.I.; Anghel, C.E.; Sava, M.; Bereanu, A.S.; Codru, I.R.; Stoica, R.; Vulcu Mihai, A.M.; Grama, A.M.; Cătană, A.C.; Boicean, A.G.; et al. Evaluating Anesthesia Practices, Patient Characteristics, and Outcomes in Electroconvulsive Therapy: A Two-Year Retrospective Study. J. Clin. Med. 2024, 13, 6253. [Google Scholar] [CrossRef]
- Steffens, D.C. Late-Life Depression, Antidepressant Treatment, and Cognition: The Short Haul and the Long Haul. Am. J. Psychiatry 2024, 181, 183–184. [Google Scholar] [CrossRef]
- Cornea, M.; Vintilă, B.I.; Bucuța, M.; Ștef, L.; Anghel, C.E.; Grama, A.M.; Lomnasan, A.; Stetiu, A.A.; Boicean, A.; Sava, M.; et al. Efficacy of Transcranial Direct Current Stimulation and Photobiomodulation in Improving Cognitive Abilities for Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2025, 14, 1766. [Google Scholar] [CrossRef]
- Lee, J.S.; Potter, G.G.; Wagner, H.R.; Welsh-Bohmer, K.A.; Steffens, D.C. Persistent Mild Cognitive Impairment in Geriatric Depression. Int. Psychogeriatr. 2007, 19, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Reitz, C.; Honig, L.H.; Schupf, N.; Tang, M.X.; Manly, J.J.; Mayeux, R.; Devanand, D.; Luchsinger, J.A. Late-Life Depression, Mild Cognitive Impairment, and Dementia. JAMA Neurol. 2013, 70, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Maina, G.; Adami, M.; Ascione, G.; Bondi, E.; De Berardis, D.; Delmonte, D.; Maffezzoli, S.; Martinotti, G.; Nivoli, A.; Ottavianelli, E.; et al. Nationwide Consensus on the Clinical Management of Treatment-Resistant Depression in Italy: A Delphi Panel. Ann. Gen. Psychiatry 2023, 22, 48. [Google Scholar] [CrossRef]
- Lomnasan, A.; Vintilă, B.I.; Bucuța, M.; Ștef, L.; Anghel, C.E.; Grama, A.M.; Cornea, M.; Boicean, A.; Ichim, C.; Paziuc, L.C.; et al. The Use of Phototherapy for the Treatment of Non-Seasonal Depression: A Systematic Review of Efficacy and Safety. J. Clin. Med. 2025, 14, 1756. [Google Scholar] [CrossRef]
- Roeh, A.; Kirchner, S.K.; Malchow, B.; Maurus, I.; Schmitt, A.; Falkai, P.; Hasan, A. Depression in Somatic Disorders: Is There a Beneficial Effect of Exercise? Front. Psychiatry 2019, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Katona, C.; Lyketsos, K.; Blazer, D.; Brodaty, H.; Rabins, P.; De Mendonça Lima, C.A.; Livingston, G. A Systematic Review of Treatments for Refractory Depression in Older People. Am. J. Psychiatry 2011, 168, 681–688. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Almheiri, E.; Alhelali, A.; Abdelnaim, M.A.; Weber, F.C.; Langguth, B.; Schecklmann, M.; Hebel, T. Effectiveness of Repetitive Transcranial Magnetic Stimulation in the Treatment of Depression in the Elderly: A Retrospective Natural Analysis. J. Clin. Med. 2023, 12, 4748. [Google Scholar] [CrossRef]
- Bhandari, A.; Lissemore, J.I.; Rajji, T.K.; Mulsant, B.H.; Cash, R.F.H.; Noda, Y.; Zomorrodi, R.; Karp, J.F.; Lenze, E.J.; Reynolds, C.F.; et al. Assessment of Neuroplasticity in Late-Life Depression with Transcranial Magnetic Stimulation. J. Psychiatr. Res. 2018, 105, 63. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Mulsant, B.H.; Thorpe, K.E.; McClintock, S.M.; Konstantinou, G.N.; Lee, H.H.; Nestor, S.M.; Noda, Y.; Rajji, T.K.; Trevizol, A.P.; et al. Effectiveness of Standard Sequential Bilateral Repetitive Transcranial Magnetic Stimulation vs Bilateral Theta Burst Stimulation in Older Adults With Depression: The FOUR-D Randomized Noninferiority Clinical Trial. JAMA Psychiatry 2022, 79, 1065–1073. [Google Scholar] [CrossRef]
- Leblhuber, F.; Steiner, K.; Fuchs, D. Treatment of Patients with Geriatric Depression with Repetitive Transcranial Magnetic Stimulation. J. Neural. Transm. 2019, 126, 1105–1110. [Google Scholar] [CrossRef]
- Lissemore, J.I.; Mulsant, B.H.; Rajji, T.K.; Karp, J.F.; Reynolds, C.F.; Lenze, E.J.; Downar, J.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. Cortical Inhibition, Facilitation and Plasticity in Late-Life Depression: Effects of Venlafaxine Pharmacotherapy. J. Psychiatry Neurosci. 2021, 46, E88–E96. [Google Scholar] [CrossRef] [PubMed]
- Lissemore, J.I.; Bhandari, A.; Mulsant, B.H.; Lenze, E.J.; Reynolds, C.F.; Karp, J.F.; Rajji, T.K.; Noda, Y.; Zomorrodi, R.; Sibille, E.; et al. Reduced GABAergic Cortical Inhibition in Aging and Depression. Neuropsychopharmacology 2018, 43, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Leuchter, M.K.; Citrenbaum, C.; Wilson, A.C.; Tibbe, T.D.; Jackson, N.J.; Krantz, D.E.; Wilke, S.A.; Corlier, J.; Strouse, T.B.; Hoftman, G.D.; et al. The Effect of Older Age on Outcomes of RTMS Treatment for Treatment-Resistant Depression. Int. Psychogeriatr. 2024, 36, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Göke, K.; Trevizol, A.P.; Ma, C.; Mah, L.; Rajji, T.K.; Daskalakis, Z.J.; Downar, J.; McClintock, S.M.; Nestor, S.M.; Noda, Y.; et al. Predictors of Remission after Repetitive Transcranial Magnetic Stimulation for the Treatment of Late-Life Depression. Psychiatry Res. 2024, 334, 115822. [Google Scholar] [CrossRef]
- Pan, W.G.; Hu, X.Y.; Zhu, D.D.; Li, L.; Bao, F.; Ren, L.; Mao, P.X.; Ma, X.; Ren, Y.P.; Tang, Y.L. The Cognitive Effects of Adjunctive Repetitive Transcranial Magnetic Stimulation for Late-Onset Depression: A Randomized Controlled Trial with 4 Week Follow-Up. Front. Psychiatry 2023, 14, 1240261. [Google Scholar] [CrossRef]
- Quinn, D.K.; Upston, J.; Jones, T.R.; Gibson, B.C.; Olmstead, T.A.; Yang, J.; Price, A.M.; Bowers-Wu, D.H.; Durham, E.; Hazlewood, S.; et al. Electric Field Distribution Predicts Efficacy of Accelerated Intermittent Theta Burst Stimulation for Late-Life Depression. Front. Psychiatry 2023, 14, 1215093. [Google Scholar] [CrossRef]
- Roth, Y.; Munasifi, F.; Harvey, S.A.; Grammer, G.; Hanlon, C.A.; Tendler, A. Never Too Late: Safety and Efficacy of Deep TMS for Late-Life Depression. J. Clin. Med. 2024, 13, 816. [Google Scholar] [CrossRef]
- Desbeaumes Jodoin, V.; Miron, J.P.; Lespérance, P. Safety and Efficacy of Accelerated Repetitive Transcranial Magnetic Stimulation Protocol in Elderly Depressed Unipolar and Bipolar Patients. Am. J. Geriatr. Psychiatry 2019, 27, 548–558. [Google Scholar] [CrossRef]
- Wathra, R.A.; Mulsant, B.H.; Daskalakis, Z.J.; Downar, J.; McClintock, S.M.; Nestor, S.M.; Rajji, T.K.; Trevizol, A.P.; Blumberger, D.M. Effect of Prior Pharmacotherapy on Remission with Sequential Bilateral Theta-Burst versus Standard Bilateral Repetitive Transcranial Magnetic Stimulation in Treatment-Resistant Late-Life Depression. Br. J. Psychiatry 2023, 223, 504–506. [Google Scholar] [CrossRef]
- Trevizol, A.P.; Goldberger, K.W.; Mulsant, B.H.; Rajji, T.K.; Downar, J.; Daskalakis, Z.J.; Blumberger, D.M. Unilateral and Bilateral Repetitive Transcranial Magnetic Stimulation for Treatment-Resistant Late-Life Depression. Int. J. Geriatr. Psychiatry 2019, 34, 822–827. [Google Scholar] [CrossRef]
- Wathra, R.A.; Men, X.; Elsheikh, S.S.M.; Marshe, V.S.; Rajji, T.K.; Lissemore, J.I.; Mulsant, B.H.; Karp, J.F.; Reynolds, C.F.; Lenze, E.J.; et al. Exploratory Genome-Wide Analyses of Cortical Inhibition, Facilitation, and Plasticity in Late-Life Depression. Transl. Psychiatry 2023, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Kaster, T.S.; Daskalakis, Z.J.; Noda, Y.; Knyahnytska, Y.; Downar, J.; Rajji, T.K.; Levkovitz, Y.; Zangen, A.; Butters, M.A.; Mulsant, B.H.; et al. Efficacy, Tolerability, and Cognitive Effects of Deep Transcranial Magnetic Stimulation for Late-Life Depression: A Prospective Randomized Controlled Trial. Neuropsychopharmacology 2018, 43, 2231–2238. [Google Scholar] [CrossRef]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-Invasive Magnetic Stimulation of Human Motor Cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic Principles of Transcranial Magnetic Stimulation (TMS) and Repetitive TMS (RTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef]
- Cappon, D.; den Boer, T.; Jordan, C.; Yu, W.; Metzger, E.; Pascual-Leone, A. Transcranial Magnetic Stimulation (TMS) for Geriatric Depression. Ageing Res. Rev. 2022, 74, 101531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mo, J.; Zhang, H.; Tang, Y.; Guo, K.; OuYang, X.; Huang, L.; Zhong, X.; Ning, Y. Efficacy and Tolerability of Repetitive Transcranial Magnetic Stimulation for Late-Life Depression: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2023, 323, 219–231. [Google Scholar] [CrossRef]
- Zangen, A.; Zibman, S.; Tendler, A.; Barnea-Ygael, N.; Alyagon, U.; Blumberger, D.M.; Grammer, G.; Shalev, H.; Gulevski, T.; Vapnik, T.; et al. Pursuing Personalized Medicine for Depression by Targeting the Lateral or Medial Prefrontal Cortex with Deep TMS. JCI Insight 2023, 8, e165271. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.Y.; Yang, L.H.; Stubbs, B.; Li, D.J.; Tseng, P.T.; Yeh, T.C.; Chen, T.Y.; Liang, C.S.; Chu, C.S. Efficacy and Tolerability of Deep Transcranial Magnetic Stimulation for Treatment-Resistant Depression: A Systematic Review and Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109850. [Google Scholar] [CrossRef]
- Chung, S.W.; Hill, A.T.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. Use of Theta-Burst Stimulation in Changing Excitability of Motor Cortex: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2016, 63, 43–64. [Google Scholar] [CrossRef]
- Chung, S.W.; Hoy, K.E.; Fitzgerald, P.B. Theta-Burst Stimulation: A New Form of TMS Treatment for Depression? Depress. Anxiety 2015, 32, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, A.I.; Camsari, D.D.; Nandakumar, A.L.; Voort, J.L.V.; Kung, S.; Lewis, C.P.; Croarkin, P.E. Accelerated TMS for Depression: A Systematic Review and Meta-Analysis. Psychiatry Res. 2019, 273, 770–781. [Google Scholar] [CrossRef]
- Kirkovski, M.; Donaldson, P.H.; Do, M.; Speranza, B.E.; Albein-Urios, N.; Oberman, L.M.; Enticott, P.G. A Systematic Review of the Neurobiological Effects of Theta-Burst Stimulation (TBS) as Measured Using Functional Magnetic Resonance Imaging (FMRI). Brain Struct. Funct. 2023, 228, 717–749. [Google Scholar] [CrossRef] [PubMed]
- Player, M.J.; Taylor, J.L.; Weickert, C.S.; Alonzo, A.; Sachdev, P.; Martin, D.; Mitchell, P.B.; Loo, C.K. Neuroplasticity in Depressed Individuals Compared with Healthy Controls. Neuropsychopharmacology 2013, 38, 2101. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Saito, T.; Asada, A.; Shimizu, S.; Iijima, K.M.; Ando, K. Microtubule Affinity-Regulating Kinase 4 with an Alzheimer’s Disease-Related Mutation Promotes Tau Accumulation and Exacerbates Neurodegeneration. J. Biol. Chem. 2020, 295, 17138–17147. [Google Scholar] [CrossRef]
- Cash, R.F.H.; Weigand, A.; Zalesky, A.; Siddiqi, S.H.; Downar, J.; Fitzgerald, P.B.; Fox, M.D. Using Brain Imaging to Improve Spatial Targeting of Transcranial Magnetic Stimulation for Depression. Biol. Psychiatry 2021, 90, 689–700. [Google Scholar] [CrossRef]
- McClintock, S.M.; Reti, I.M.; Carpenter, L.L.; McDonald, W.M.; Dubin, M.; Taylor, S.F.; Cook, I.A.; O’Reardon, J.; Husain, M.M.; Wall, C.; et al. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (RTMS) in the Treatment of Depression. J. Clin. Psychiatry 2018, 79, 35–48. [Google Scholar] [CrossRef]
- Duprat, R.J.; Linn, K.A.; Satterthwaite, T.D.; Sheline, Y.I.; Liang, X.; Bagdon, G.; Flounders, M.W.; Robinson, H.; Platt, M.; Kable, J.; et al. Resting FMRI-Guided TMS Evokes Subgenual Anterior Cingulate Response in Depression. Neuroimage 2025, 305, 120963. [Google Scholar] [CrossRef]
| Authors | Study | Country | Year | Key Words | Study Size | Outcome Measure | |
|---|---|---|---|---|---|---|---|
| 1 | Almheiri et al. [19] | Effectiveness of Repetitive Transcranial Magnetic Stimulation in The Treatment of Depression in the Elderly: A Retrospective Natural Analysis | Germany | 2023 | late-life depression, geriatric depression, TMS, efficacy | 78 pacients | HRDS21 MRI |
| 2 | Bhandari et al. [20] | Assessment of Neuroplasticity in Late-Life Depression with Transcranial Magnetic Stimulation | Canada USA | 2023 | late-life depression, geriatric depression, TMS, efficacy | 56 pacients | MARDS |
| 3 | Blumberger et al. [21] | Effectiveness of Standard Sequential Bilateral Repetitive Transcranial Magnetic Stimulation vs. Bilateral Theta Burst Stimulation in Older Adults With Depression The FOUR-D Randomized Noninferiority Clinical Trial | Canada | 2022 | late-life depression, geriatric depression, TMS, efficacy | 172 pacients | MADRAS, HRSD-17, QIDS-SR-16 MRI |
| 4 | F. Leblhuber et al. [22] | Treatment of patients with geriatric depression with repetitive transcranial magnetic stimulation | Austria | 2019 | late-life depression, geriatric depression, TMS | 19 pacients | HAM-D MRI |
| 5 | Lissemore et al. [23] | Cortical inhibition, facilitation and plasticity in late-life depression: effects of venlafaxine pharmacotherapy | Canada | 2020 | late-life depression, geriatric depression, TMS | 68 pacients | MADRS EMG |
| 6 | Lissemore et al. [24] | Reduced GABAergic cortical inhibition in aging and depression | Canada | 2018 | late-life depression, geriatric depression, TMS | 92 pacients | HDRS-21 SSI |
| 7 | Leuchter et al. [25] | The effect of older age on outcomes of rTMS treatment for treatment-resistant depression | USA | 2024 | late-life depression, geriatric depression, rTMS | 207 eldery pacients | HRDS-21 SCID MRI |
| 8 | Goke et al. [26] | Predictors of remission after repetitive transcranial magnetic stimulation for the treatment of late-life depression | Canada USA JAPAN | 2024 | Repetitive transcranial magnetic stimulation Late-life depression | 164 pacients | HDRS-21, PHQ-9, BDI-II |
| 9 | Pan et al. [27] | The cognitive effects of adjunctive repetitive transcranial magnetic stimulation for late-onset depression: a randomized controlled trial with 4 week follow-up. | China | 2023 | 58 pacients | IDS-SR, POMS, HDRS, PHQ | |
| 10 | Quinn et al. [28] | Electric field distribution predicts efficacy of accelerated intermittent theta burst stimulation for late-life depression | USA | 2023 | late-life depression, geriatric depression, TMS, efficacy theta burst | 25 pacients | IDS-C-30, Functional MRI, Electric field modeling |
| 11 | Roth et al. [29] | Never Too Late: Safety and Efficacy of Deep TMS for Late-Life Depression | USA | 2024 | late-life depression, geriatric depression, TMS, efficacy | 247 pacients | MADRS |
| 12 | Jodoin et al. [30] | Safety and efficacy of accelerated rTMS protocol in elderly depressed unipolar and bipolar patients | USA | 2018 | late-life depression, geriatric depression, TMS, efficacy | 19 eldery pacients | MADRAS |
| 13 | Wathra et al. [31] | Effect of prior pharmacotherapy on remission with sequential bilateral theta-burst versus standard bilateral repetitive transcranial magnetic stimulation in treatment-resistant late-life depression | UK | 2023 | late-life depression, geriatric depression, TMS, efficacy | 164 pacients | MADRAS, HRSD-17, QIDS-SR-16 MRI |
| 14 | Trevizol et al. [32] | Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression | Canada | 2018 | late-life depression, geriatric depression, TMS, efficacy | 43 pacients | MADRAS, HRSD-17, QIDS-SR-16 MRI |
| 15 | Wathra et al. [33] | Exploratory genome-wide analyses of cortical inhibition, facilitation, and plasticity in late-life depression | Canada | 2023 | late-life depression, geriatric depression, TMS, efficacy | 79 pacients | MADRAS EMG |
| 16 | Tyler S. Kaster et al. [34] | Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial | Canada | 2018 | late-life depression, geriatric depression, TMS, efficacy | 80 pacients | MADRAS EMG |
| Study | Device | Target Area | Frequency | Intensity of RMT | Protocol | Remission Rate | Response Rate |
|---|---|---|---|---|---|---|---|
| Leblhuber et al. [22] | Theracell apparatus (Guth Meditec, Salach, Germany) | Bilateral PFC | 3 Hz | 0.08 T | 30 min per session 10 sessions, 2 weeks HAMD-7 | Not reported | Not reported |
| Jodoin et al. [30] | MagPro X100 (MagVenture, Farum, Denmark) | DLPFC | 20 Hz | 110% | 15 min per session (3000 pulses/session), 20 to 30 sessions, twice daily, 3–5 days/week MADRS, HAM-A | 63% | 73.7% |
| Blumberger et al. [21] | MagPro X100 (MagVenture) | Bilateral DLPFC | 1 Hz and 10 Hz | 120% | 600 pulses over 10 minutes to the right DLPFC, followed by 3000 pulses: 4 s on, 26 s off over 37.5 min to the left DLPFC; 20 initial daily sessions over 4 weeks (an additional 10 daily sessions over 2 additional weeks if they did not achieve remission) MADRS, HRSD-17, QIDS-SR-16 | 21.4–32.9% | 29.6–35.7 |
| Bhandari et al. [20] | Magstim 200 (Magstim Company Ltd., Sheffield, UK) (Two stimulators) | Left motor cortex | Not specified, using PAS protocol | Adjusted for test stimulus | Focus on motor cortex with PAS protocol MADRS, MMSE, CIRS-G | Not reported | Not reported |
| Trevizol et al. [32] | Magventure RX-100 (MagVenture) | DLPFC | 10 Hz for high frequency unilateral, 1 Hz for bilateral | 120% | 15 sessions initially, with an additional 15 sessions if non-remitting 5 sessions/week; 3 weeks HDRS | 40% bilateral/0% unilateral/ sham 0% | 45 bilateral/0% unilateral/sham 16.7% |
| Leuchter et al. [25] | MagPro X100, Magstim Horizon, Magstim Super Rapid2, or Neurostar | Left DLPFC | 10 Hz | 120% | 30 sessions beginning with HFL IDS, POMS, PHQ-9, HDRS | 25–27% | 25–36% |
| Pan et al. [27] | Magstim (eight-coil device) | DLPFC | 10 Hz | 120% | 20 min per session (800 pulses) 4 weeks HDRS, RBANS | Not reported | Not reported |
| Lissemore et al. [23] | Magstim 200 (Two stimulators, Bistim module) | Left motor cortex | Not specified, uses various TMS paradigms (SICI, ICF, CSP, PAS) | 80%/140% | Assesses cortical physiology pre- and post-venlafaxine treatment with various TMS paradigms. MADRS | Not reported | Not reported |
| Lissemore et al. [24] | Magstim 200 (Two stimulators, Bistim module) | Left motor cortex | Not specified (focus on SICI, ICF, CSP) | 80%/140% | Measures SICI, ICF, CSP, and PAS with precise coil positioning for cortical excitability. MADRS, CIRS-G | Not reported | Not reported |
| Wathra et al. [31] | Magstim 200 (Two stimulators, Bistim module) | Left motor cortex | Not specified (focus on PAS) | 80%/140% | Uses PAS with sensory threshold for peripheral nerve stimulation and MEP amplitude measures. MADRS | Not reported | Not reported |
| Study | Therapeutic Approach | Additional Group Comparison | Neuroplasticity Measures | Cortical Target | Nerve Targeted | Stimulation Parameters | Remission Rate | Response Rate |
|---|---|---|---|---|---|---|---|---|
| Bhandari et al. (2018) [20] | Directly measures PAS-induced synaptic plasticity (LTP-like effects) | Age-matched healthy controls | PAS-LTP (MEP potentiation after PAS) | Left motor cortex | Right median nerve | PAS: 180 pairs, 25 ms delay, 300% sensory threshold | Not reported | Not reported |
| Lissemore et al. (2020) [23] | Examines whether venlafaxine treatment affects cortical plasticity | No | PAS-LTP, SICI, ICF, CSP | Left motor cortex | Right median nerve | PAS: 180 pairs, 25 ms delay, 3× participant’s sensory threshold | Not reported | Not reported |
| Lissemore et al. (2018) [24] | Examines GABAergic inhibition (which regulates neuroplasticity) | Age-matched and younger healthy controls, younger depressed adults | SICI (GABA A), CSP (GABA B), ICF (glutamate) | Left motor cortex | None (Cortical inhibition focus) | TMS inhibition protocols: SICI (2 ms), CSP (140% RMT), ICF (10 ms) | Not reported | Not reported |
| Wathra et al. (2023) [33] | Examines the genetic basis of TMS-induced cortical changes in LLD, focusing on BDNF polymorphisms and key neurotransmitter system genes. | No | SICI, CSP, ICF | Left motor cortex | Right median nerve | PAS: 180 pairs, 25 ms delay, no participant’s sensory threshold provided (focus on motor inhibition) | Not reported | Not reported |
| Patient Profile | Recommended Protocol | Rationale |
|---|---|---|
| Mild-to-Moderate Depression, No Major Comorbidities | Standard high-frequency (10–20 Hz) rTMS over left DLPFC | Most common, well-tolerated protocol; strong evidence base; good balance of efficacy and safety |
| Severe Depression, Treatment-Resistant | Bilateral rTMS (e.g., 1 Hz right + 10 Hz left) or Deep TMS | Higher remission rates observed; bilateral protocols may better modulate network-level dysfunction. Deep TMS (e.g., BrainsWay H1 coil) showed up to 60% remission with longer duration |
| Polypharmacy or Medication Intolerant | Monotherapy TMS (without concurrent pharmacotherapy) | Several studies show clinical improvement without meds, making TMS suitable as a standalone treatment |
| Time-Constrained or Inpatient Setting | Accelerated TBS (iTBS, cTBS protocols) | Rapid delivery (3–5 min per session), allowing multiple sessions/day; suitable for faster symptom reduction |
| Comorbid Cognitive Decline or Mild Cognitive Impairment | Protocols with neurocognitive monitoring (e.g., Pan [27], Jodoin [30]) | Some protocols included cognitive batteries (RBANS, MMSE); iTBS and high-frequency rTMS generally well-tolerated cognitively |
| Patients with High Sensitivity to Pain or Discomfort | Standard rTMS with moderate intensity (≤110% RMT) | Avoid deep TMS or high-pulse protocols like H1L coil (Kaster [34]); standard coils better tolerated |
| Patients with Poor Treatment Adherence | Protocols with short duration or low side effects (e.g., iTBS) | Shorter protocols improve adherence. Adverse effects are minor and transient across most protocols |
| Interest in Cognitive Enhancement or Neuroplasticity | PAS-enhanced TMS or protocols with motor cortex targets | Some studies (e.g., Bhandari [20], Lissemore [23,24] explore cortical plasticity changes using paired associative stimulation. Though clinical relevance remains limited. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Băcilă, C.-I.; Cornea, M.; Lomnasan, A.; Anghel, C.E.; Grama, A.M.; Dobre, C.E.; Rusu, S.; Vintilă, B.I. Efficacy and Safety of Transcranial Magnetic Stimulation for Treating Late-Life Depression: A Scoping Review. J. Clin. Med. 2025, 14, 3609. https://doi.org/10.3390/jcm14103609
Băcilă C-I, Cornea M, Lomnasan A, Anghel CE, Grama AM, Dobre CE, Rusu S, Vintilă BI. Efficacy and Safety of Transcranial Magnetic Stimulation for Treating Late-Life Depression: A Scoping Review. Journal of Clinical Medicine. 2025; 14(10):3609. https://doi.org/10.3390/jcm14103609
Chicago/Turabian StyleBăcilă, Ciprian-Ionuț, Monica Cornea, Andrei Lomnasan, Claudia Elena Anghel, Andreea Maria Grama, Cristina Elena Dobre, Silvia Rusu, and Bogdan Ioan Vintilă. 2025. "Efficacy and Safety of Transcranial Magnetic Stimulation for Treating Late-Life Depression: A Scoping Review" Journal of Clinical Medicine 14, no. 10: 3609. https://doi.org/10.3390/jcm14103609
APA StyleBăcilă, C.-I., Cornea, M., Lomnasan, A., Anghel, C. E., Grama, A. M., Dobre, C. E., Rusu, S., & Vintilă, B. I. (2025). Efficacy and Safety of Transcranial Magnetic Stimulation for Treating Late-Life Depression: A Scoping Review. Journal of Clinical Medicine, 14(10), 3609. https://doi.org/10.3390/jcm14103609






