5.1. Changes in Corneal Parameters in the Group of Patients with Type 2 Diabetes After Cataract Removal Surgery Using the Phacoemulsification Method
The aim of the present study was to evaluate changes in the corneal endothelium following uncomplicated cataract surgery performed using the phacoemulsification technique. Previous studies addressing this issue have reported inconsistent findings. However, many of these investigations did not account for several preoperative and postoperative factors that may influence outcomes. Key variables often omitted include cataract severity, glycemic control (as measured by HbA1c), duration and type of diabetes (type 1 vs. type 2), presence of diabetic retinopathy, and cumulative dissipated energy (CDE) during surgery. Variability in results may also be attributed to small sample sizes and ethnic differences. Additionally, most prior studies assessed endothelial changes at only a single postoperative time point.
5.1.1. Visual Acuity (Vis)
In this study, preoperative visual acuity was significantly lower in patients with type 2 diabetes compared to the control group. Following cataract surgery via the phacoemulsification technique, both groups demonstrated improvement in visual acuity at each postoperative follow-up. At the 14-day follow-up, visual acuity in the diabetic group remained slightly lower than in the control group; however, this difference was not statistically significant. Notably, this disparity coincided with a more pronounced increase in central corneal thickness (CCT) in the diabetic group, suggesting more severe postoperative corneal edema. Clinically, this may reflect transient endothelial dysfunction, as the corneal endothelium plays a key role in maintaining stromal hydration through its pump function.
Visual acuity continued to improve at later follow-up visits (3 and 6–8 months postoperatively) in both groups. These improvements paralleled changes in CCT: a subclinical increase in CCT was observed at 3 months, followed by a return to baseline values by the 6–8-month mark. The stabilization of visual acuity at these time points corresponded with normalization of CCT, further supporting the association between corneal hydration status and visual recovery.
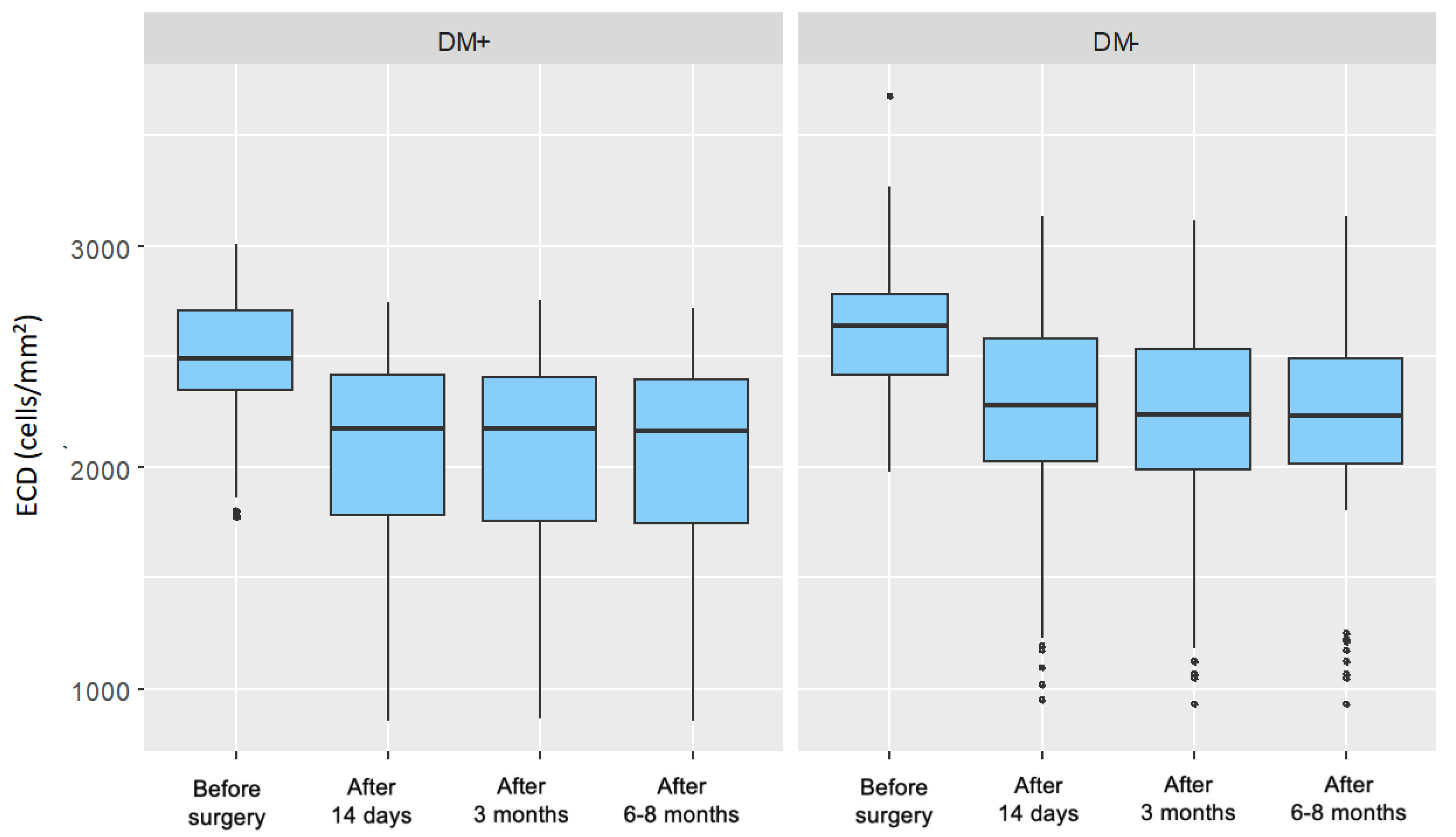
5.1.2. Endothelial Cell Density (ECD)
In this study, the preoperative endothelial cell density (ECD) was significantly lower in patients with type 2 diabetes than in the control group. At all postoperative follow-up points, both groups showed a significant decline in ECD compared to baseline values. However, comparison of the changes in ECD (ΔECD) between the groups at each follow-up revealed no statistically significant differences in absolute values.
When expressed as a percentage of baseline, the ECD decrease was greater in the diabetic group (18.44%) compared to the control group (15.12%). The lack of statistical significance in absolute ΔECD between the groups may be attributed to the comparable cataract severity (nuclear density < grade 5 on the LOCS III scale) and similar levels of cumulative dissipated energy (CDE) during surgery. Importantly, all diabetic participants had well-controlled glycemia.
Several studies have investigated corneal endothelial cell loss in diabetic patients undergoing cataract surgery via phacoemulsification. Al-Sharkawy et al. reported similar ECD loss (~8%) in both diabetic and non-diabetic groups [
14]. Similarly, Lee et al. found comparable ΔECD values among non-diabetic patients and diabetic patients with varying stages of retinopathy 6 months postoperatively [
12].
He et al. retrospectively analyzed 133 eyes and reported a greater ECD reduction in diabetic patients (15%) than in non-diabetic patients (11%). This study also accounted for intraoperative factors (CDE, BSS use, surgical duration) but included both type 1 and type 2 diabetic patients without considering disease duration, and had a relatively short follow-up of 1 month [
15].
Langwińska-Wośko et al. observed a greater reduction in ECD in diabetic patients (14%) compared to non-diabetic patients (9%), while Tang et al.’s meta-analysis of 13 studies (1923 eyes) found preoperative ECD to be significantly lower in diabetics, with more pronounced postoperative loss at all time points (up to 3 months) [
16,
17]. Unlike the present study, those studies did not differentiate patients by diabetes duration, type, or glycemic control.
Sahu et al. compared ECD outcomes between patients with well-controlled type 2 diabetes (HbA1c < 7%) and controls, also considering CDE [
18]. Although their follow-up was shorter (up to 3 months), they found a significantly greater ECD loss in the diabetic group. Notably, both their study and the present one showed the greatest ECD decline shortly after surgery, followed by stabilization.
The current findings align with the general trend of postoperative ECD reduction and subsequent stabilization. However, in contrast to Sahu et al., the present study did not find a statistically significant difference in ΔECD between the groups.
Other studies, such as that by Morikubo et al., reported significantly greater ECD loss in diabetic patients [
6]. However, their study was limited by a short follow-up (1 month) and a lack of data on glycemic control or retinopathy status, potentially explaining discrepancies with our findings.
Hugod et al. also reported a significantly greater ECD decline in the diabetic group at 3 months, while Beato et al., studying patients with well-controlled type 2 diabetes, found no significant difference in ΔECD between groups—findings consistent with those of the present study [
5,
19].
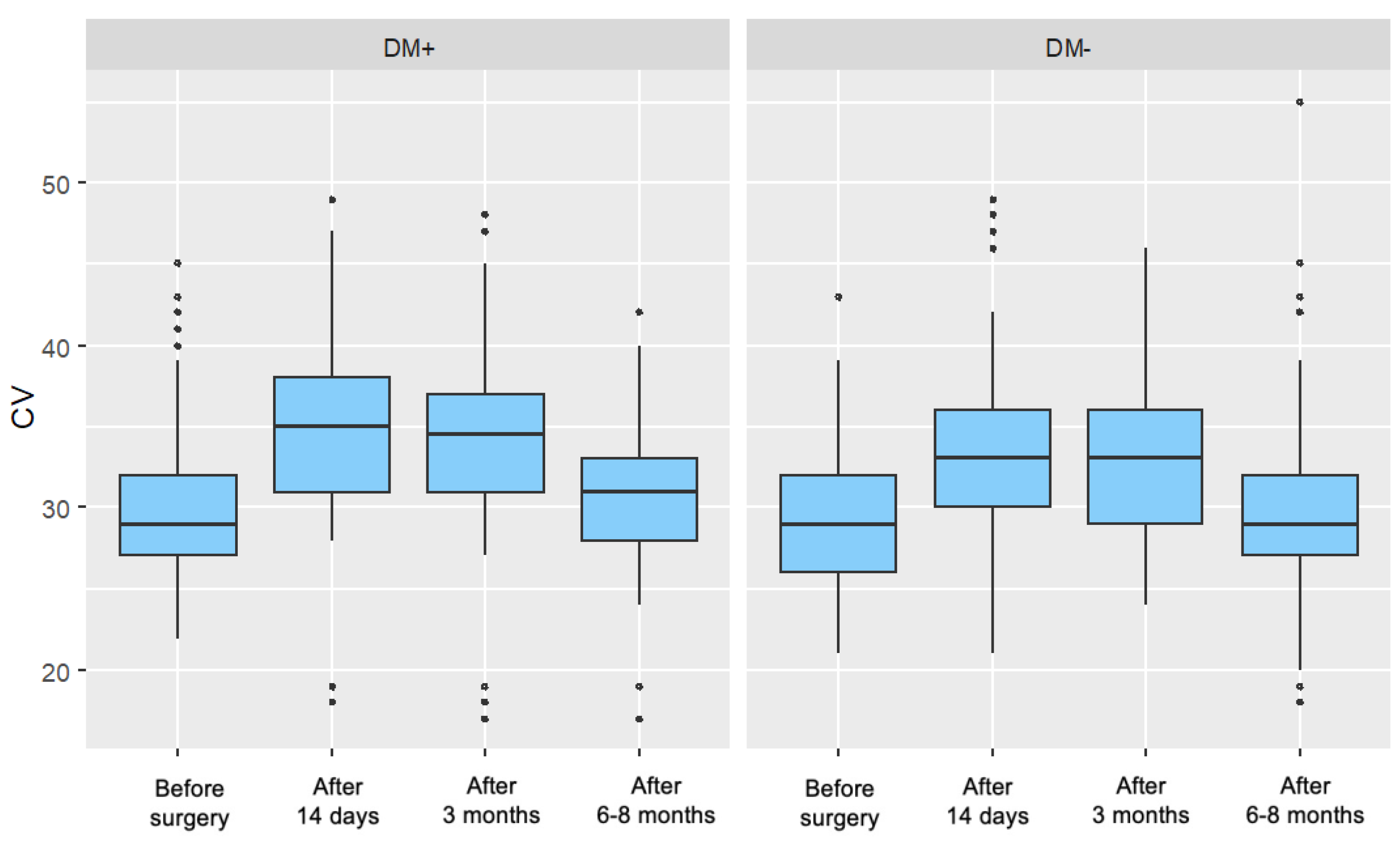
5.1.3. Coefficient of Variation of Cells (CV)
In the present study, a significant increase in the coefficient of variation of endothelial cell size (CV) was observed in both the diabetic and control groups at 14 days and 3 months postoperatively. At the 6–8-month follow-up, CV values in the diabetic group remained significantly elevated compared to baseline, while in the control group, they had returned to levels comparable with the preoperative measurement.
This postoperative increase in CV reflects enhanced polymegathism, likely representing an endothelial regenerative response to intraoperative stress. The prolonged elevation of CV in diabetic patients may suggest a delayed or impaired regenerative process in this population. Despite these trends, no statistically significant differences were found between the groups in the magnitude of CV change (ΔCV) at any of the follow-up time points.
Sahu et al. reported similar preoperative CV values in both groups and noted a postoperative increase over a 3-month period, consistent with the current study. However, they observed a smaller increase in the diabetic group, which they interpreted as evidence of a blunted regenerative response. This finding was not replicated in the present study or in other literature [
18].
Tang et al. also reported increased CV values postoperatively in both groups. Notably, the increase was significantly greater in diabetic patients at the 1-week and 1-month follow-ups but not at 3 months, suggesting a transient disparity in endothelial response [
17].
In contrast, Morikubo et al. and Hugod et al. did not observe significant postoperative changes in CV in either group, which differs from the findings of the present study [
6,
17]. These discrepancies may be related to differences in follow-up duration, patient selection, or study methodology.
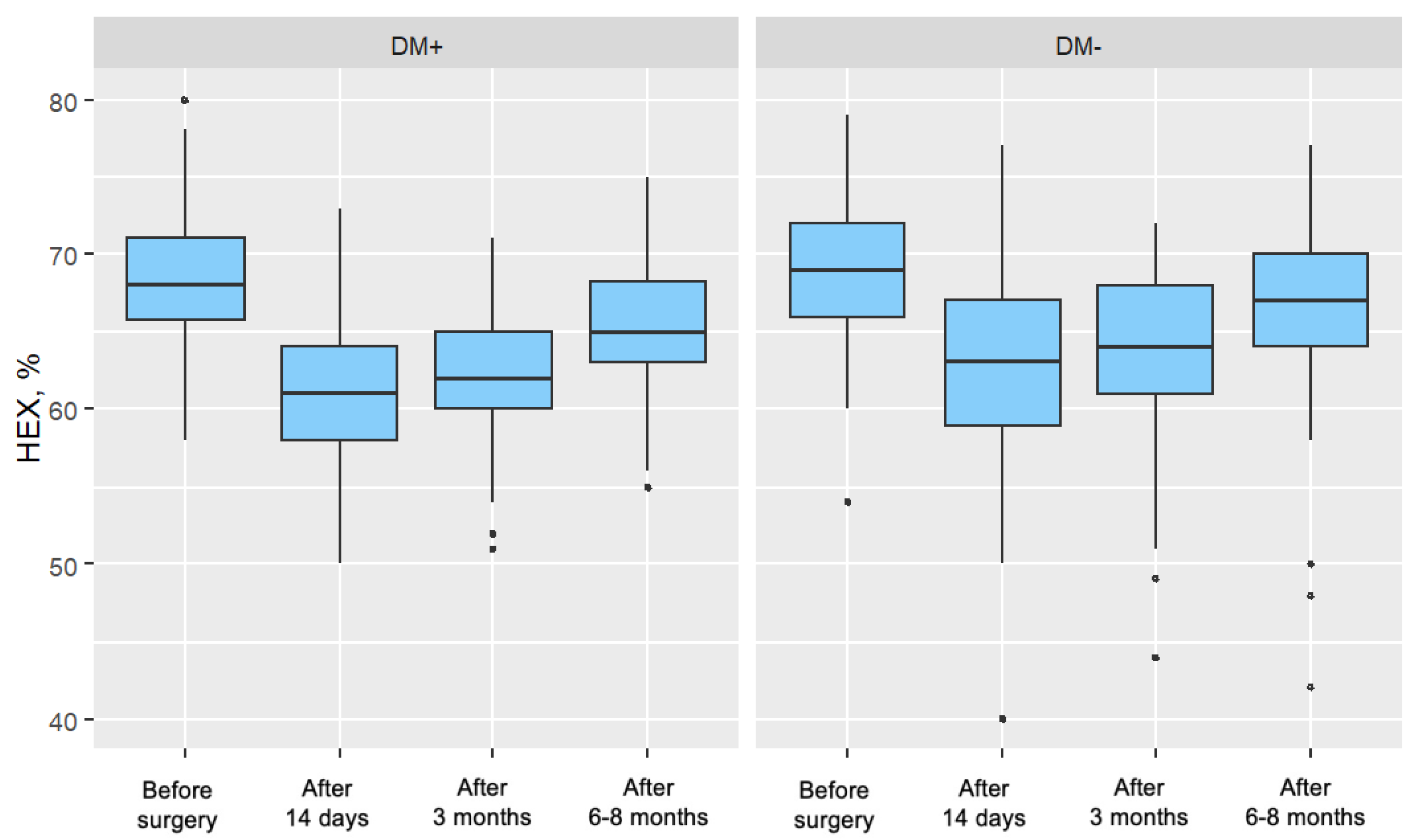
5.1.4. Percentage of Hexagonal Cells (%HEX)
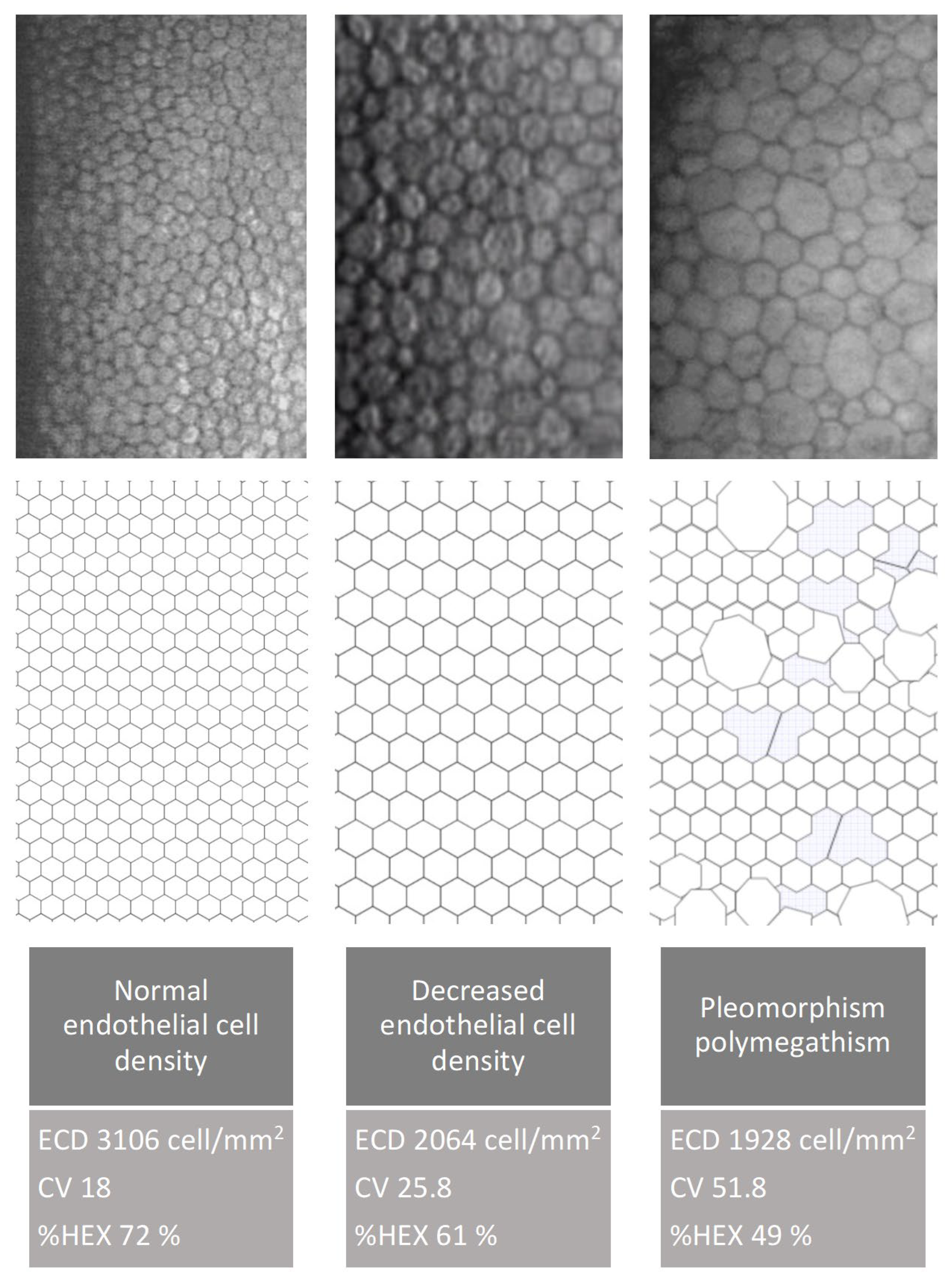
Cataract surgery induces trauma to the corneal endothelium, resulting in decreased endothelial cell density (ECD). To maintain coverage of the posterior corneal surface, surviving cells enlarge, leading to increased variation in cell size and loss of their characteristic hexagonal shape. Over time, with regeneration, endothelial cell morphology—including cell shape and size—tends to return toward preoperative values.
In the present study, no significant differences in %HEX were observed between groups in the preoperative period. Postoperatively, a significant reduction in %HEX was noted in both groups, with the decrease being more pronounced in patients with type 2 diabetes. This more substantial reduction may reflect a delayed or impaired regenerative response in the diabetic group following phacoemulsification.
Sahu et al. reported decreases in %HEX in both diabetic and control groups (4.93% and 5.28%, respectively), though these changes did not reach statistical significance 18. Similar trends were observed by Lee et al. and Morikubo et al.; however, both studies were limited by a short postoperative follow-up (1 month) [
6,
12]. In contrast, Hugod et al. found a significant reduction in %HEX only in diabetic patients, suggesting a potentially greater vulnerability of the diabetic endothelium. The authors proposed that ethnic and metabolic differences may partly explain the variability in endothelial responses across studies [
5].
It is important to note that while ECD remains the primary metric for assessing endothelial cell status, it does not fully capture the dynamic changes involved in endothelial regeneration. In contrast, morphological parameters such as CV and %HEX provide more sensitive indicators of the regenerative processes that occur after cataract surgery.
5.1.5. Central Corneal Thickness (CCT)
At 14 days postoperatively, central corneal thickness (CCT) was significantly elevated in both groups compared to baseline. However, the increase (ΔCCT) was significantly more pronounced in the type 2 diabetes group, indicating greater corneal edema in the early postoperative period.
By the 3-month follow-up, CCT values had decreased in both groups compared to the 14-day measurement, though they remained significantly higher than baseline. At the 6–8-month follow-up, CCT values in both groups had returned to baseline levels, and no significant intergroup differences were observed.
These findings suggest that the greater early postoperative corneal edema observed in diabetic patients, reflected in higher CCT values and slightly reduced visual acuity, was transient. In later follow-ups, both CCT and visual acuity (Vis) values stabilized and equalized between groups. This underscores a reversible, stress-induced endothelial dysfunction rather than permanent damage in diabetic patients with well-controlled glycemia.
CCT and Vis are functional indicators of corneal endothelial status. In this study, higher CCT and lower Vis in the diabetic group at 14 days postoperatively corresponded with early endothelial dysfunction. However, the normalization of these parameters by 3 months and beyond, despite ongoing differences in endothelial morphology (CV and %HEX), supports the conclusion that corneal endothelial integrity was preserved.
These findings are consistent with results from Sahu et al., who observed significantly greater CCT increases in diabetic patients at 1 week postoperatively, with gradual reductions over time and minimal intergroup differences by 3 months [
18]. Similar patterns were reported by Morikubo et al., Hugod et al., and Tang et al. [
5,
6,
17]. Tang et al. additionally noted a prolonged elevation in CCT in diabetic patients up to 3 months postoperatively, consistent with the trend observed in the present study.
It is important to note that subclinical endothelial cell changes, such as those reflected in CV and %HEX parameters, may persist even when functional metrics like CCT and Vis return to normal. This highlights the regenerative nature of the endothelial response and suggests that, in patients with well-controlled type 2 diabetes and preserved baseline ECD, the risk of long-term corneal decompensation following phacoemulsification is minimal.
5.1.6. Impact of Cumulative Dissipated Energy (CDE)
In the present study, no statistically significant difference in cumulative dissipated energy (CDE) during phacoemulsification was observed between the type 2 diabetes group and the control group. This finding is consistent with data reported by Tang et al., who also found no intergroup differences in CDE values [
17]. However, the literature remains limited regarding the influence of CDE on corneal endothelial parameters.
A significant negative correlation was found between CDE and baseline visual acuity (Vis) in both groups. This suggests that poorer preoperative visual acuity, often due to denser or harder cataracts, was associated with the use of higher ultrasound energy during phacoemulsification.
In both diabetic and non-diabetic patients, CDE was negatively correlated with endothelial cell density (ECD) at each postoperative time point. This indicates that higher ultrasound energy exposure is associated with greater endothelial cell loss, supporting the hypothesis that intraoperative energy use directly impacts endothelial integrity.
CDE did not show a significant correlation with lens thickness in either group, suggesting that lens thickness alone is not a sufficient predictor of energy usage during surgery.
For the percentage of hexagonal cells (%HEX), a correlation with CDE was observed at the 3-month follow-up. In the diabetic group, this was a weak positive correlation, whereas in the control group, a weak negative correlation was observed. Although intriguing, these findings have not been reported in other studies and should be interpreted with caution pending further research in larger cohorts.
Comparisons with the study by Sahu et al. reveal partial alignment. While both studies observed a correlation between CDE and ECD in the control group, Sahu et al. did not find a similar correlation in the diabetic group, contrary to the current study’s findings. Additionally, Sahu et al. reported a positive correlation between CDE and CV changes in the control group, a relationship not confirmed here. Both studies identified a correlation between CDE and %HEX in the control group at 3 months, though the correlation was weak [
18].
It is important to highlight that certain intraoperative factors, such as total fluid usage, surgical duration, and anterior chamber depth, were not accounted for in this study. These variables may also influence endothelial outcomes and should be considered in future research.