A Nationwide Epidemiological Study of Chronic Kidney Disease Prevalence in a High-Risk Patient Population Without Prior Diagnosis in Primary Health Care in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
- Age 60–75 years (individual criterion even without comorbidities);
- Younger patients meeting at least one of the following conditions:
- ○
- A history of kidney disease (other than CKD—structural urinary tract disease, recurrent kidney calculi, drug-induced nephrotoxicity, radiation nephritis, polycystic kidney disease, APOL1-mediated kidney disease, prior acute kidney injury and Alport syndrome);
- ○
- A history of diabetes;
- ○
- A history of hypertension;
- ○
- Cardiovascular disease other than a history of hypertension;
- ○
- Obesity.
2.2. Exclusion Criteria
- Age < 18 and >75 years;
- A diagnosis of CKD.
2.3. Epidemiological Study Design
- the measurement of serum creatinine levels with an estimation of eGFR and
- the determination of albuminuria in a urine sample.
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Group
3.2. Risk Factors for Developing CKD
3.2.1. Results of Univariate Logistic Regression Analysis
3.2.2. Results of Multivariate Logistic Regression Analysis
4. Discussion
Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| ACR | Albumin-to-creatinine ratio |
| eGFR | Estimated glomerular filtration rate |
| BMI | Body mass index |
| OR | Odds ratio |
| P | Statistical significance |
| SD | Standard deviation |
References
- Król, E.; Rutkowski, B. Chronic kidney disease–classification, epidemiology and diagnosis. Forum Nefrol. 2008, 1, 1–6. (In Polish) [Google Scholar]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.R.; Aeddula, N.R. Chronic Kidney Disease; StatPearls: Tampa, FL, USA, 2022. [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, 1–276. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef]
- Rutkowski, B.; Czekalski, S.; Sułowicz, W.; Wiecek, A.; Król, E.; Szubert, R.; Kraszewska, E. Epidemiology of kidney diseases in Poland–pilot program (PolNef). Przegląd Lek. 2004, 61, 22–24. (In Polish) [Google Scholar]
- Konsultacje Programu Polityki Zdrowotnej pn. “Regionalny Program Zdrowotny Zapobiegania i Wczesnego Wykrywania Przewlekłej Choroby Nerek Wśród Mieszkańców Województwa Dolnośląskiego”; Taryfikacji nr 1/2020 z Dnia; Opinia Prezesa Agencji Oceny Technologii Medycznych: Warsaw, Poland, 31 January 2020.
- Development of DAiS MZ Based on Data from the National Health Fundation. Available online: https://analizy.mz.gov.pl/app/mpz_2020_poz (accessed on 1 April 2024).
- Borg, R.; Carlson, N.; Søndergaard, J.; Persson, F. The Growing Challenge of Chronic Kidney Disease: An Overview of Current Knowledge. Int. J. Nephrol. 2023, 2023, 9609266. [Google Scholar] [CrossRef]
- Brück, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Völzke, H.; Ärnlöv, J.; Kastarinen, M.; Guessous, I.; Vinhas, J.; Stengel, B.; et al. CKD Prevalence Varies across the European General Population. J. Am. Soc. Nephrol. 2016, 7, 2135–2147. [Google Scholar] [CrossRef]
- Renke, M.; Parszuto, J.; Rybacki, M.; Wolyniec, W.; Rutkowski, P.; Rutkowski, B.; Walusiak-Skorupa, J.; Dębska-Ślizień, A. Chronic kidney disease–The relevant information for an occupational physician. Med. Pract. 2018, 69, 67–75. [Google Scholar] [CrossRef]
- Fraser, S.D.; Blakeman, T. Chronic kidney disease: Identification and management in primary care. Pragmat. Obs. Res. 2016, 7, 21–32. [Google Scholar] [CrossRef]
- Jazienicka-Kiełb, A.; Babicki, M.; Krajewska, M.; Oko, A.; Kłoda, K.; Mastalerz-Migas, A. Assessment of primary care physicians’ knowledge of chronic kidney disease in Poland. Front. Public Health 2022, 10, 1032240. [Google Scholar] [CrossRef]
- Masajtis-Zagajewska, A.; Kurek, R.; Modrzyńska, K.; Coker, T.; Nowicki, M. The Clinical and Economic Burden of Chronic Kidney Disease in Poland: Inside Patient-Level Microsimulation Modelling of CKD. J. Clin. Med. 2025, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Jadoul, M.; Aoun, M.; Masimango Imani, M. The major global burden of chronic kidney disease. Lancet Glob. Health 2024, 12, e342–e343. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Okpechi, I.G.; Levin, A.; Ye, F.; Damster, S.; Arruebo, S.; Donner, J.-A.; Caskey, F.J.; Cho, Y.; Davids, M.R.; et al. An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob. Health 2024, 12, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Kaze, A.D.; Ilori, T.; Jaar, B.G.; Echouffo-Tcheugui, J.B. Burden of chronic kidney disease on the African continent: A systematic review and meta-analysis. BMC Nephrol. 2018, 19, 125. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. In Kidney International Supplements; Elsevier: Amsterdam, The Netherlands, 2022; Volume 12, pp. 7–11. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Rothenbacher, D. Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health 2008, 8, 117. [Google Scholar] [CrossRef]
- Wieliczko, M.; Kulicki, P.; Matuszkiewicz-Rowińska, J. Classification, epidemiology and causes of chronic kidney disease. Wiadomości Lek. 2014, 65, 393–396. (In Polish) [Google Scholar]
- Ji, A.; Pan, C.; Wang, H.; Jin, Z.; Lee, J.H.; Wu, Q.; Jiang, Q.; Cui, L. Prevalence and Associated Risk Factors of Chronic Kidney Disease in an Elderly Population from Eastern China. Int. J. Environ. Res. Public Health 2019, 16, 4383. [Google Scholar] [CrossRef]
- Zdrojewski, T.; Rutkowski, M.; Bandosz, P.; Gaciong, Z.; Jędrzejczyk, T.; Solnica, B.; Pencina, M.; Drygas, W.; Wojtyniak, B.; Grodzicki, T.; et al. Prevalence and control of cardiovascular risk factors in Poland. Assumptions and objectives of the NATPOL 2011 Survey. Kardiol. Pol. 2013, 71, 381–392. [Google Scholar] [CrossRef]
- Persson, F.; Charles, M.; Povlsen, J.V.; Knudsen, S.T. Improving frequency of urinary albumin testing in type 2 diabetes in primary care–an analysis of cross-sectional studies in Denmark. Prim. Care Diabetes 2021, 15, 1007–1011. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Alicic, R.Z.; Duru, O.K.; Jones, C.R.; Daratha, K.B.; Nicholas, S.B.; McPherson, S.M.; Neumiller, J.J.; Bell, D.S.; Mangione, C.M.; et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: An analysis of the CURE-CKD registry. JAMA Netw. Open 2019, 2, e1918169. [Google Scholar] [CrossRef] [PubMed]
- Zmieniające Zarządzenie w Sprawie Warunków Zawarcia i Realizacji Umów o Udzielanie Świadczeń Opieki Zdrowotnej w Rodzaju Podstawowa Opieka Zdrowotna; ZARZĄDZENIE NR 124/2022/DSOZ; Prezesa Narodowego Funduszu Zdrowia: Warsaw, Poland, 29 September 2022.
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease–A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Chen, Y.; Dabbas, W.; Gangemi, A.; Benedetti, E.; Lash, J.; Finn, P.W.; Perkins, D.L. Obesity Management and Chronic Kidney Disease. Semin. Nephrol. 2021, 4, 392–402. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Y.; Rasheed, H.; Walker, V.; Sugawara, Y.; Li, J.; Leng, Y.; Elsworth, B.; E Wootton, R.; Fang, S.; et al. Trans-ethnic Mendelian-randomization study reveals causal correlation between cardiometabolic factors and chronic kidney disease. Int. J. Epidemiol. 2022, 50, 1995–2010. [Google Scholar] [CrossRef]
- Gupta, A.; Nagaraju, S.P.; Bhojaraja, M.V.; Swaminathan, S.M.; Mohan, P.B. Hypertension in Chronic Kidney Disease: An Update on Diagnosis and Management. South. Med. J. 2023, 116, 237–244. [Google Scholar] [CrossRef]
- Hamrahian, S.M.; Falkner, B. Hypertension in Chronic Kidney Disease. Adv. Exp. Med. Biol. 2017, 956, 307–325. [Google Scholar] [CrossRef]
- Sternlicht, H.; Bakris, G.L. The Kidney in Hypertension. Med. Clin. N. Am. 2017, 101, 207–217. [Google Scholar] [CrossRef]
- Guo, W.; Song, Y.; Sun, Y.; Du, H.; Cai, Y.; You, Q.; Fu, H.; Shao, L. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011–2018. Front. Endocrinol. 2022, 13, 1071465. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, R.D.; Morgan, A.R.; Whyte, M.B.; Hanif, W.; Bain, S.C.; Davies, S.; Dashora, U.; Yousef, Z.; Patel, D.C.; et al. A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Adv. Ther. 2022, 39, 33–43. [Google Scholar] [CrossRef]
- Nordheim, E.; Geir Jenssen, T. Chronic kidney disease in patients with diabetes mellitus. Endocr. Connect. 2021, 10, R151–R159. [Google Scholar] [CrossRef]
- Joshi, R.; Subedi, P.; Yadav, G.K.; Khadka, S.; Rijal, T.; Amgain, K.; Rajbhandari, S. Prevalence and risk factors of chronic kidney disease among patients with type 2 diabetes mellitus at a tertiary care hospital in Nepal: A cross-sectional study. BMJ Open 2023, 13, e067238. [Google Scholar] [CrossRef] [PubMed]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef] [PubMed]
- Schefold, J.C.; Filippatos, G.; Hasenfuss, G.; Anker, S.D.; von Haehling, S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat. Rev. Nephrol. 2016, 12, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; Van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef]
- Ludność Rezydująca–Informacja o Wynikach Narodowego Spisu Powszechnego Ludności i Mieszkań 2021–21.12.2022 r; Główny Urząd Statystyczny: Warsaw, Poland, 2023.
- Ludność. Stan i Struktura Oraz Ruch Naturalny w Przekroju Terytorialnym w 2023 r; Główny Urząd Statystyczny: Warsaw, Poland, 2024. [Google Scholar]


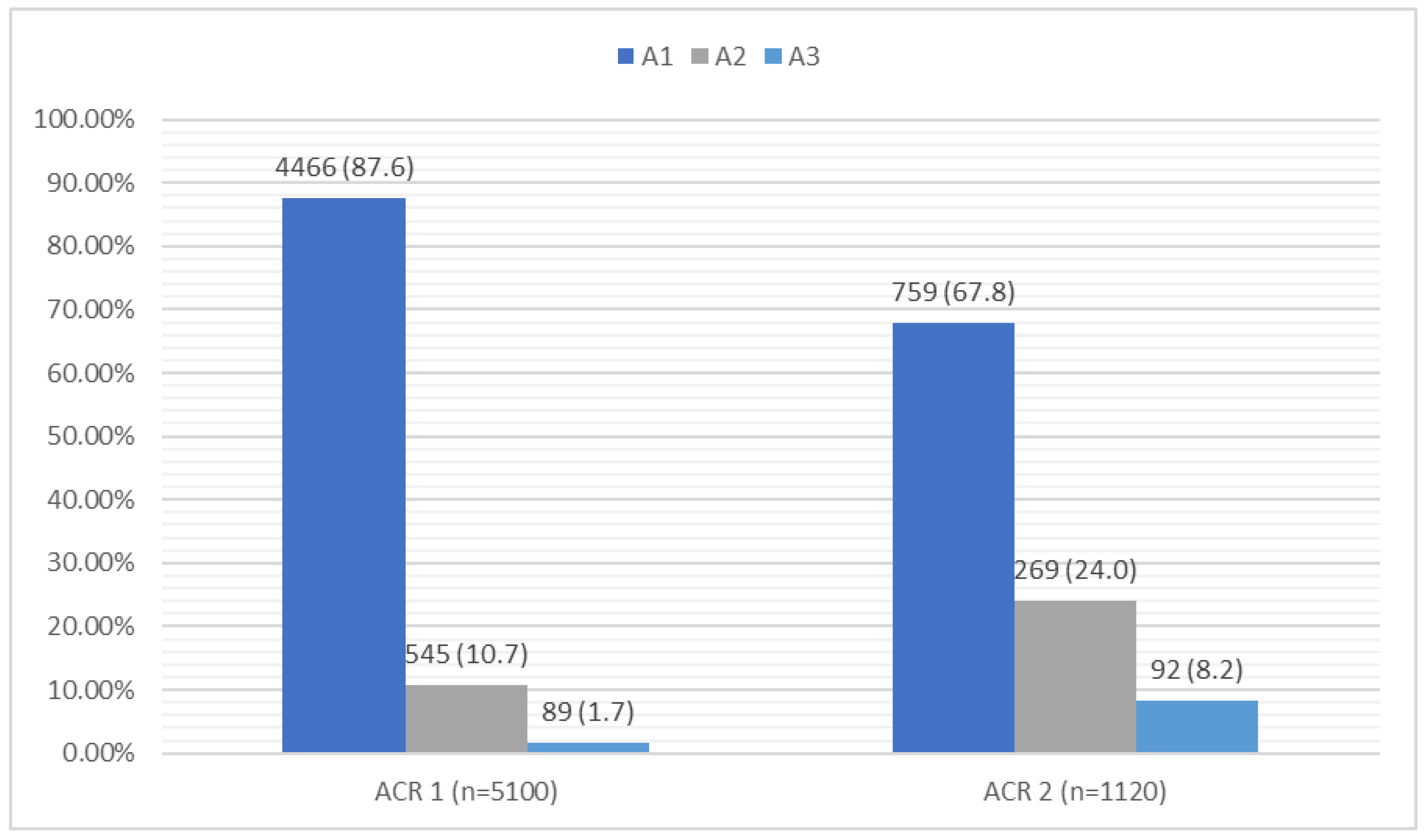

| Variables | The Whole Group N (%) M ± SD | CKD N (%) M ± SD | Non-CKD N (%) M ± SD | p | |
|---|---|---|---|---|---|
| Age | 60.7 ± 10.6 | 64.5 ± 9.1 | 60.1 ± 10.7 | <0.001 | |
| BMI | 29.9 ± 5.6 | 30.5 ± 5.6 | 29.9 ± 5.6 | 0.004 | |
| Sex | Female | 2759 (54.1) | 384 (13.9) | 2375 (86.1) | 0.537 |
| Male | 2341 (45.9) | 340 (14.5) | 2001 (85.5) | ||
| Place of residence | Village | 3937 (77.2) | 557 (14.2) | 3380 (85.8) | 0.068 |
| Up to 500 thousand | 511 (10.0) | 87 (17.0) | 424 (83.0) | ||
| >500 thousand | 652 (12.8) | 80 (12.3) | 572 (87.7) | ||
| Risk factors for CKD | Age 60–75 | 3046 (59.7) | 508 (16.6) | 2558 (83.4) | <0.001 |
| Obesity | 1894 (37.1) | 277 (14.6) | 1617 (85.4) | 0.499 | |
| Diabetes | 1041 (20.4) | 232 (22.3) | 809 (77.7) | <0.001 | |
| Hypertension | 3377 (66.2) | 526 (15.6) | 2851 (84.4) | <0.001 | |
| Kidney disease other than CKD | 271 (5.3) | 36 (13.3) | 235 (86.7) | 0.656 | |
| Heart failure | 129 (2.5) | 48 (37.2) | 81 (62.8) | <0.001 | |
| Cardiovascular diseases other than hypertension and heart failure | 350 (6.9) | 96 (27.4) | 254 (72.6) | <0.001 | |
| The sum of risk factors | 2.0 ± 1.1 | 2.4 ± 1.2 | 1.9 ± 1.0 | <0.001 | |
| Variable | OR [95Cl] | p |
|---|---|---|
| Age, years | 1.04 [1.03, 1.06] | <0.001 |
| BMI | 1.01 [1.01, 1.03] | 0.007 |
| Place of residence, village | 1.17 [0.92, 1.52] | 0.773 |
| Place of residence, <500 thousand residents | 1.47 [1.06, 2.04] | 0.024 |
| Sex, female | 1.05 [0.89, 1.23] | 0.536 |
| Age 60–75 | 1.67 [1.41, 1.98] | <0.001 |
| Obesity | 1.05 [0.89, 1.24] | 0.499 |
| Diabetes | 2.08 [1.75, 2.47] | <0.001 |
| Hypertension | 1.42 [1.19, 1.69] | <0.001 |
| Kidney disease other than CKD | 0.92 [0.64, 1.32] | 0.658 |
| Heart failure | 3.76 [2.61, 5.43] | <0.001 |
| Cardiovascular diseases other than hypertension and heart failure | 2.47 [1.93, 3.18] | <0.001 |
| The sum of risk factors | 1.58 [1.53, 1.63] | <0.001 |
| Variable | OR [95Cl] | p | R2 Nagelkerke |
|---|---|---|---|
| Age, years | 1.06 [1.05, 1.08] | <0.001 | 0.099 |
| BMI | 1.01 [1.01, 1.02] | 0.022 | |
| Place of residence, village | 1.26 [1.06, 1.50] | 0.009 | |
| Place of residence, <500 thousand residents | 1.55 [1.24, 1.95] | <0.001 | |
| Sex, female | 0.92 [0.80, 1.00] | 0.069 | |
| Age 60–75 | 1.67 [1.41, 1.98] | <0.001 | |
| Obesity | 0.91 [0.79, 1.04] | 0.184 | |
| Diabetes | 1.73 [1.54, 1.94] | <0.001 | |
| Hypertension | 1.16 [1.00, 1.36] | 0.047 | |
| Kidney disease other than CKD | 2.37 [1.83, 3.08] | <0.001 | |
| Heart failure | 1.94 [1.57, 2.41] | <0.001 | |
| Cardiovascular diseases other than hypertension and heart failure | 1.42 [1.22, 1.68] | <0.001 | |
| The sum of risk factors | 2.01 [1.32, 3.07] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jazienicka, A.; Babicki, M.; Krajewska, M.; Oko, A.; Kłoda, K.; Biesiada, A.; Mastalerz-Migas, A. A Nationwide Epidemiological Study of Chronic Kidney Disease Prevalence in a High-Risk Patient Population Without Prior Diagnosis in Primary Health Care in Poland. J. Clin. Med. 2025, 14, 3600. https://doi.org/10.3390/jcm14103600
Jazienicka A, Babicki M, Krajewska M, Oko A, Kłoda K, Biesiada A, Mastalerz-Migas A. A Nationwide Epidemiological Study of Chronic Kidney Disease Prevalence in a High-Risk Patient Population Without Prior Diagnosis in Primary Health Care in Poland. Journal of Clinical Medicine. 2025; 14(10):3600. https://doi.org/10.3390/jcm14103600
Chicago/Turabian StyleJazienicka, Alicja, Mateusz Babicki, Magdalena Krajewska, Andrzej Oko, Karolina Kłoda, Aleksander Biesiada, and Agnieszka Mastalerz-Migas. 2025. "A Nationwide Epidemiological Study of Chronic Kidney Disease Prevalence in a High-Risk Patient Population Without Prior Diagnosis in Primary Health Care in Poland" Journal of Clinical Medicine 14, no. 10: 3600. https://doi.org/10.3390/jcm14103600
APA StyleJazienicka, A., Babicki, M., Krajewska, M., Oko, A., Kłoda, K., Biesiada, A., & Mastalerz-Migas, A. (2025). A Nationwide Epidemiological Study of Chronic Kidney Disease Prevalence in a High-Risk Patient Population Without Prior Diagnosis in Primary Health Care in Poland. Journal of Clinical Medicine, 14(10), 3600. https://doi.org/10.3390/jcm14103600






