Mineralocorticoid Receptor Antagonists in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Study Eligibility and Inclusion Criteria
2.3. Data Extraction
2.4. Risk of Bias
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment and Risk of Bias
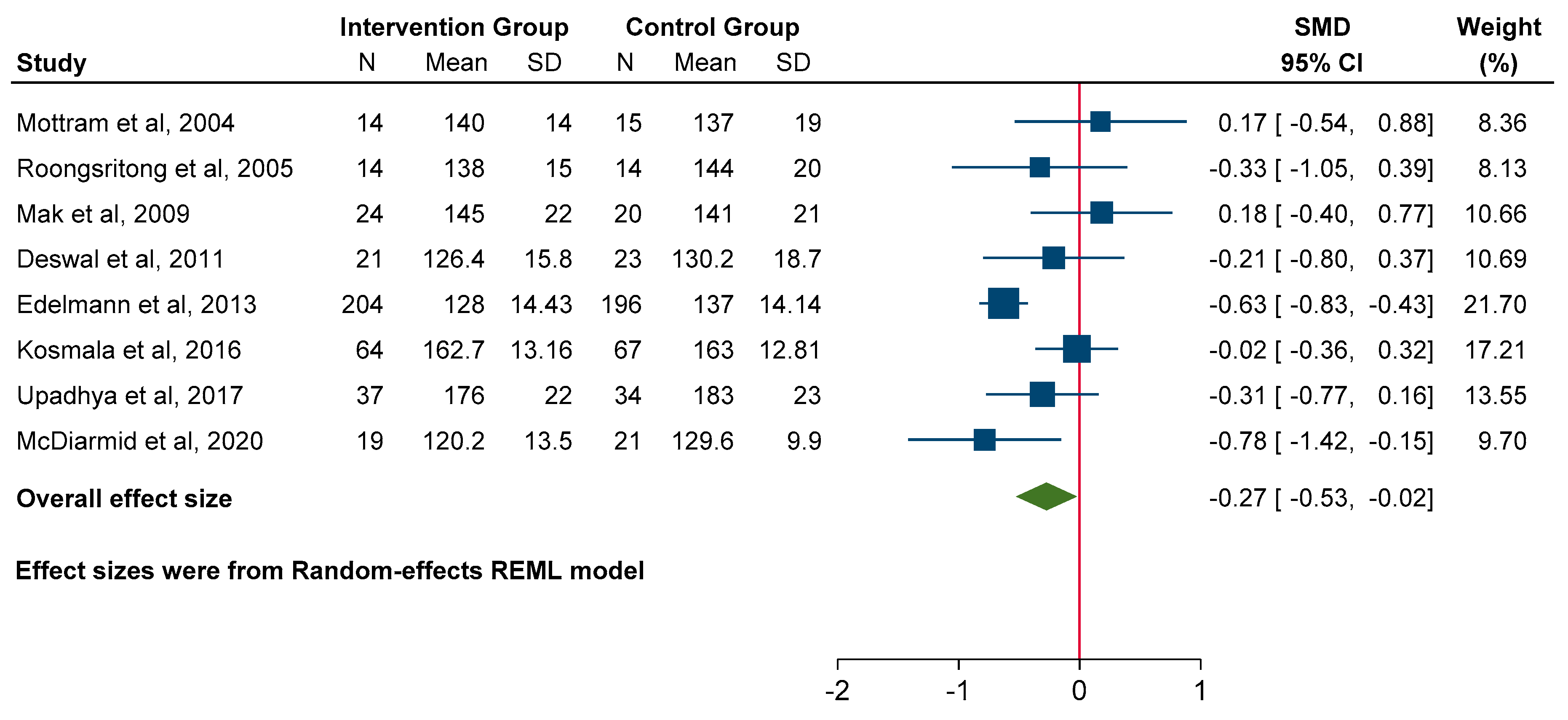
3.4. Diastolic Function
3.5. Effect on Blood Pressure
3.6. Functional Parameters
3.7. Quality-of-Life Measures
3.8. Adverse Events
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HFpEF | Heart failure with preserved ejection fraction |
| MRA | Mineralocorticoid receptor antagonists |
| RCT | Randomised controlled trials |
| NYHA | New York Heart Association |
| LVEF | Left ventricular ejection fraction |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| 6MWD | 6 min walk distance |
| MLWHFQ | Minnesota living with heart failure questionnaire |
| KCCQ | Kansas City cardiomyopathy questionnaire |
| QoL | Quality of life |
References
- Pieske, B.; Tschope, C.; de Boer, R.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Hear. J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Zannand, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. N. Engl. J. Med. 1999, 314, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Filippatos, B.G.; Ferreira, J.P.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Khariton, Y.; Malik, A.O.; Khumri, T.; Umpierrez, G.; et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 2021, 27, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Meta, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Vizzardi, E.; Regazzoni, V.; Caretta, G.; Gavazzoni, M.; Sciatti, E.; Bonadei, I.; Trichaki, E.; Raddino, R.; Metra, M. Mineralocorticoid receptor antagonist in heart failure: Past, present and future perspectives. Int. J. Cardiol. Heart Vessel. 2014, 3, 6–14. [Google Scholar] [CrossRef][Green Version]
- Brown, N.J. Eplerenone: Cardiovascular Protection. Circulation 2003, 107, 2512–2518. [Google Scholar] [CrossRef]
- Cittadini, A.; Monti, M.G.; Isgaard, J.; Casaburi, C.; Strömer, H.; Di Gianni, A.; Serpico, R.; Saldamarco, L.; Vanasia, M.; Saccà, L. Aldosterone receptor blockade improves left ventricular remodeling and increases ventricular fibrillation threshold in experimental heart failure. Cardiovasc. Res. 2003, 58, 555–564. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Jbi.global. Critical Appraisal Tools | Joanna Briggs Institute. 2021. Available online: https://jbi.global/critical-appraisal-tools (accessed on 20 February 2024).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane. 2021. Available online: https://www.training.cochrane.org/handbook (accessed on 20 February 2024).
- Mottram, P.M.; Haluska, B.; Leano, R.; Cowley, D.; Stowasser, M.; Marwick, T.H. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation 2004, 110, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Roongsritong, C.; Sutthiwan, P.; Bradley, J.; Simoni, J.; Power, S.; Meyerrose, G.E. Spironolactone improves diastolic function in the elderly. Clin. Cardiol. 2005, 28, 484–487. [Google Scholar] [CrossRef]
- Mak, G.J.; Ledwidge, M.T.; Watson, C.J.; Phelan, D.M.; Dawkins, I.R.; Murphy, N.F.; Patle, A.K.; Baugh, J.A.; McDonald, K.M. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J. Am. Coll. Cardiol. 2009, 54, 1674–1682. [Google Scholar] [CrossRef]
- Deswal, A.; Richardson, P.; Bozkurt, B.; Mann, D.L. Results of the Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction trial (RAAM-PEF). J. Card. Fail. 2011, 17, 634–642. [Google Scholar] [CrossRef]
- Edelmann, F.; Wachter, R.; Schmidt, A.G.; Kraigher-Krainer, E.; Colantonio, C.; Kamke, W.; Duvinage, A.; Stahrenberg, R.; Durstewitz, K.; Löffler, M.; et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-DHF randomized controlled trial. JAMA 2013, 309, 781–791. [Google Scholar] [CrossRef]
- Kurrelmeyer, K.M.; Ashton, Y.; Xu, J.; Nagueh, S.F.; Torre-Amione, G.; Deswal, A. Effects of spironolactone treatment in elderly women with heart failure and preserved left ventricular ejection fraction. J. Card. Fail. 2014, 20, 560–568. [Google Scholar] [CrossRef]
- Shah, A.M.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Deswal, A.; Anand, I.S.; Fleg, J.L.; Pitt, B.; Pfeffer, M.A.; Solomon, S.D. Prognostic Importance of Changes in Cardiac Structure and Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circ. Heart Fail. 2015, 8, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, W.; Rojek, A.; Przewlocka-Kosmala, M.; Wright, L.; Mysiak, A.; Marwick, T.H. Effect of Aldosterone Antagonism on Exercise Tolerance in Heart Failure With Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2016, 68, 1823–1834. [Google Scholar] [CrossRef]
- Kosmala, W.; Przewlocka-Kosmala, M.; Marwick, T.H. Association of Active and Passive Components of LV Diastolic Filling With Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Mechanistic Insights From Spironolactone Response. JACC Cardiovasc. Imaging 2019, 12, 784–794. [Google Scholar] [CrossRef]
- Upadhya, B.; Hundley, W.G.; Brubaker, P.H.; Morgan, T.M.; Stewart, K.P.; Kitzman, D.W. Effect of Spironolactone on Exercise Tolerance and Arterial Function in Older Adults with Heart Failure with Preserved Ejection Fraction. J. Am. Geriatr. Soc. 2017, 65, 2374–2382. [Google Scholar] [CrossRef]
- McDiarmid, A.K.; Swoboda, P.P.; Erhayiem, B.; Bounford, K.A.; Bijsterveld, P.; Tyndall, K.; Fent, G.J.; Garg, P.; Dobson, L.E.; Musa, T.A.; et al. Myocardial Effects of Aldosterone Antagonism in Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2020, 9, e011521. [Google Scholar] [CrossRef]
- Shantsila, E.; Shahid, F.; Sun, Y.; Deeks, J.; Calvert, M.; Fisher, J.P.; Kirchhof, P.; Gill, P.S.; Lip, G.Y.H. Spironolactone in Atrial Fibrillation With Preserved Cardiac Fraction: The IMPRESS-AF Trial. J. Am. Heart Assoc. 2020, 9, e016239. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, X.; Dong, M.; Gong, S.; Shang, Z.; Jia, X.; Chen, W.; Yang, J.; Li, J. Effects of spironolactone in heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Medicine 2018, 97e, 11942. [Google Scholar] [CrossRef]
- Kapelios, C.J.; Murrow, J.R.; Nührenberg, T.G.; Montoro Lopez, M.N. Effect of mineralocorticoid receptor antagonists on cardiac function in patients with heart failure and preserved ejection fraction: A systematic review and meta-analysis of randomized controlled trials. Heart Fail. Rev. 2019, 24, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- US National Library of Medicine. Spironolactone in the Treatment of Heart Failure (SPIRIT-HF). 29 January 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04727073 (accessed on 11 June 2024).
- van Dalen, B.M.; Chin, J.F.; Motiram, P.A.; Hendrix, A.; Emans, M.E.; Brugts, J.J.; Westenbrink, B.D.; de Boer, R.A. Challenges in the diagnosis of heart failure with preserved ejection fraction in individuals with obesity. Cardiovasc. Diabetol. 2025, 24, 71. [Google Scholar] [CrossRef]
- Kittipibul, V.; Lam, C.S.P. Heart failure with preserved ejection fraction and atrial fibrillation: Epidemiology, pathophysiology, and diagnosis interplay. Heart Fail. Rev. 2025, 30, 1–11. [Google Scholar] [CrossRef]
- La Fazia, V.M.; Pierucci, N.; Mohanty, S.; Chiricolo, G.; Natale, A. Atrial fibrillation ablation in heart failure with preserved ejection fraction. Card. Electrophysiol. Clin. 2025, 17, 53–62. [Google Scholar] [CrossRef]
- Pfeffer, M.; Claggett, B.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; Gordeev, I.; et al. Regional Variation in Patients and Outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.E.; Tromp, J.; Shah, S.J.; Lam, C.S.; Lewis, G.D.; Borlaug, B.A.; Sharma, K.; Pandey, A.; Sweitzer, N.K.; Kitzman, D.W. Phenomapping in heart failure with preserved ejection fraction: Insights, limitations, and future directions. Cardiovasc. Res. 2022, 118, 3403–3415. [Google Scholar] [CrossRef] [PubMed]









| Study (Year) | Country | Sample Size (MRA/Control) | Mean Age (Years) | LVEF (%) | Intervention | Duration | Key Outcomes Reported |
|---|---|---|---|---|---|---|---|
| Mottram (2004) [14] | Australia | 15/15 | 62 ± 7 | >50 | Spironolactone 25 mg daily | 6 months | Echo (E/A, EDT), BP |
| Roongsritong (2005) [15] | USA | 15/15 | ~72 | ≥45 | Spironolactone 25 mg daily | 4 months | Echo (E/A, EDT), BNP, PICP |
| Mak (2009) [16] | Ireland | 24/20 | 80 ± 7.8 | >45 | Eplerenone 25–50 mg daily | 12 months | Echo, biomarkers, NYHA class, QoL |
| Deswal (2011) [17] | USA | 23/23 | ~70 | ≥50 | Eplerenone 25–50 mg daily | 6.5 months | Echo, biomarkers, 6MWD, QoL |
| Edelmann (2013) [18] | Germany, Austria | 213/209 | 67 ± 8 | ≥50 | Spironolactone 25 mg daily | 12 months | Echo, biomarkers, 6MWD, peak VO2, QoL |
| Kurrelmeyer (2014) [19] | USA | 24/24 | 70 | ≥50 | Spironolactone 25 mg daily | 6 months | Echo, biomarkers, 6MWD, QoL |
| Shah (2015) [20] | USA, Russia, Georgia | 121/118 | ~69 | ≥45 | Spironolactone 15–45 mg daily | 18 months | Echo |
| Kosmala (2016) [21] | Australia | 75/75 | 67 ± 9 | >50 | Spironolactone 25 mg daily | 6 months | Echo, biomarkers, peak VO2 |
| Kosmala (2019) [22] | Australia | 51/54 | 64 ± 8 | >50 | Spironolactone 25 mg daily | 6 months | Echo, biomarkers, peak VO2 |
| Upadhya (2017) [23] | USA | 42/38 | 71 ± 1 | ≥50 | Spironolactone 25 mg daily | 9 months | Echo, CMR, biomarkers, 6MWD, QoL |
| McDiarmid (2020) [24] | UK | 27/24 | 75 ± 7.3 | >50 | Spironolactone 25 mg daily | 6 months | Echo, CMR, biomarkers |
| Shantsila (2020) [25] | UK | 125/125 | 72.3 ± 7.4 | ≥55 | Spironolactone 25 mg daily | 2 years | Echo, biomarkers, 6MWD, QoL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaheen, M.; Ferdous, F.; Amarasekera, A.T.; Petutschnigg, J.; Edelmann, F.; Tan, T.C. Mineralocorticoid Receptor Antagonists in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 3598. https://doi.org/10.3390/jcm14103598
Zaheen M, Ferdous F, Amarasekera AT, Petutschnigg J, Edelmann F, Tan TC. Mineralocorticoid Receptor Antagonists in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(10):3598. https://doi.org/10.3390/jcm14103598
Chicago/Turabian StyleZaheen, Mithila, Fardin Ferdous, Anjalee T. Amarasekera, Johannes Petutschnigg, Frank Edelmann, and Timothy C. Tan. 2025. "Mineralocorticoid Receptor Antagonists in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 10: 3598. https://doi.org/10.3390/jcm14103598
APA StyleZaheen, M., Ferdous, F., Amarasekera, A. T., Petutschnigg, J., Edelmann, F., & Tan, T. C. (2025). Mineralocorticoid Receptor Antagonists in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(10), 3598. https://doi.org/10.3390/jcm14103598






